Abstract
Introduction
Tick-borne pathogens cause a spectrum of disease manifestations in both dogs and humans. Recognizing regional and temporal shifts in exposure are important as tick distributions change. To better delineate regional exposure to canine tick-borne pathogens, an expanded set of species-specific peptides were used to detect Anaplasma phagocytophilum (Aph), Anaplasma platys (Apl), Ehrlichia canis (Ec), Ehrlichia chaffeensis (Ech), Ehrlichia ewingii (Eew), and Borrelia burgdorferi (Bb) antibodies in canine serum.
Methods
Archived canine serum samples (n=6,582) collected during 2008–2010 and in 2012 from the US, Canada, and the Caribbean were retrospectively screened for antibodies against Ehrlichia and Anaplasma species-specific peptides. Overall, regional and temporal seroprevalence rates were determined.
Results
Overall Bb and Eew were the most seroprevalent pathogens. During 2008–2010, seroprevalence rates increased overall for Aph and Ech, and regionally, Bb and Aph seroprevalence rates increased in the South. Canada had unexpectedly high seroprevalence rates for Ec and Apl. The most common co-exposures were Eew+Ech, followed by Aph+Bb and Eew+Bb.
Conclusions
This study demonstrated significant shifts in canine vector-borne disease seroprevalence rates. The use of specific peptides facilitated improved geographic delineation of tick-borne pathogen distributions among dogs, which may enhance epidemiological surveillance of vector-borne pathogens shared by dogs and humans.
Canine vector-borne diseases (CVBDs) are prevalent in the US, Canada, and the Caribbean. Tick-borne pathogens, including Anaplasma phagocytophilum (Aph), Anaplasma platys (Apl), Ehrlichia canis (Ec), Ehrlichia chaffeensis (Ech), Ehrlichia ewingii (Eew), and Borrelia burgdorferi (Bb), infect dogs and humans, resulting in clinical or subclinical infections Citation1–(Citation7) . As tick distributions change through ecosystem fluctuations, wildlife migration, and increased international transport of companion animals, diagnosing and managing dog and human tick-borne diseases has become medically complex and more challenging. Previous studies indicate that Bb seroreactive dogs are effective sentinels for human Lyme disease risk (Citation7, Citation8). Recognizing risk factors and the prevalence of single and co-exposures within a particular region is epidemiologically important for public health and diagnostically important for clinicians. Spatio-temporal tick-borne pathogen surveillance should identify high-risk areas for vector-borne pathogen exposure, facilitate the diagnosis of regionally neglected pathogens, and better elucidate co-infection risks.
In 2001, IDEXX Laboratories, Inc., developed rapid, in-house ELISA platforms (SNAP®3Dx®, SNAP®4Dx®, and SNAP® 4Dx® Plus), allowing veterinarians to screen for CVBDs (heartworm disease, Lyme disease, ehrlichiosis, and anaplasmosis). Species-specific peptides developed to detect canine antibodies to Ec, Ech, Eew, Aph, and Apl were used to manufacture a proprietary, research prototype ELISA SNAP assay (SNAP M-A), showing seroreactivity to individual Anaplasma spp. and Ehrlichia spp. Citation9–(Citation12) . Archived canine serum samples submitted between 2008 and 2010 and in 2012 by veterinarians from dogs with suspected tick-borne disease to the Vector-Borne Disease Diagnostic Laboratory at North Carolina State University (VBDDL–NCSU) were tested using the SNAP M-A. Regional and temporal seroprevalences within the US, Canada, and the Caribbean and common co-exposures between these pathogens are reported.
Methods
Canine serum samples
Archived canine serum samples (n=6,582) submitted to the VBDDL–NCSU for serological testing against tick-borne pathogens between January 2008–December 2010 (n=6,270; 95.3%) and January–March 2012 (n=312; 4.7%) were available for SNAP M-A testing and analysis. Samples submitted from the same dog within 5 weeks of the initial submission were excluded. Available information included signalment (age, breed, and sex), date of sample collection, and owner or veterinary practice address. Regions, states, and provinces are defined in .
