Abstract
We purposively selected 39 sampling sites along the Mara River and its two perennial tributaries of Amala and Nyangores and sampled snails. In addition, water physicochemical parameters (temperature, turbidity, dissolved oxygen, conductivity, alkalinity, salinity and pH) were taken to establish their influence on the snail abundance and habitat preference. Out of the 39 sites sampled, 10 (25.6%) had snails. The snail species encountered included Biomphalaria pfeifferi Krauss – the intermediate host of Schistosoma mansoni Sambon, Bulinus africanus – the intermediate host of Schistosoma haematobium, and Lymnaea natalensis Krauss – the intermediate host of both Fasciola gigantica and F. hepatica Cobbold. Ceratophallus spp., a non-vector snail was also encountered. Most (61.0%) of the snails were encountered in streamside pools. Schistosomiasis-transmitting host snails, B. pfeifferi and B. africanus, were fewer than fascioliasis-transmitting Lymnaea species. All the four different snail species were found to be attached to different aquatic weeds, with B. pfeifferi accounting for over half (61.1%) of the snails attached to the sedge, followed by B. africanus and Lymnaea spp., accounting for 22.2 and 16.7%, respectively. Ceratophallus spp. were non-existent in sedge. The results from this preliminary study show that snails intermediate hosts of schistosomiasis and fascioliasis exists in different habitats, in few areas along the Mara River, though their densities are still low to have any noticeable impacts on disease transmission in case they are infected. The mere presence of the vector snails in these focal regions calls for their immediate control and institution of proper regulations, management, and education among the locals that can help curtail the spread of the snails and also schistosomiasis and fascioliasis within the Mara River basin.
Both Schistosoma mansoni and S. haematobium are endemic in Kenya Citation1 Citation2, with the latter being more widely distributed. In Africa, S. haematobium is transmitted by the bulinid snail of Bulinus species, including B.((a) globosus, B.(a) africanus, B.(a) nasutus, B. truncatus, B. forskalii Citation3, and also B. senegalensis Citation4. Intestinal schistosomiasis due to S. mansoni and urinary schistosomiasis due to S. haematobium are common in most areas surrounding Lake Victoria and along the coastal belt of Kenya, respectively, where prevalence ranges between 50 and 90% Citation1 Citation4–Citation6.
Fascioliasis is also one of the most important disease agents of livestock and humans throughout the world and shows promise of remaining so for years to come, causing enormous economic losses through mortalities, condemned livers, reduced milk and meat production, secondary bacterial infection, and expensive anthelmintic treatment Citation7. Traditionally regarded as a disease of livestock, fascioliasis is now recognized as an important emerging zoonotic disease of humans Citation8. Prior to 1992, the total number of reported human cases of fascioliasis was estimated to be less than 3,000; however, more current figures indicate that between 2.4 and 17 million people are infected, with a further 91.1 million living at risk of infection Citation9. Human infections have been reported in areas where animal fascioliasis is endemic, and transmission occurs where rural farming communities regularly share the same water source with domestic animals or consume water-based vegetation grown in endemic areas Citation10.
Although control measures such as use of drugs and molluscicide have been intermittently used to contain spread of the disease such as schistosomiasis Citation11, prevalence continue to soar Citation12. Regardless, the contribution of other intermediary snail focal points such as rivers and their tributaries, and even adjacent terrestrial aquatic habitats frequently associated with human contact have been ignored. Particularly, no schistosomiasis or fascioliasis snails survey has been done along the Mara River which stimulated our study. This study aimed at investigating the presence, and abundance of schistosomiasis and fascioliasis host snails and their relation to the vegetation types along the Mara River. Physicochemical parameters such as temperature, turbidity, dissolved oxygen (DO), conductivity, salinity, and pH, and their influence on abundance of schistosomiasis and fascioliasis snails were also determined.
