Abstract
Introduction
Extended-spectrum beta-lactamases (ESBL) and AmpC beta-lactamases (AmpC) are of concern for veterinary and public health because of their ability to cause treatment failure due to antimicrobial resistance in Enterobacteriaceae. The main objective was to assess the relative contribution (RC) of different types of meat to the exposure of consumers to ESBL/AmpC and their potential importance for human infections in Denmark.
Material and methods
The prevalence of each genotype of ESBL/AmpC-producing E. coli in imported and nationally produced broiler meat, pork and beef was weighted by the meat consumption patterns. Data originated from the Danish surveillance program for antibiotic use and antibiotic resistance (DANMAP) from 2009 to 2011. DANMAP also provided data about human ESBL/AmpC cases in 2011, which were used to assess a possible genotype overlap. Uncertainty about the occurrence of ESBL/AmpC-producing E. coli in meat was assessed by inspecting beta distributions given the available data of the genotypes in each type of meat.
Results and discussion
Broiler meat represented the largest part (83.8%) of the estimated ESBL/AmpC-contaminated pool of meat compared to pork (12.5%) and beef (3.7%). CMY-2 was the genotype with the highest RC to human exposure (58.3%). However, this genotype is rarely found in human infections in Denmark.
Conclusion
The overlap between ESBL/AmpC genotypes in meat and human E. coli infections was limited. This suggests that meat might constitute a less important source of ESBL/AmpC exposure to humans in Denmark than previously thought – maybe because the use of cephalosporins is restricted in cattle and banned in poultry and pigs. Nonetheless, more detailed surveillance data are required to determine the contribution of meat compared to other sources, such as travelling, pets, water resources, community and hospitals in the pursuit of a full source attribution model.
Extended-spectrum beta-lactamases (ESBL) were defined by the EFSA Panel on Biological Hazards (BIOHAZ) as plasmid-encoded enzymes found in the bacterial family Enterobacteriaceae, commonly in Escherichia coli and Klebsiella pneumonia. ESBL confer resistance to a variety of beta-lactam antibiotics, including penicillins; second-, third-, and fourth-generation cephalosporins; and monobactams (Citation1). The BIOHAZ panel also stated that AmpC beta-lactamases (AmpC) are intrinsic cephalosporinases found on the chromosomal DNA of many gram-negative bacteria. However, the number of AmpC enzymes that are plasmid borne is increasing (Citation1). An example of such plasmidic AmpC is CMY-2.
Burden of disease
The World Health Organization (WHO) estimated 25,000 deaths each year in the European Union (EU) due to infections with antibiotic-resistant bacteria (Citation2). The increase of antimicrobial resistance caused by ESBL/AmpC is particularly worrying due to their resistance to third- and fourth-generation cephalosporins, which have been considered critically important to human medicine (Citation3).
Infections caused by ESBL/AmpC-producing bacteria are increasing worldwide (Citation4). The European Antimicrobial Resistance Surveillance Network (EARS-Net) stated that no country among the 28 countries reporting to this program has demonstrated decreasing trends of third-generation cephalosporin-resistant E. coli over the past few years (Citation5). Moreover, in 2011, more than half of the countries reported proportions between 85 and 100% of third-generation cephalosporin-resistant E. coli to be ESBL-producing (Citation5). In Denmark, a steady increase of third-generation cephalosporin-resistant E. coli from human bloodstream infections has been observed from 2005 (1.1%) through 2011 (8.5%) (Citation6).
The importance of the increase of ESBL/AmpC among E. coli septicemias can also be reflected in the augmented case fatality risk (Citation7), although it should be noted that these bacteraemic cases are often associated with a critical underlying disease, delay in appropriate treatment and older age (Citation8).
In general, the economic burden of antimicrobial resistance is significant (Citation9). Moreover, ESBL/AmpC resistance leads to an increase in the use of carbapenems (Citation4). The use of this class of drugs is related to the emergence of carbapenemases (Citation10), which have the ability to hydrolyze all the beta-lactams and are therefore of concern (Citation1).