Table 1 Distribution of all samples collected between 2008–2010 and 2012 (n=6,582) by region (gray shading) and state with seroreactivity to Ehrlichia canis (Ec), E. ewingii (Eew), E. chaffeensis (Ech), Anaplasma platys (Apl), A. phagocytophilum (Aph), Borrelia burgdgorferi (Bb), Anaplasma spp. (A-genus), and Ehrlichia spp. (E-genus)
Serology
All canine sera were retrospectively tested by SNAP M-A for the simultaneous and individual detection of specific Ec, Ech, Eew, Aph, Apl, and Bb antibodies. Included on SNAP M-A are two additional spots containing a combination of Anaplasma spp. synthetic peptides, labeled A-genus, and Ehrlichia spp. (Ec and Ech only) synthetic peptides, labeled E-genus. SNAP M-A uses a reversible chromatographic flow of sample and automatic, sequential flow of wash solution and enzyme substrate. Archived canine serum stored at −80°C was thawed to room temperature prior to mixing four drops of serum with 4–5 drops of SNAP M-A conjugate. The mixture was allowed to move across a flow matrix where peptide-specific antibody could bind to peptide-HRP conjugate before color reactant release. Color development indicating a positive reaction was read after 15 min.
Statistical analysis
Seroprevalence, defined as the number of seropositive samples divided by the number of samples tested, was calculated by region, month, and year. The Chi-squared test or Fisher exact test was used to determine significant differences in the proportions of seroreactivity by region, month, and year. Multiple comparisons were performed using the Multtest procedure in SAS/STAT v.9.3 (SAS Institute, Cary, NC). Regions were assigned into the following categories based on owner or veterinary hospital address: Northeast, Mid-Atlantic, South, Midwest, West, Canada, and the Caribbean region, which includes all countries and territories in and around the Caribbean Sea. State-wide seroprevalence was calculated for states with at least 30 sample submissions and depicted in heat maps (openheatmap.com). The proportion of co-exposures, defined as the number of dogs with two or more seropositive results divided by the total number of dogs, was calculated. The following positive species-specific peptide combinations were not considered co-exposures: E-genus+Ech, E-genus+Ec, A-genus+Apl, or A-genus+ Aph. Odds ratios (ORs) and 95% confidence intervals (95% CI) were used as measures of association between exposure to one pathogen and exposure to a second pathogen (representing concurrent or sequential co-exposures). Level of significance was established at p<0.05. Statistical analyses were performed using SAS/STAT 9.3 (SAS Institute Inc., Cary, NC).
Results
A total of 6,582 dog serum samples were tested, including 6,268 (95.2%) from the US, representing 43 states; 285 (4.3%) from Canada, representing seven provinces; and 29 (0.44%) from the Caribbean region (). Exposure to at least one tick-borne pathogen was documented in 1,198 (18.2%) dogs. Of the 6,582 sera tested, exposures included Bb (n=545, 8.3%), Eew (n=251, 3.8%), Aph (n=227, 3.4%), Ech (n=202, 3.1%), Ec (n=117, 1.8%), and Apl (n=99, 1.5%) (). E-genus and A-genus antibodies were detected in 327 (5.0%) and 238 (3.6%) dogs, respectively. Of the E-genus and A-genus antibody positives, 50 (15.3%) and 32 (13.4%) dogs, respectively, did not have species-specific antibodies, which could represent dogs with low Ehrlichia and Anaplasma species-specific antibody titers or potentially, seroreactivity to a species, such as Ehrlichia muris or the Panola mountain Ehrlichia not specifically tested for in this study.
Seroprevalences by region are reported in . The greatest proportion of samples were submitted from the South (n=3,011, 45.7%), followed by the Midwest (n=1,162, 17.7%), the Mid-Atlantic (n=1,065, 16.2%), the Northeast (n=532, 8.1%), the West (n=498, 7.6%), Canada (n=285, 4.3%), and the Caribbean (n=29, 0.44%). Regional comparisons documented significantly higher Bb exposure frequencies in the Northeast (n=122, 22.9%) and Mid-Atlantic (n=236, 22.2%), as compared to the Midwest (n=66, 5.7%; p<0.001 and p<0.001, respectively) and the South (n=100, 3.3%; p<0.001 and p<0.001, respectively). Aph seroprevalence was significantly higher in the Northeast (n=69, 13%) and the Mid-Atlantic (n=58, 5.5%) when compared with other regions in the US (p<0.01, all comparisons). Eew and Ech exposures were most prevalent in Mid-Atlantic (n=61, 5.7%; n=59, 5.5%, respectively) and Southern dogs (n=156, 5.2%; n=129, 4.3%, respectively) compared to the Midwest (n=14, 1.2%; n=8, 0.7%, respectively) (p<0.001 for all comparison listed) and did not significantly differ across the Mid-Atlantic and Southern regions. Ec prevalence was low among all US and Canadian regions (ranging from 0.5 to 3.2%), with the highest prevalence in the West (n=12; 2.4%) and Canada (n=9, 3.2%). The Caribbean had a significantly higher Ec seroprevalence (n=8, 27.6%) than all other regions (p<0.001, all comparisons). The Apl seroprevalence ranged from a high of 10.3% (n=3) in the Caribbean to a low of 0.6% (n=7) in the Midwest.