We selected 39 sites, purposively, along the Mara River of Kenya and Tanzania, which included sites along the two perennial tributaries (Amala and Nyangores) and/or any other adjacent terrestrial aquatic habitats (pools and small ponds) and determined the presence or absence of snails. The selection of the sites was purposive because whenever a suitable and safe area along the Mara River (free of hippos, crocodiles, and other hazards) were reached, the area was searched for sites which could be suitable breeding habitats for snails and sampled. The geographical locations of all the sampling sites were taken at access points (). Snail sampling was conducted by one man searching each site for 10 min using a standard flat-wire mesh scoop with a mesh size of 2 mm, as per the methods of Ouma et al. Citation13 with modifications. The snails were preserved individually in flat-bottomed glass vials (with 90% Ethanol) and taken to the laboratory at Kenya Medical Research Institute (KEMRI) and identified to species level by use of appropriate key. Physicochemical parameters (DO, pH, temperature, conductivity, turbidity, and salinity) were measured in situ by use of a hand-held multi-parameter-YSI meter (YSI Model 650-01 m Environmental Monitoring Systems, Yellow Springs, OH).
Fig. 1. Map of the sampling sites and distribution of snail species along the Mara River from July to August, 2011, n=39.

Out of a total of 39 sites sampled, 10 had snails representing 25.6% of the total sites sampled. The snail species encountered in these water bodies included B. pfeifferi Krauss, the intermediate host of S. mansoni Sambon, B. africanus the intermediate host of S. haematobium, L. natalensis Krauss, the intermediate host of F. gigantica and F. hepatica Cobbold, and Ceratophallus spp. a non-vector snail (). The abundance of each snail species along the Mara River is given in .
Fig. 2. Mean snail species abundance (%) in habitats along the Mara River from July to August, 2011, n=39.
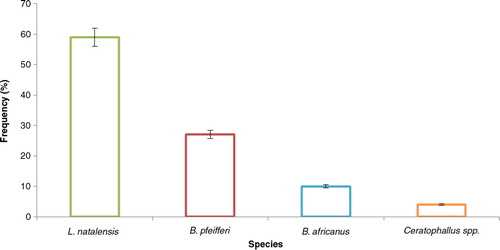
Table 1. Mean (SE) distribution of snails on different habitats along the Mara River basin, July–August 2011, n=39
In these sampling sites, L. natalensis was found to be the most dominant snail species accounting for 59.0% of the total snails and they were mainly encountered in streamside pools particularly at Tenwek Falls sampling site. Other habitats that harbored L. natalensis included dams located along Mara River, such as Mulot dam and also puddles adjacent to the Mara River. Biomphalaria pfeifferi were the second most dominant snail species within the Mara River basin accounting for 27.0% of the snail population encountered, most of which were encountered in swamps. Other habitats that harbored the B. pfeifferi snail species included stream side pools, dams, and puddles, in that order (). Bulinus africanus snail species were also encountered in three habitat types, namely stream side pools, puddles, and dams, though they represented only 10% of the snail population recorded in the study. Ceratophallu spp. which are non-vector snails were the least occurring, accounting for only 4.0% of the total snail population within the basin.
The highest number of snails (62) representing 62.0% of all the snails encountered was collected from temporary streamside pools, which discharged their waters onto the main Mara River. Swamps, dams, and puddles recorded 16, 12, and 10%, respectively, of all the snails sampled (). A Chi-square test revealed that bilharzia-transmitting host snails; B. pfeifferi and Bulinus spp. were fewer than fascioliasis-transmitting snails Lymnaea species, Pearson Chi-square χ 2=8.834, p≤0.001. The population density of L. natalensis did not correlate with population density of B. pfeifferi Krauss and B. africanus species combined (r=−0.0121, p=0.78).
The snails were either found attached to aquatic weeds, dead logs, and open puddles/rock pools, or floating freely on water (). The majority of the snails were found to be free floating in areas without any vegetation, of which Lymnaea spp. accounted for 87.1%, of the floating snails, while B. pfeifferi and B. africanus accounted for 9.7 and 3.2%, respectively. Consequently, Lymnaea spp. were the most dominant (88.9%) of the snails found attached to freely floating dead logs, while B. africanus and B. pfeifferi accounted for 7.4 and 3.7%, respectively.
Table 2. Snail distribution by vegetation types and habitat preference along the Mara River basin, July–August 2011, n=39
Only 6 out of the 100 snails encountered were found attached to hippo grass, while 18 out of 100 were attached to sedges. Only two B. pfeifferi snails were found to be either free floating or attached to the freely floating logs. All the four different snail species were found to be attached to different aquatic weeds, with B. pfeifferi accounting for over half (61.1%) of the snails attached to the sedge, followed by B. africanus and Lymnaea spp. which accounted for 22.2 and 16.7%, respectively. Ceratophallu spp. was non-existent in the sedges ().