Possible ESBL/AmpC-producing bacteria sources
There are several potential ESBL/AmpC-producing bacteria sources that can be summarized in five major groups: foodborne, direct animal-to-human transmission, human-to-human transmission, environment and infections obtained abroad during travelling. Travelling is considered a source by itself since surveillance programs are normally set at a national level. Moreover, it is more difficult to unveil the specific source when infection is acquired abroad. To our knowledge, the true importance of each of these possible sources for ESBL/AmpC dissemination and their relative relevance for human cases is unknown. Recently, a possible link to food and food-producing animals was made (Citation1), stressing the importance of scrutinizing the role of food animal products to human ESBL/AmpC-producing bacterial infections.
The Danish situation
Denmark, like other Nordic countries, has always been considered to have a low prevalence of antimicrobial resistance in the livestock population, due to the conscious use and restrictive measures related to antibiotics. The ‘Yellow card’ initiative (Citation11) and the voluntary ban on the use of cephalosporin in pigs (Citation12) are examples of the precautionary Danish policy regarding the antimicrobial use in animal production.
From 2000 to 2008, DANMAP reports consistently showed a very low prevalence of third-generation cephalosporin-resistant bacteria both in live animals and in Danish meat (below 2% of positive samples) (Citation13–Citation21). Meat samples with 5 g of weight were collected at wholesale and retail outlets and a non-selective culture method was used.
To detect ESBL/AmpC-producing bacteria, an antibiotic enriched culture method is preferred (Citation1). In 2009, the first surveillance study using an enriched culture method was performed, followed by a genetic background investigation (Citation22). In relation to the sample weight and place of sample collection, the original procedure was kept. Since then, this protocol has been repeated yearly, revealing an increasing prevalence of ESBL/AmpC-producing E. coli in Danish broiler meat from 3% in 2009 (data not published) to 44% in 2011 (Citation23). In the same period, the prevalence in imported broiler meat ranged from 36 to 50% (Citation22–Citation24). This has raised concern that meat may be an increasingly important source of human cases in Denmark.
Hence, the aim of this study was to determine the relative contribution (RC) of various meat types to the exposure of Danish meat consumers to ESBL/AmpC-producing E. coli and their potential importance for human infections in Denmark.
Material and methods
A relative exposure assessment was performed. The approach was to compare the distributions of genotypes in humans and the most common meat sources in Denmark.
Meat consumption patterns
Data about the fresh and frozen meat available for consumption in 2009, 2010 and 2011 from the ‘Annual Report on Zoonoses in Denmark 2011’ were consulted and divided into six categories: imported and domestically produced broiler meat, pork and beef (Citation25) (). Consumption was calculated as:
Fig. 1. Fresh and frozen meat available for consumption in Denmark of Danish (DK) and imported (IMP) origin between 2009 and 2011. Source: DTU 2011.
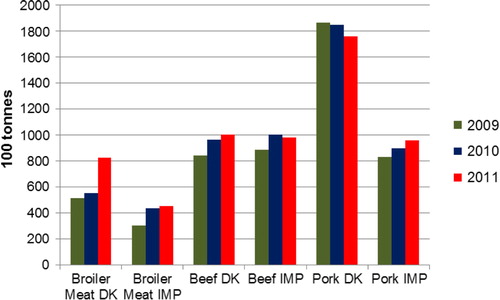
Domestically produced – exported +imported (Citation25).
ESBL/AmpC-producing E. coli occurrence in meat
The data describing the ESBL/AmpC prevalence were collected from the Danish surveillance program for antibiotic use and antibiotic resistance (Citation22–Citation24). In the DANMAP program, meat samples (5 g per sample for all three species) are collected at wholesale/retail level and detection of ESBL/AmpC-producing E. coli is conducted using a selective culture method using antimicrobials. This is a qualitative method with a very high sensitivity.
RC of each type of meat to human exposure
To calculate the contribution of each type of meat and each genotype to human exposure, the prevalence of ESBL/AmpC genotypes was weighted by the amount of meat available for consumption: Weighted Prevalence (WP)=Prevalence * Meat available for consumption. All the WPs were added up to obtain the total pool of ESBL/AmpC in meat (TP). Finally, the WP of each genotype was divided by the ESBL/AmpC Total Pool: RC=WP/TP.
Due to the low number of positive samples for pork and beef and the low number of samples tested in general, the 3 years of sampling were analyzed collectively. However, the described procedure was also applied to each year separately.