Due to the lack of complete 2012 data for the entire year, significant differences in overall and regional seroprevalences were evaluated by year and month using only data from years 2008 (n=2,327; 35.4%), 2009 (n=2,184; 33.2%), and 2010 (n=1,759; 27%) (). There were significant differences in the overall Aph, Anaplasma spp., and Ech seroprevalences by year (p<0.0001, p=0.0024, and p=0.0004, respectively). Overall Ech exposure appeared to decline from 2008 to 2009, but increased in 2010, while Aph increased. Regionally, significant increases in seroprevalence were observed in the Mid-Atlantic, including Aph (p=0.0026), and the South, including Aph and Bb (p<0.0001 and p<0.0001, respectively). The South also had significant changes in Ech and Eew seroprevalences, with a decline in Eew and Ech exposure in 2009 followed by an increase in 2010 (p=0.0191 and p=0.0001, respectively). No significant changes or trends were observed when seroprevalences were compared between months (data not shown). Seroprevalence was determined for each state within the US (). States with no sample submissions included HI, AK, MT, ID, SD, and ND. Heat maps of the US were generated when in-state seroprevalence data were based upon ≥30 submissions (Figs. – .
Fig. 1 Seroprevalence by state of Borrelia burgdorferi (Bb) or Anaplasma phagocytophilum (Aph) in dogs suspected of canine vector-borne disease.
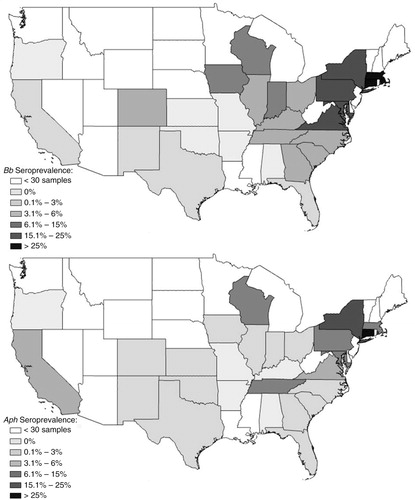
Fig. 2 Seroprevalence by state of Ehrlichia canis (Ec) or Anaplasma platys (Apl) in dogs suspected of canine vector-borne disease.
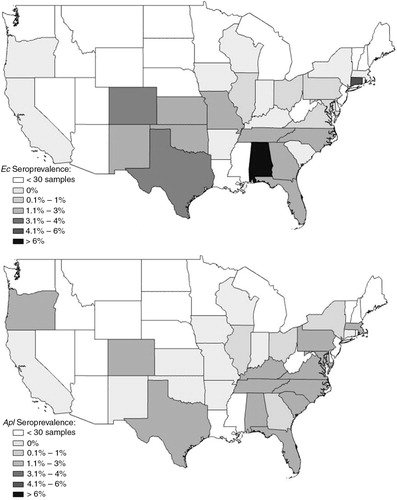
Fig. 3 Seroprevalence by state of Ehrlichia ewingii (Eew) or E. chaffeensis (Ech) in dogs suspected of canine vector-borne disease.