The findings of physicochemical parameters of water carried out from the different sites within the Mara River basin are presented in . The water temperature varied between 16.6 and 32.0°C, with highest water temperature recorded at Kwebuse village (site 4) and the lowest at Bomet town sampling site. Similar patterns were observed for conductivity which showed variation among sampling sites, ranging between 38.0 µS/cm at Silibwet sampling site and 8,290 µS/cm at Kwebuse village IV sampling site in Tanzania. Except for Kwebuse village Citation4, which recorded a salinity of 4.0 mg/l, Morito site 4, with 0.7 mg/l, and Mangore swamp 2 and Chepterer bridge both with a salinity of 0.1 mg/l, salinity in all the other six sites was not detectable, that is, it was 0.00 mg/l. However, hardness, turbidity, and DO showed the opposite trend, while pH values did not show any remarkable variation between all the sampling sites except Kwebuse village (site 4) which recorded an extremely acidic pH of 2.91. The DO levels which are important in determining the water quality of aquatic systems, varied greatly along the Mara River basin, ranging between 3.64 and 8.23 mg/l. Alkalinity also varied greatly between sites ranging between 45 and 120 mg/l ().
Table 3. Sampled sites with snails and their corresponding water physicochemical parameters along the Mara River basin, July–August 2011, n=39
Statistical analyses showed that there were significant differences in mean turbidity, alkalinity, and DO in water sample containing B. pfeifferi Krauss and B. africanus as compared to those with L. natalensis (t=4.76, p=0.013; t=5.55, p=0.001; and t=6.12, p≤0.001, respectively), but no significant differences were observed in pH and conductivity (t=−1.14, p=0.445; t=−2.056, p=0.173). However, temperature showed a rather borderline significant difference (t=−1.241, p=0.0549).
This study showed that fascioliasis host snails L. natalensis was the dominant species (59%) followed by schistosomiasis-causing B. pfeifferi (27%) and Bulinus (a) africanus (10%) in the studied sites within Mara River basin. The least common snail was the non-vector snail, Ceratophallus spp., which was found in some sites in Tanzania and some sites in Kenya all within the Mara River basin. The snails showed a spatial distribution pattern with instances of clustering observed with 10 of the 39 sites accounting for most of the snails. The current study findings were fairly consistent with those by Opisa et al. Citation14 which found that spatial distribution of vector snails within informal settlements in Kisumu city (along river Nyamasaria) in the western part of Kenya was clustered, with only a few sites accounting for most of the snails, and that Bulinus (a) africanus and B. pfeifferi snails were more abundant along the streams and inland sites, respectively.
The three different disease-transmitting vector snail species encountered within the Mara River basin were found mainly on streamside pools, puddles, dams, and swamps, which characteristically have lower flow rates than those from rivers and streams, whose water velocity is swift. Most snails were found attached to the vegetation at the streamside pools while others were collected at dam sites and temporary puddles adjacent to the Mara River tributaries. No host snails were found in the swift waters of the Mara River during the sampling period. Snails abound in shallow stagnant or slow flowing water where they feed on organic waste and aquatic vegetation that are abundant in such microhabitats. Our findings are therefore consistent with those reported by Alves Citation15 and Moore Citation16.
Lymnaea natalensis were mainly confined to streamside pools particularly at Tenwek falls sampling site – a site that was near the turbine feeders and accessible to people and livestock, along Nyangores tributary on the Kenyan side of the Mara River. Tenwek falls had a wide variety of microhabitats especially at the lower edges next to the electricity-generating tunnels. The water overflow and human activities, as well as many livestock contact points around this area probably influenced the snail abundance by contributing organic wastes. Puddles, dams, and swamps harbored some snails. In addition, the Mara River crosses the Maasai Mara Game Park, which is home to thousands of wild animals which may further contribute additional organic waste to the remaining water pockets and positively influence the abundance of L. natalensis.
Preston and Castelino Citation17 reported that the snail population increased during the rainy season. However, we also know that snail populations decrease during heavy rains especially along the river, stream banks, and flood plains thus lowering the levels of schistosomiasis in such periods Citation18 Citation19. Water flow in lotic environment has been reported to be the most important factor that influences the distribution of the snails, including the intermediate hosts of schistosomiasis (Citation20.
Kapkimolwa bridge sampling site, along Amala tributary, is a water collection point for domestic use, such as washing of utensils, watering livestock, bathing among other anthropogenic activities and thus is polluted by organic waste, making this location a possible high-risk habitat for schistosomiasis vector snails.