Uncertainty distributions
Using the software @Risk 4.5 Palisade Corporation®, beta distributions were created to assess the 95% credibility interval (CI) for each genotype. The main objective of this procedure was to conduct an uncertainty analysis and hereby estimate how high the true prevalence could be given the available data. Beta distributions were used in previous risk assessments to take into account the uncertainty and variability of estimates (Citation26).
The beta distributions were made through a simulation using 1,000,000 iterations. The distributions were defined by (s+1, n−s+1), where s represents the number of positive samples for each genotype in each type of meat from 2009 to 2011, and n represents the total number of samples tested for ESBL/AmpC-producing E. coli in each type of meat within the same 3-year period.
This procedure was of particular relevance because some genotypes have not been found in the Danish sampling surveillance, but still may have been present in meat and could have played a role for ESBL/AmpC-producing E. coli human cases. Their distribution was set as (0+1, n−0+1). Some genotypes were not found in meat during the surveillance program, but were found in live animals. The higher the number of samples tested and found negative, the lower the maximum prevalence that could be in the meat.
Results
RC of each type of meat to human exposure
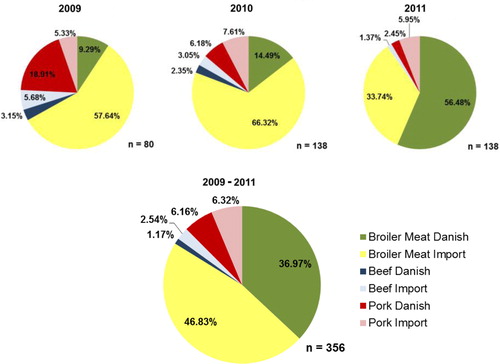
During the period 2009–2011, broiler meat contributed the most to the total human exposure to ESBL/AmpC representing 83.8% of the total exposure. Danish broiler meat contributed with 37.0% and imported broiler meat with 46.8%. Danish and imported pork constituted 6.2 and 6.3% of the ESBL/AmpC positive meat available for consumption, respectively. Beef was less important, representing 1.2% in beef of Danish origin and 2.5% of imported origin. The RC of Danish broiler meat increased markedly from 2009 to 2011, while the RC of pork and imported broiler meat decreased in the same period ().
Fig. 2. Relative contribution of each type of meat and their respective origin to human ESBL/AmpC- producing E. coli exposure, based on Danish data from 2009 to 2011 (Danmap, 2009, 2010, 2011).
The RC of each genotype varied among the 3 years (). Most important was the increase in the role of CMY-2 in Danish broiler meat from 4.7% in 2009 to 11.8% in 2010, increasing to 53.5% of the total exposure in 2011. Moreover, CMY-2 constituted the genotype that meat consumers were most exposed to (58.3% across the 3-year period). Broiler meat was the source that contributed the most to this specific exposure (56.8% from broiler meat, 0.8% from pork, and 0.7% from beef).
Table 1 Relative contribution (RC) of each type of meat for human exposure considering the origin and the genotypes found in DANMAP surveillance from 2009 to 2011
CTX-M-1 was the most frequently isolated genotype in pork and beef at 8.2 and 2.5% of the total exposure, respectively. Including broiler meat, CTX-M-1 represented 28.8% of the total ESBL/AmpC-positive meat on the Danish market from 2009 to 2011.
Considering the origin of positive meat for ESBL/AmpC, a major shift occurred from 2010 to 2011. Both in 2009 and 2010, Danish meat represented less than one third of the ESBL/AmpC meat contributing to human exposure (31.4 and 23.0%, respectively). In 2011, the RC for human exposure of the Danish meat rose to 58.9% and the imported meat declined to 41.1%. However, across the 3-year period, imported meat still contributed the most to human exposure (55.7%).
Genotype overlapping between meat and human infections
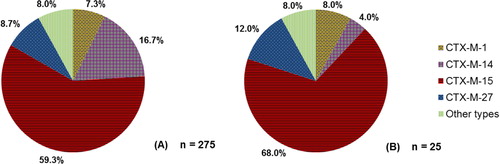
In humans, the so-called ‘pandemic’ CTX-M-15 genotype dominated in 2011 in Denmark, both in blood and urine ESBL-producing E. coli isolates, with 68.0 and 59.3%, respectively (Citation23) (). CTX-M-1 was detected in 7.3% of urine and 8.0% of blood isolates (Citation23).