Table 2 Seroprevalence per year between 2008 and 2010 in the US, Canada, and Caribbean to Ehrlichia canis (Ec), E. ewingii (Eew), E. chaffeensis (Ech), Anaplasma platys (Apl), A. phagocytophilum (Aph), Borrelia burgdgorferi (Bb), Anaplasma spp. (A-genus), and Ehrlichia spp. (E-genus)
Co-exposures, defined as seroreactivity to more than one Anaplasma spp., Ehrlichia spp., or Bb, were detected in 261 dogs (4.0%). Seroreactivity to two pathogens occurred in 207 dogs (3.1%); three pathogens in 44 dogs (0.7%); four pathogens in seven dogs (0.1%); and five pathogens in three dogs (0.05%). The most common co-exposures included Eew+Ech (n=91, 1.4%); Aph+Bb (n=76, 1.2%); and Eew+Bb (n=41, 0.6%), in contrast to Ec+Apl (n=18, 0.3%) (). Notable regional co-exposures included Aph+Bb in the Northeast (n=33; 6.2%), Eew+Ech in the South (n=62; 2.1%) and Mid-Atlantic (n=22; 2.1%). The Mid-Atlantic had the highest co-exposure seroprevalence rates for several unexpected pathogen combinations including Eew+Bb (n=19; 1.8%), Ech+Bb (n=17; 1.6%), and Ech+Aph (n=12; 1.1%) (). ORs identified associations among CVBD co-exposures (). The highest ORs were found among pathogens known to share a common tick vector (Eew+Ech: OR=31.9, 95% CI=23.2–43.8; Ec+Apl: OR=14.3, 95% CI=8.3–24.8). The OR for Aph+Bb (OR=6.2, 95% CI=4.6–8.3) was lower by comparison. The lower ORs were found among unexpected combinations of pathogens (Ec+Bb: OR =0.2, 95% CI=0.05–0.8; Apl+Bb: OR=2.2, 95% CI=1.3–3.7; and Ec+Ech: OR=2, 95% CI=0.9–4.5) ().
Table 3 Co-exposures from all samples collected between 2008–2010 and 2012 (n=6,582) with corresponding seroprevalence (%) and odds ratios (OR) with 95% confidence interval (CI) to Ehrlichia canis (Ec), E. ewingii (Eew), E. chaffeensis (Ech), Anaplasma platys (Apl), A. phagocytophilum (Aph), and Borrelia burgdgorferi (Bb)
Discussion
This study utilized a panel of species-specific, CVBD peptides to determine regional seroprevalences in dogs with suspected tick-borne pathogen exposure. Ec, Ech, Eew, Aph, Apl, and Bb peptides were designed to detect species-specific antibodies, so as to facilitate identification of unique patterns of CVBD exposure in dog sera from the US, Canada, and the Caribbean Citation9–(Citation12) . Significant regional changes and various co-exposure patterns were identified overall, regionally and during 2008–2010; however, significant patterns were not observed between months or seasons (data not shown) of the year, likely because these data do not represent infection onset. Limitations of this study include the following: Sample submission was not proportional across regions with a near majority of specimens submitted from the Southern region (45.7%) compared to the Northeast (8%), the West (7.6%), Canada (4.3%), and the Caribbean (0.4%). Specimens were regionalized based on local veterinary hospital or owner zip codes, and individual dog travel histories were not available. All samples from NCSU-College of Veterinary Medicine were regionalized according to owner zip codes; however, 21% (n=1,353) of samples submitted from other veterinary teaching hospitals may not accurately represent local exposure, since clients may travel farther distances for specialized services offered at large teaching hospitals. As this convenience sample was submitted to the VBDDL from dogs suspected of a CVBD, seroprevalence rates are most likely higher than in the general dog population.