There were significant differences in turbidity, alkalinity, temperature, and DO in water samples harboring B. pfeifferi Krauss and B. africanus as compared to sites harboring L. natalensis, but no significant differences were observed in pH and conductivity. Appleton's review of 1978 suggested that water temperature is the most important abiotic factor in lentic environments, whereas in lotic environments current velocity is the key factor that determines snail abundance. Cañete et al. Citation21 reported that temperature plays an important role in Lymnaea spp. abundance, while Kazibwe et al. Citation22 observed a positive correlation between B. sudanica abundance and water temperature.
There were also significant differences in alkalinity on water bodies with the two different species of snails. High alkalinity is believed to be associated with organic pollution, and Alves Citation15 confirmed that most snails were abundant in water bodies polluted by human excreta and sewerage from domestic waste. The abundance of organic matter increases growth and abundance of algae, known to be one the best food for most snails Citation23. pH is rarely a factor limiting the distribution of snails in aquatic systems Citation21, while pulmonate snail species are thought to increase with increase in DO Citation24. However, Utzinger et al. Citation25 concluded that the distribution of freshwater snails is as a result of more complex interactions of different habitat factors.
The non-vector, Ceratophallus spp., was found in some habitats in Tanzania and also on the Kenyan side of the Mara River basin. It was not possible to determine the most preferred habitats within the Mara River basin for Ceratophallus spp. owing to their limited numbers. In contrast, a Chi-square test established that the population of schistosomiasis host snail B. pfeifferi and Bulinus spp. were fewer than fascioliasis-transmitting Lymnaea spp. within the Mara River basin. No correlation was observed between the abundance of L. natalensis and that of B. pfeifferi Krauss and B. africanus species combined, although all the three species were found condensed into colonies in similar habitats, namely dams, stream side pools, and puddles.
Statistical analysis found significant differences in mean turbidity, alkalinity, and DO in water samples containing B. pfeifferi Krauss and B. africanus as compared to those with L. natalensis but no significant differences were observed in pH and conductivity. Temperature correlations demonstrated a borderline significant difference.
Vegetation preference was another factor. We observed that plants were more abundant and constant in habitats where Biomphalaria spp. and B. africanus were abundant, while open rock pools and streamside pools sites were basically preferred by L. natalensis and Ceratophallus species. The aquatic and marginal plants have been reported to provide an egg laying medium and food for freshwater snails Citation26. These combinations of factors have also been shown to favor growth of populations of Biomphalaria Citation27.
The possibility of using the Ghanaian strain of an ampullariid snail, Lanistes varicus, for the biological control of the main snail host of S. mansoni, B. pfeifferi, has been investigated Citation28, and shows potential as a competitive inhibitor for vector snail populations. If determined to have little or no negative effects on the ecology of the Mara River ecosystem, this method of control could probably be one of the best if implemented and used for controlling the schistosomiasis snails. Also ideally, a biological control method with no or low impact on other indigenous species would be preferred. As yet, little is known of the predators and parasites of the Schistosoma intermediate hosts, but such agents could be suitable as bio control agents. To date, the most promising results have been obtained from experimental introductions of competitive snail species. For example, Pomacea and Marisa species have been introduced to Africa and Asia in an attempt to control other medically problematic snails in the family Planorbidae: Bulinus species and Biomphalaria species, which serve as intermediate hosts for trematoda parasites. In addition, a predatory species, the ampullariid snail, Lanistes varicus, has been evaluated as a biological control method under laboratory conditions and the results are promising. With further investigation into this snail's impact on the ecosystem, it may be of value in the reduction in number of the snail hosts of the trematodes that cause human schistosomiasis.
During our sampling period, schistosomiasis- and fascioliasis-transmitting snails were found in random focal sites/microhabitats along the Mara River, and the local communities were unaware of the health risks associated with their presence in the communities. Our perception based on discussions with the local residents during the time of sampling confirmed our suspicions that most individuals were unaware of the threat posed by the vector snails and they were not able to identify them within their environment. Therefore, there is need for more community education focused on teaching the identification of vector snail morphology and establishing a reporting system for monitoring the main snail hosts of the Schistosoma species. This system could be used to assist a regional control programs in monitoring snail bed expansion within the study area and implement control measures as needed. Such a community-based vector reporting system could contribute to understanding, monitoring, and implementing snail vector spread control measures.