Fig. 3. (A) Proportion of various genotypes detected in ESBL-producing E. coli urinary tract infections in humans in Denmark in 2011. (B) Proportion of various genotypes detected in ESBL-producing E. coli bloodstream infections in humans in Denmark in 2011. Source: DANMAP 2011.
CMY-2 was detected in human infections, but it was not possible to calculate its relative prevalence in urinary tract infections and septicemias. Nevertheless, the number of positive samples was low (data not published).
Considering both the human data and the results from the RC of each genotype to the human exposure through meat, the genotype overlapping between the two reservoirs is low.
Prevalence estimations for each genotype
CTX-M-15 was the genotype most commonly found in human cases. It was found on rare occasions in Danish pigs and cattle at slaughter: two positive samples in 2009 (n=786) and one in 2011 (n=777) in pigs, and one positive sample in 2010 (n=192) and another in 2011 (n=186) in cattle. However, this genotype had not been detected in meat samples previously. Through our calculations, this ESBL gene was estimated to be present in Danish pork and beef with prevalences below 0.7 and 1.0%, respectively, given the available data ().
Table 2 Lower limit, mode and upper limit of prevalence calculated for each genotype in each type of meat through beta distribution with a 95% CI
The prevalence of CTX-M-1 was 0.2% in imported pork (Citation23), but there was a high degree of uncertainty due to the low number of positive samples and total number of samples taken. The estimated upper limit of prevalence for this genotype was 3.8% in imported pork. CTX-M-14 was also estimated to be present in imported pork, with a prevalence below 1.3%. It should be noted that both CTX-M-1 and CTX-M-14 have been found in meat and in human cases in Denmark.
Imported broiler meat was the type of meat where the highest diversity of ESBL/AmpC genes was found in the DANMAP program. CMY-2 and CTX-M-1 had an upper limit of the estimated prevalence of 24.5 and 17.9%, respectively.
Discussion
We evaluated the RC of various types of meat for the exposure of consumers to ESBL/AmpC-producing E. coli and their potential importance for human cases. We concluded that broiler meat was the type of meat that contributed the most to human exposure (83.8%), while CMY-2 was the genotype with the highest RC (58.3% of the total exposure through meat). However, this genotype has only been found on rare occasions in Danish human infections.
A definitive cause-effect association cannot be established through our approach. A genotype overlay is not proof that the sources were the actual route of exposure, so overlaps are indicative of a physical link between the source and the exposed individual that warrant further investigations. On the contrary, the absence of a genotype overlap, considering an appropriate sample scheme, provides evidence against a possible link. Therefore, inferences can be made with caution. With this in mind, the limited genotype overlap between the two reservoirs indicates that meat might play a minor role for human cases of ESBL/AmpC-producing E. coli.
Data and methods
The meat consumption patterns were measured as the fresh and frozen meat available for consumption, not as the meat that was actually consumed. However, it was assumed that waste of meat occurred in the same proportion for all types of meat.
The bacterial capacity to survive in frozen and fresh meat might differ (Citation27). However, data were not available to take the survival in different types of meat and storage types into account. Therefore, this might result in potential bias.
It was not possible to extend the study to other types of meat, such as turkey or horse meat, due to the lack of data on ESBL/AmpC-producing E. coli prevalences. Nonetheless, the presented exposure assessment covered the three types of meat that represent the vast majority of meat consumed in Denmark.
The RC of meat to human exposure
Some changes were observed in the RC of each genotype from 2009 to 2011. Mainly minor shifts happened due to the sporadic detection of some ESBL/AmpC genes, and these were probably due to the limited sample sizes. The most evident change occurred with the increasing contribution of CMY-2 in Danish broiler meat from 4.7% in 2009 to 53.5% in 2011. This was due to the increasing prevalence of this genotype in Danish broiler meat, especially from 2010 to 2011 (Citation23).