Bb (8.3%), the etiologic agent of Lyme disease, was the most seroprevalent pathogen in this convenience sample of dogs (n=6,582). This finding is consistent with a recent study involving a large cohort of dogs from the US that reported an overall canine Bb seroprevalence, defined as seroreactivity to C6 peptide, of 7.2% (509,195/6,996,197) (Citation13). This is an increase from an earlier, similar study, which showed an overall canine Bb seroprevalence of 5.1% (49,817/982,336) (Citation14). Lyme disease is the most prevalent tick-borne disease in humans in the US and has historically been confined to Northeast and upper Midwestern regions of the country (Citation15, Citation16). Notably, we documented a statistical increase in Bb seroprevalence from 2008 to 2010 in the South ((p<0.0001) (), a region not historically endemic for Bb infection. A study by Duncan et al. using a convenience sample from sick dogs submitted for testing to the VBDDL between 2001 and 2003 measured a lower seroprevalence of Bb, defined as C6 seroreactivity, in individual Southern states, including, NC (0.4%), VA (8.7%), and MD (14.4%) than the Bb seroprevalences reported in this study (NC, 5.4%; VA, 20.3%; MD, 24.9%) (Citation8). Notably, the seroprevalence of Bb in northern states was more similar between the two studies (25% vs. 22.2%, respectively, in PA) suggesting the differences in the South are more likely due to prevalence changes and less likely testing variations. Our study found the Bb seroprevalence in dogs from the Mid-Atlantic (n=236; 22.2%), a region bordering the South, to approximate the Bb seroprevalence in the Northeast (n=122; 22.9%) (p=0.99). Furthermore, one third (n=1,014; 34%) of the samples from the South in this study were collected from dogs residing in NC, a state that borders VA, where according to the CDC, an increase in Lyme disease incidence had been reported in recent years (Citation17). Recently, VA established five counties along the NC border endemic for Lyme disease (Citation18, Citation19). The increased Bb seroprevalence observed in dogs from the Southern US supports a potential trend for Bb expansion southward, warranting further studies to monitor Lyme disease in both dogs and humans south of Mid-Atlantic States. The CDC reports the approximate distribution of I. scapularis extends from Texas to the Southeast, Mid-Atlantic, Northeast, and upper Midwestern states, and a recent report has documented population increases in Canada (Citation20, (Citation21). We found a Bb seroprevalence of 2.1% ((n=6) within our Canadian dog population (n=285). Previous studies measuring canine seroreactivity to C6 peptide reported lower Bb seroprevalences in Canada; canine sera collected from southern Ontario and Quebec between 2000 and 2003 (n=108) reported Bb seroprevalence as 1.85%, while another study in 2008 that included all provinces found an overall seroprevalence of 0.72% (n=624) (Citation22, Citation23). The increased seroprevalence could be related to differences in testing platforms, health status of the dogs, population number and distribution differences and possibly a northern movement of Bb infected ticks. In 2009, Lyme disease became a nationally reportable disease in Canada, with reports of increasing incidence in people (Citation24, (Citation25). Interestingly, a study in dogs using SNAP® 3Dx® and 4Dx® showed the incidence of Lyme in dogs from ON in 2006 (0.36) and 2007 (0.58) is approximate to the incidence reported in people from ON in 2006 (0.35) and 2007 (0.58) (Citation25, Citation26). These data further support the use of dogs as sentinels for Bb exposure in people.
This study documented a significant increase in canine exposure to Aph in the US from 2008 to 2010 (p<0.0001) (), suggesting a progressively increased risk for human Aph exposure. These data are supported by the substantial (53%) increase of reported human granulocytic anaplasmosis cases described by the CDC from 2009 to 2010 Citation27–(Citation29) . Furthermore, canine Aph seroprevalences were high in the Northeast (n=69; 13%), Mid-Atlantic (n=58; 5.4%) and the Midwestern state, WI (n=6; 10.3%) emphasizing the potential utility of dog data for establishing real-time regional human Aph exposure risk. The South had a higher Aph seroprevalence (2.1%) than previous reports that documented Anaplasma spp. (n=496; 0.5% and n=1,631,332; 0.9%) (Citation13, Citation14); the discrepancy, in part, could be due to a greater number of sick dogs in this sample set, while the former studies included a larger population of healthy dogs. We identified a significant increase in Aph seroprevalence from 2008 to 2010 in the Mid-Atlantic (p<0.0001) and the South (p<0.0001), consistent with Bb seroprevalence trends for the Southern region. Like Bb, Aph is not endemic in the South. Studies reporting the molecular presence of Aph in ticks from the South found Aph DNA in 1.3% of I. scapularis ticks and 2.7% of A. americanum ticks collected from rodents in Florida (Citation30); another study found 1.6% Aph DNA in I. scapularis ticks collected in SC, GA and FL, with the highest prevalence (20%) identified in ticks collected along the GA coast, a documented avian flyway (Citation31).