For focal snail control, using molluscicides should be considered Citation29 Citation30 as an effective way to augment chemotherapy of schistosomiasis. To implement this as a focal control method, studies are first carried out to identify sites of snail colonization and only at such sites are chemicals applied periodically. Applications are usually restricted to places frequently used by the local population for swimming, washing, bathing, and so on. Currently, only one chemical molluscicide, niclosamide, is acceptable for operational use in snail control programs. However, other molluscicides, including some of plant origin, are being evaluated. Because of its high cost, niclosamide is used only sparingly in local control programs. At low concentrations, it is highly toxic to snails and their egg masses. For practical use, a concentration of 0.6–1 mg/l is recommended with an exposure time of 8 hours. The compound is safe to handle and after dilution is non-toxic to water plants and crops; however, it is very toxic to fish. Fish killed by the molluscicide can be safely eaten. When used focally and seasonally, molluscicide application should not cause any serious negative impact on the environment.
It is fortunate that some of the focal points of vector snail distribution within the Mara River basin have been detected early from results of our study, making it possible to monitor their future dynamics and effectively design intervention measures aimed at eliminating them during future disease control programs from the focal points within the Mara River basin.
Conflict of interest and funding
The authors declare that there are no conflicts of interest.
Acknowledgements
East Africa Community – Lake Victoria Basin Commission Secretariat (EAC-LVBC) provided funds for this study. We are grateful to Maseno University, Kenya Medical Research Institute (KEMRI) schistosomiasis laboratory, and Division of Vector-Borne Diseases, Kisumu, Kenya, for providing the time, material, and/or technical support.
References
- Doumenge JP, Mott KE, Cheung C, Villenave D, Chapuis O, Perrin MF, et al.Atlas of the global distribution of schistosomiasis. CEGET-CNRS/OMS-WHO, Presses Universitaires de Bordeaux. , 1987, pp. 249–255. Available from: http://whqlibdoc.who.int/temp/schistosomiases/2867810604_31.pdf [cited 29 July 2013].
- Handzel T, Karanja DM, Addiss DG, Hightower AW, Rosen DH, Colley DG, et al.. Geographic distribution of schistosomiasis and soil-transmitted helminths in Western Kenya: implications for anthelminthic mass treatment. 2003; 69: 318–23. [PubMed Abstract].
- Miguel E, Kremer M. Worms: education and health externalities in Kenya. NBER Working Paper No. 8481. CambridgeMA: National Bureau of Economic Research., 2001.
- World Health Organization. 1993. Study group on the ecology of intermediate snail hosts of bilharziasis: report. WHO Technical Report Series, 120. Geneva: World Health Organization., pp. 1–38.
- Pamba HO. Schistosomiasis in Nyanza Province Kenya, Rusinga ISLAND. 1974; 51: 594–9. [PubMed Abstract].
- Odiere MR, Rawago FO, Ombok M, Secor WE, Karanja DMS, Mwinzi PN, et al.. High prevalence of schistosomiasis in Mbita and its adjacent islands of Lake Victoria, western Kenya. 2012; 5: 278. 10.3402/iee.v4.24281. [PubMed Abstract] [PubMed CentralFull Text].
- Schmidt GD, Roberts LS. Foundations of parasitology. Times Mirrow/Mosby College. St Louis MO, 1985
- Robinson MW, Dalton JP. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. 2009; 364: 2763–76. 10.3402/iee.v4.24281. [PubMed Abstract] [PubMed CentralFull Text].
- Keiser J, Utzinger J. Emerging foodborne trematodiasis. 2005; 11: 1507–14. 10.3402/iee.v4.24281. [PubMed Abstract] [PubMed CentralFull Text].
- Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. 2005; 79: 207–16. 10.3402/iee.v4.24281. [PubMed Abstract].
- Mkoji GM, Mungai BN, Koech DK, Hofkin BV, Loker ES, Kihara JH, et al.. Does the snail Melanoides tuberculata have a role in biological control of Biomphalaria pfeifferi and other medically important African pulmonates?. 1992; 86: 201–4. [PubMed Abstract].