Considering that cephalosporins have not been used in poultry in Denmark for more than 10 years, it is possible that the origin of the problem is upstream in the production pyramid. This hypothesis has also arisen in Sweden (Citation28, Citation29). Danish and Swedish broiler parents come from the same Swedish breeding stock, which in turn is supplied by a Scottish grand-parent breeding company, where cephalosporins have been used prophylactically until recently. In Denmark, an all in/all out policy and strict biosecurity measures are implemented. Nevertheless, transmission between flocks might be possible and should be dealt with: proper actions related to all logistics behind broiler feeding, technical support and broiler transportation to the slaughterhouses ((cages and trucks) which might help to limit the dissemination between flocks. Moreover, it should be investigated whether the animal feed might act as an important source of ESBL/AmpC-producing E. coli. However, the fact that CMY-2 and CTX-M-1 were also the genotypes detected in Swedish broiler meat (Citation30) and both of them being the only genotypes persistently detected in Danish broiler meat from 2009 to 2011, supports the hypothesis of a common source upstream in the production pyramid. In contrast, imported broiler meat presented a substantial variety of genotypes (Citation22–Citation24), possibly due to the different importing countries and various causes of ESBL/AmpC emergence.
In general, the use of other antimicrobials in the broiler production may facilitate the spread and maintenance of ESBL/AmpC-producing E. coli through co-resistance patterns (Citation29). Moreover, cross-contamination through the environment, humans and animals, as well as off-label use of cephalosporins are other ways of spreading resistance.
Denmark has one of the lowest antimicrobial uses in livestock of the EU and demonstrates a very prudent use of cephalosporins in animals (Citation31). The discontinuation of the use of cephalosporins in 2010 in pigs is the most likely explanation for the reduction in the ESBL/AmpC-producing E. coli prevalence seen in pigs in 2011 (Citation12). Other factors could also have contributed to this diminution, that is, the ‘Yellow card’ initiative, which took place almost at the same time and initially led to a 25% decrease in the use of antimicrobials in pigs (Citation11, Citation32).
The homogeneity of the ESBL/AmpC-producing E. coli genotypes from imported beef is mostly likely a result of the low number of positive samples: one positive sample per year out of 298 samples analyzed. In Danish beef just two positive samples from different genotypes were found from 2009 to 2011 (data not published). The total number of Danish beef collected samples during the 3-year period was 398. Due to the low number of positive samples, beef is the type of meat in which the uncertainty about the true ESBL/AmpC genotype distribution is the highest.
Genotype overlaps between meat and human infections
In humans, the so-called ‘pandemic’ CTX-M-15 genotype dominated in 2011 in Denmark, both in blood and urine ESBL-producing E. coli isolates, with 68.0 and 59.3%, respectively (Citation23) (). CTX-M-1 was detected in 7.3% of urine and 8.0% of blood isolates (Citation23). However, it should be noted that, particularly for human ESBL-producing E. coli septicemia, the number of cases that these results are based on is quite small (n=25). Consequently, it is possible that the true ESBL genotype distribution in Denmark is slightly different from what was found, and some genotypes may not have been detected.
ESBL/AmpC genes found in humans and meat were, as previously described, only congruent to a limited extent. Although some genotypes were not found in meat, they were found in live animals, indicating their possible presence in meat. That is the case of CTX-M-15, which was detected in very low prevalence in Danish pigs and Danish cattle. Its low prevalence probably explains why it was not detected in meat. This was one of the main motives that propelled us to perform an uncertainty analysis through the use of beta distributions. As expected, the mode prevalence was equal or very similar to the prevalence found in DANMAP and the uncertainty was higher with smaller sample sizes. The maximum expected prevalence for ESBL/AmpC genes not detected in meat, considering the DANMAP sampling within the 3-year period, was 0.7% for Danish pork, 0.9% for imported pork, 1.0% for Danish beef, 1.4% for imported beef, 0.9% for Danish broiler meat and 0.7% for imported broiler meat. Through our calculations, these are the upper 95% credibility limits for the expected prevalences for CTX-M-15 in each type of meat. Such low prevalence points against a relevant role of meat for the CTX-M-15-producing E. coli infections in humans. Nonetheless, more should be known about the duration of carriage, dominance in the human gut microflora and different reservoir adaptability and ESBL/AmpC-producing E. coli's diverse ability to survive in the human gut environment.
CMY-2 was detected in human infections, but it was not possible to calculate its relative prevalence in urinary tract infections and septicemias due to lack of information. Nevertheless, the positive number of samples in humans was low (data not published). Considering the contribution of CMY-2-producing E. coli for human exposure through meat, its relative prevalence in human infections must be calculated in the upcoming years.
A quantitative exposure assessment to third-generation cephalosporin-resistant E. coli through the consumption of broiler meat performed in Belgium (Citation33) also highlighted the lack of some important data to evaluate the impact of animal foodborne sources to human exposure.