Despite similar Aph and Bb seroprevalence trends and a significant Aph+Bb co-exposure pathogen association (OR=6.2; 95% CI=4.6–8.3), overall the Aph (3.5%) and Bb (8.3%) seroprevalences differed significantly (p≤0.001). Correspondingly, the prevalence of Aph DNA in I. scapularis ticks collected in NJ was much lower than Bb (6.1% (n=9) and 50.3% (n=74), respectively) (Citation32). In this study, Bb seroprevalence was found to be similar among dogs from the Mid-Atlantic (22.2%) and the Northeast (22.9%) (p=0.99); however, the Aph seroprevalence differed significantly between the two regions (Mid-Atlantic; 5.4% (n=58) and Northeast; 13% (n=69) (p≤0.001), potentially reflecting a less prevalent Aph infection of ticks in the Mid-Atlantic when compared to the Northeast.
We identified Eew (3.8%) as the most common Ehrlichia exposure in dogs, followed by Ech (3.1%) and Ec (1.8%), which is consistent with a 2010 study that found Eew (5.1%) as the most seroprevalent Ehrlichia spp. pathogen in a large population of dogs from North America (n=8,622), when compared to Ech (2.8%) and Ec (0.8%) (Citation9). A similar study in dogs from the south central US (n=143) detected much higher Eew (44.8%) and Ech (17.5%) seroprevalences and a similar Ec (1.4%) seroprevalence (Citation33). In this study, overall Ech seroprevalence varied significantly over time with an initial decrease and then increase in 2010; seroprevalence rates were determined to be 3.3% (n=77) in 2008, 2.0% (n=43) in 2009, and 4.1% (n=72) in 2010 (p=0.0004) (). This pattern was observed in three regions, the South, Mid-Atlantic and Midwest, in which A. americanum ticks are prevalent. Reported Ech human monocytic ehrlichiosis (HME) cases increased before a significant drop in 2010 Citation27–(Citation29) , which did not mirror our canine Ech seroprevalence. Regionally, however, the high prevalence of HME cases was largely similar to dog Ech seroprevalence, with highest exposure risk in the South and Mid-Atlantic Citation27–(Citation29) . In the South, canine Eew seroprevalence also showed statistically significant changes over 2008 (n=57; 5.5%), 2009 (n=37; 3.6%), and 2010 (n=48; 6.3%) (p=0.02) (), which mirrored the trend for human Eew cases (Eew ehrlichiosis) for 2008–2010 Citation27–(Citation29) . The difficulty in clinically distinguishing between HME and Eew ehrlichiosis, along with the low number of human Eew infection reports could complicate comparisons made between canine and human Eew exposure (Citation5, Citation28); nevertheless, reports of high canine Eew seroprevalences should prompt more consideration for greater Eew exposure risk in humans throughout much of the Central and Southern US. In 2008, CDC made Eew ehrlichiosis a reportable disease in humans (Citation29.
Overall, Ec (n=117; 1.8%) and Apl (n=99; 1.5%) had the lowest seroprevalences in dogs from the US. Exposure frequencies were high in the Caribbean (n=8; 27.6% and n=3; 10.3%, respectively), as expected, where R. sanguineus, the known vector for Ec and potential vector for Apl, is prevalent. A previous study reported high Ec sero- (43.8%) and PCR (24.7%) prevalences in the Caribbean (Citation4). R. sanguineus is rarely documented in Canada (Citation34); however, Canada had unexpectedly high Ec and Apl seroprevalences (n=9; 3.2% and n=5; 1.8%, respectively), potentially due to a reporting bias from low numbers tested in this study or because dogs had traveled to or were transported from Ec and Apl endemic regions. Efforts to relocate homeless animals, particularly from tropical regions, including the Caribbean, to the Northeastern US and Canada have increased. For example, in 2003 the Save a Sato Foundation, which aims to relocate homeless dogs in Puerto Rico to the US, transported roughly 14,000 dogs to the US (Citation35). Relocating animals to shelter environments in non-endemic US regions and Canada could create R. sanguineus infestations within kennels, significantly impacting the prevalence rates of foreign tick-borne pathogen strains within the local dog population and exposing people to foreign, zoonotic pathogens.