- Nagi S, Chadeka EA, Sunahara T, Mutungi F, Justin YK, Kaneko S, et al.. Risk factors and spatial distribution of Schistosoma mansoni infection among primary school children in Mbita District, Western Kenya. 2014; 8: e2991. doi: 10.3402/iee.v4.24281. 10.3402/iee.v4.24281. [PubMed Abstract] [PubMed CentralFull Text].
- Ouma JH, Sturrock RF, Klumpp RK, Kariuki HC. A comparative evaluation of snail sampling and cercariometry to detect Schistosoma mansoni transmission in a large scale, longitudinal field study in Machakos, Kenya. 1989; 6: 349–55. doi: 10.3402/iee.v4.24281. 10.3402/iee.v4.24281.
- Opisa S, Odiere MR, Jura WGZO, Karanja MS, Mwinsi PNM. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. 2011; 4: 226. doi: 10.3402/iee.v4.24281. 10.3402/iee.v4.24281. [PubMed Abstract] [PubMed CentralFull Text].
- Alves W. Bilharziasis in Africa: a review. 1957; 3: 123–7.
- Moore IJ. Effects of water currents on freshwater snails Stagnicola palustris and Physa propinqua. 1964; 45: 558–64. 10.3402/iee.v4.24281.
- Preston M, Castelino B. A study of the epidemiology of bovine fascioliasis in Kenya and its control using N-tritylmorpholine. 1997; 133: 600–8.
- Woodhall DM, Wiegand RE, Wellman M, Matey E, Abudho B, Karanja DM, et al.. Use of geospatial modeling to predict Schistosoma mansoni prevalence in Nyanza Province, Kenya. 2013; 8: e71635. 10.3402/iee.v4.24281. [PubMed Abstract] [PubMed CentralFull Text].
- Wu XH, Zhang SQ, Xu XJ, Huang YX, Steinmann P, Utzinger J, et al.. Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People's Republic of China. 2008; 57: 271–6. doi: 10.3402/iee.v4.24281. 10.3402/iee.v4.24281. [PubMed Abstract].
- Appleton CC. Review of literature on abiotic factors influencing the distribution and life cycles of bilharziasis intermediate host snails. 1978; 11: 1–25.
- Cañete R, Yong M, Sánchez J, Wong L, Gutiérrez A. Population dynamics of intermediate snail hosts of Fasciola hepatica and some environmental factors in San Juan Martinez Municipality, Cuba. 2004; 99: 257–62. 10.3402/iee.v4.24281.
- Kazibwe F, Makanga B, Rubaire-Akiiki C, Ouma J, Kariuki C, Kabatereine NB, et al.. Ecology of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert, Western Uganda: snail distribution, infection with schistosomes and temporal associations with environmental dynamics. 2006; 568: 433–44. 10.3402/iee.v4.24281.
- Rosso AL, McCune B. Exploring the effects of mollusk herbivory on an epiphytic lichen community. 2003; 20: 15–30.
- Ofoezie IE. Distribution of freshwater snails in the man-made Oyan Reservoir, Ogun State, Nigeria. 1999; 416: 181–91. 10.3402/iee.v4.24281.
- Utzinger J, Mayombana C, Mez K, Tanner M. Evaluation of chemical and physical morphological factors as potential determinants of Biomphalaria pfefferi (Krauss, 1848) distribution. 1997; 92: 323–8. 10.3402/iee.v4.24281. [PubMed Abstract].
- Ndifon GT, Ukoli FMA. Ecology of freshwater snails in Southwestern Nigeria. I. distribution and habitat preferences. 1989; 171: 231–53. 10.3402/iee.v4.24281.
- Hilali AM, Desouqi LA, Wassila M, Daffalla AA, Fenwick A. Snails and aquatic vegetation in Gezira irrigation canals. 1985; 88: 75–81. [PubMed Abstract].
- Anto F, Bosompem K, Kpikpi J, Adjuik M, Edoh D. Experimental control of Biomphalaria pfeifferi, the intermediate host of Schistosoma mansoni, by the ampullariid snail Lanistes varicus. 2005; 99: 203–9. 10.3402/iee.v4.24281. [PubMed Abstract].
- Fenwick A. Experience in mollusciciding to control schistosomiasis. 1987; 3: 70–3. 10.3402/iee.v4.24281. [PubMed Abstract].
- Klumpp RK, Chu KY. Focal mollusciciding: an effective way to augment chemotherapy of schistosomiasis. 1987; 3: 74–6. 10.3402/iee.v4.24281. [PubMed Abstract].