Control options
Our results suggest that meat does not have as high impact on human ESBL/AmpC-producing E. coli infections in Denmark as previously thought. This might be related to the actions taken in Denmark to limit the use of cephalosporins in livestock. Hence, this does not exclude the importance of animal production, and even meat, for ESBL/AmpC dissemination, so a precautionary approach should be taken.
In Denmark, there are limited additional options to control ESBL/AmpC occurrence through the reduction of the use of cephalosporins in livestock. However, other antimicrobials can co-select ESBL/AmpC-producing bacteria. Therefore, measures to promote the reduction of antimicrobial use in general, such as the ‘Yellow card’ scheme, may have some influence. Regarding the high ESBL/AmpC-producing E. coli prevalences in Danish broiler meat, measures have recently been taken to assure the non-use of cephalosporins in the top of the production pyramid (Jan Dahl, Danish Agriculture and Food Council, personal communication, 2013).
Moreover, actions taken to limit cross-contamination during slaughter should always be encouraged.
A judicious use of resources must be done. In that sense, the role of other sources should be assessed including the annual human consumption of cephalosporins. In 2009, the human consumption of cephalosporins in Denmark was 2,740 kg compared to 95 kg in pigs (Citation22). This would allow us to point to the reservoir where most effort should be applied to control ESBL/AmpC-producing E. coli. presents some general measures to control ESBL/AmpC-producing E. coli and ESBL/AmpC-producing bacteria in Denmark.
Table 3 Suggested measures to control ESBL/AmpC-producing bacteria in Denmark
Meat might be the source for at least a small part of the human cases, but to obtain more precise conclusions, further studies are needed. ESBL/AmpC-producing bacteria is a complex subject and their emergence, ecology, and dynamics is nowadays poorly understood.
A wider study about the ESBL/AmpC genotypes in human cases in Denmark is recommended including an extensive characterization of human patients, that is, through a detailed and recorded patient history which may enable further investigations on the epidemiology of ESBL/AmpC-producing bacteria. Additionally, studying the risk factors for the colonization of healthy humans could be a relevant step for source attribution and important to prioritize control measures. However, new and more discriminatory molecular methods may be needed to distinguish different reservoirs and transmission routes.
The relevance of ESBL/AmpC-producing bacteria carriage for the occurrence of community acquired and nosocomial infections should also be assessed. This includes not only further investigations on the risk of transmission of ESBL/AmpC genetic material from bacteria in consumed food to commensal gut flora, but also an assessment of the relevance of ESBL/AmpC-producing bacteria carriage for the development of severe infections caused by the same bacteria.
Conclusion
In this study, broiler meat was the type of meat with the highest RC for human exposure (83.8%), followed by pork (12.5%) and beef (3.7%).
We conclude that the genotype overlap between human and meat from poultry, pigs and cattle is low, suggesting that meat might not have such a relevant role for ESBL/AmpC-producing E. coli human infections in Denmark in 2009–2011, as previously thought. This is most likely related to the limited use of cephalosporins in Danish livestock.
Conflict of interest and funding
The authors have no conflicts of interest.
Acknowledgements
We thank the Technical University of Denmark and the Statens Serum Institute of Denmark for their collaboration. Moreover, we acknowledge the European Union for funding an ERASMUS internship.
References
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J. 2011; 9: 2322.
- WHO. Tackling antibiotic resistance from a food safety perspective in Europe. 2011. 88 pp. Available from: http://www.euro.who.int/__data/assets/pdf_file/0005/136454/e94889.pdf [cited 10 June 2013]..
- WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically important antimicrobials for human medicine. 2011. 3rd Rev. 38 pp. Available from: http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf [cited 10 June 2013].
- Pitout JDD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008; 8: 159–66. [PubMed Abstract].
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2011. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2012; 212 pp. Available from: http://www.ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-surveillance-europe-2011.pdf [cited 24 May 2013]..
- European Centre for Disease Prevention and Control. EARS-net interactive database. Available from: http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/database/Pages/tables_report.aspx [cited 17 April 2013]..
- Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing. E. coli. J Infect. 2007; 55: 254–9.
- Rottier WC, Ammerlaan HSM, Bonten MJM. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012; 67: 1311–20. [PubMed Abstract].