Co-infections complicate interpretation of the clinical manifestations typically associated with single tick-borne diseases in both canine and human medicine. Co-infections can occur from simultaneous or sequential exposure to several tick species, or when multiple pathogens are transmitted by a single tick (Citation2, Citation3) (Citation36). In our study, co-exposures were defined as dogs seropositive to two or more vector-borne pathogens. Overall, the co-exposure seroprevalence rates were low. Combinations with the highest seroprevalence rates were among pathogens known to share a common tick vector such as Eew+Ech in A. americanum and Aph+Bb in I. scapularis. Regional co-exposure seroprevalences were highest in areas where the respective shared tick species are endemic, including Eew+Ech in the South and Mid-Atlantic, and Aph+Bb in the Northeast. Interestingly, the Mid-Atlantic had the highest co-exposure seroprevalence rates for several unexpected pathogen combinations including Eew+Bb, Ech+Bb, and Ech+Aph (). These co-exposure combinations and seroprevalence rates highlight the Mid-Atlantic as a potential region where I. scapularis and A. americanum ticks and their respective pathogens coalesce. As tick species migrate and habitats overlap, co-exposures will likely be more common with the potential for more disease severity. When monitoring tick-borne diseases in regions like the Mid-Atlantic, co-infections should be considered.
In conclusion, this study provides further support for the use of dogs in tick-borne pathogen human surveillance risks for several zoonotic infections of human and veterinary medical importance. Over a relatively brief time period, we demonstrated significant shifts in CVBD seroprevalence rates including overall increases in Aph and Ech, increases in Aph in the Mid-Atlantic and the South and increases in Bb in the South. Furthermore, by recognized species-specific seroprevalence, expected and unique co-exposures were identified and highlight the potential for tick-borne pathogen co-infections. Combining dog and human tick-borne disease surveillance data could enhance both public health and animal health.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Disclaimer
Barbara Qurollo's fellowship in Vector-Borne Disease Research at the College of Veterinary Medicine, North Carolina State University is supported by IDEXX Laboratories. Ramaswamy Chandrashekar, Melissa J. Beall, Brett A. Stillman, Jiayou Liu, and Brendon Thatcher are employees of IDEXX Laboratories, and Edward B. Breitschwerdt is a consultant to the company in the area of tick-borne infectious diseases.
Acknowledgements
We thank Tonya Lee for editorial assistance. We thank the Vector-borne Disease and Diagnostic Laboratory at North Carolina State University, Raleigh, NC, for access to archived canine serum.
References
- Zhang XF, Zhang JZ, Long SW, Ruble RP, Yu XJ. Experimental Ehrlichia chaffeensis infection in beagles. Med Microbiol. 2003; 52: 1021–6.
- Gaunt S, Beall M, Stillman B, Lorentzen L, Diniz P, Chandrashekar R, etal. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors. 2010; 3: 33–43. [PubMed Abstract] [PubMed CentralFull Text].
- Beall MJ, Chandrashekar R, Eberts MD, Cyr KE, Diniz PP, Mainville C, etal. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis. 2008; 8: 455–64. [PubMed Abstract].
- Yabsley MJ, McKibben J, Macpherson CN, Cattan PF, Cherry NA, Hegarty BC, etal. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet Parasitol. 2008; 151: 279–85. [PubMed Abstract].
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, etal. Ehrlichia ewingii, a newly recognized agent of human Ehrlichiosis. N Engl J Med. 1999; 341: 148–55. [PubMed Abstract].
- Azad AF, Beard CB. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis. 1998; 4: 179–86. [PubMed Abstract] [PubMed CentralFull Text].
- Mead P, Goel R, Kugeler K. Canine serology as adjunct to human Lyme disease surveillance canine serology as adjunct to human Lyme disease surveillance. Emerg Infect Dis. 2011; 17: 1710–12. [PubMed Abstract] [PubMed CentralFull Text].
- Duncan AW, Correa MT, Levine JF, Breitschwerdt EB. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 2004; 4: 221–9. [PubMed Abstract].
- Beall MJ, Alleman RA, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, etal. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors. 2012; 5: 29. [PubMed Abstract] [PubMed CentralFull Text].
- O'Connor TP, Esty KJ, Hanscom JL, Shields P, Philipp MT. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin Diagn Lab Immunol. 2004; 11: 458–62. [PubMed Abstract] [PubMed CentralFull Text].
- Stillman B, Beall M, Shields P, Hegarty B, Breitschwerdt E, Chandrashekar R. Performance comparison of species-specific peptide-based assays with immunofluorescence assays for detection of canine antibodies to Anaplasma and Ehrlichia spp. American College of Veterinary Internal Medicine. J Vet Intern Med. 2010; 24: 765.