- de Kraker MEA, Davey PG, Grundmann H. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011; 8: e1001104. [PubMed Abstract] [PubMed CentralFull Text].
- Wilcox MH. The tide of antimicrobial resistance and selection. Int J Antimicrob Agents. 2009; 34: S6–10. [PubMed Abstract].
- Alban L, Dahl J, Andreasen M, Petersen JV, Sandberg M. Possible impact of the “yellow card” antimicrobial scheme on meat inspection lesions in Danish finisher pigs. Prev Vet Med. 2013; 108: 334–41. [PubMed Abstract].
- Agersø Y, Aarestrup FM. Voluntary ban on cephalosporin use in Danish pig production has effectively reduced extended-spectrum cephalosporinase-producing Escherichia coli in slaughter pigs. J Antimicrob Chemother. 2013; 68: 569–72.
- DANMAP. DANMAP 2000 – Consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2001; 56 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2000/danmap_2000.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2001 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2002; 68 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2001/danmap_2001.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2002 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2003; 68 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2002/danmap_2002.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2003 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2004; 83 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2003/danmap_2003.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2004 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2005; 95 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2004/danmap_2004.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2005 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2006; 100 pp. Available from: http://www.food.dtu.dk//media/Institutter/Foedevareinstituttet/Publikationer/Pub-2005/danmap_2005.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2006 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2007; 101 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2006/danmap_2006.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2007 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2008; 108 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2007/danmap_2007.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2008 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2009; 132 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2008/danmap_2008.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2009 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2010; 136 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2009/DANMAP%202009.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2011 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2012; 140 pp. Available from: http://www.danmap.org/Downloads/~/media/Projekt%20sites/Danmap/DANMAP%20reports/Danmap_2011.ashx [cited 17 May 2013]..
- DANMAP. DANMAP 2010 – Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2011; 160 pp. Available from: http://orbit.dtu.dk/fedora/objects/orbit:89418/datastreams/file_6329669/content [cited 17 May 2013]..
- National Food Institute, Technical University of Denmark. Annual report on Zoonoses in Denmark 2011. 2012; 62 pp. Available from: http://www.food.dtu.dk/~/media/Institutter/Foedevareinstituttet/Publikationer/Pub2012/Annual%20report%20on%20Zoonoses%20in%20Denmark%202011.ashx [cited 12 June 2013]..
- Alban L, Nielsen EO, Dahl J. A human health risk assessment for macrolide-resistant Campylobacter associated with the use of macrolides in Danish pig production. Prev Vet Med. 2008; 83: 115–29. [PubMed Abstract].
- Black EP, Hirneisen KA, Hoover DG, Kniel KE. Fate of Escherichia coli O157:H7 in ground beef following high-pressure processing and freezing. J Appl Microbiol. 2010; 108: 1352–60. [PubMed Abstract].
- The National Veterinary Institute. SVARM 2010. Swedish Veterinary Antimicrobial Resistance Monitoring. 2011; 62 pp. Available from: http://www.sva.se/upload/Redesign2011/Pdf/Om_SVA/publikationer/1/Svarm2010.pdf [cited 18 July 2013]..
- Börjesson S, Bengtsson B, Jernberg C, Englund S. Spread of extended-spectrum beta-lactamase producing Escherichia coli isolates in Swedish broilers mediated by an incI plasmid carrying blaCTX-M-1. Acta Vet Scand. 2013; 55: 3.
- Börjesson S, Egervärn M, Lindblad M, Englund S. Frequent occurrence of extended-spectrum beta-lactamase and transferable AmpC beta-lactamase-producing Escherichia coli on domestic chicken meat in Sweden. Appl Environ Microbiol. 2013; 79: 2463–6.
- European Medicines Agency. Sales of veterinary antimicrobial agents in 19 EU/EEA countries in 2010 (EMA/88728/2012). 2012; 74 pp. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/10/WC500133532.pdf [cited 5 June 2013]..
- Aarestrup F. Sustainable farming: get pigs off antibiotics. Nature. 2012; 486: 465–6. [PubMed Abstract].
- Depoorter P, Persoons D, Uyttendaele M, Butaye P, De Zutter L, Dierick K, etal. Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium. Int J Food Microbiol. 2012; 159: 30–8. [PubMed Abstract].