- Qurollo B, Chandrashekar R, Hegarty B, Beall M, Stillman B, Liu J, etal. Clinical utility of an expanded panel of species-specific peptides for determining vector-borne pathogen co-infection exposure. Proceedings of the 2nd Symposium of the International Society for Companion Animal Infectious Disease, San Francisco, CA, 14–17 November 2012, Abstract P17..
- Little SE, Beall MJ, Bowman DD, Chandrashekar R, Stamaris J. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma pp. and Ehrlichia spp. in the United States, 2010–2012. Parasit Vectors. 2014; 7: 257. [PubMed Abstract] [PubMed CentralFull Text].
- Bowman D, Little SE, Lorentzen L, Shields J, Sullivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet Parasitol. 2009; 160: 138–48. [PubMed Abstract].
- Bacon RM, Kugeler KJ, Mead PS. Centers for Disease Control and Prevention (CDC). Surveillance for Lyme disease-United States, 1992–2006. MMWR Surveill Summ. 2008; 57: 1–9. [PubMed Abstract].
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, etal. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the eastern United States. Am J Trop Med Hyg. 2012; 86: 1062–71. [PubMed Abstract] [PubMed CentralFull Text].
- CDC. Lyme disease incidence rates by state, 2003–2012. Available from: http://www.cdc.gov/lyme/stats/chartstables/incidencebystate.html [cited 27 February 2014]..
- Department of Health, Commonwealth of Virginia. Available from: http://www.vdh.state.va.us/clinicians/pdf/05-17-11 Clinicians%27 Letter - Lyme Disease.pdf [cited 27 February 2014]..
- Herman-Giddens ME. Yale Lyme disease risk maps are not accurate for the south in 2012. Am J Trop Med Hyg. 2012; 86: 1085. [PubMed Abstract] [PubMed CentralFull Text].
- Stromdahl EY, Hickling GJ. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health. 2012; 59: 48–64. [PubMed Abstract].
- National Center for Emerging and Zoonotic Infection Diseases. Available from: http://www.cdc.gov/ticks/geographic_distribution.html#blacklegged [cited 27 February 2014]..
- Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J. 2006; 47: 1194–200. [PubMed Abstract] [PubMed CentralFull Text].
- Villeneuve A, Goring J, Marcotte L, Overvelde S. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia canis and Dirofilaria immitis among dogs in Canada. Can Vet J. 2011; 52: 527–30. [PubMed Abstract] [PubMed CentralFull Text].
- Public Health Agency of Canada. Available from: http://www.phac-aspc.gc.ca/id-mi/lyme-fs-eng.php#s9 [cited 27 February 2014]..
- Public Health Ontario. Technical report: update on Lyme disease, prevention and control. 2012. Available from: http://www.publichealthontario.ca/en/eRepository/PHO Technical Report Update on Lyme Disease Prevention and Control Final 20030212.pdf [cited 27 February 2014]..
- IDEXX Laboratories. 2007 Canadian incidence study – incidence of heartworm, Ehrlichia canis, and Lyme disease in dogs across Canada as determined by the IDEXX SNAP® 3Dx® test. 2008; IDEXX Laboratories. 1–13. Available from: http://www.bridgelin.ca/bridgelin2/files/2602.pdf [cited 27 February 2014]..
- CDC. Summary of notifiable diseases: United States, 2010. MMWR Morb Mortal Wkly Rep. 2012; 59: 1–111.
- CDC. Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011; 58: 1–100.
- CDC. Summary of notifiable diseases: United States, 2008. MMWR Morb Mortal Wkly Rep. 2010; 57: 1–94.
- Clark KL. Anaplasma phagocytophilum in small mammals and ticks in northeast Florida. J Vector Ecol. 2012; 37: 262–8. [PubMed Abstract].
- Fang QQ, Mixson TR, Hughes M, Dunham B, Sapp J. Prevalence of the agent of human granulocytic Ehrlichiosis in Ixodes scapularis (Acari: Ixodidae) in the coastal southeastern United States. J Med Entomol. 2002; 39: 251–5. [PubMed Abstract].
- Schulze TL, Jordan RA, Schulze CJ, Mixon T, Papero M. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J Med Entomol. 2005; 42: 450–6. [PubMed Abstract].
- Little SE, O'Conner TP, Hempstead J, Saucier J, Reichard MV, Meinkoth JH, etal. Ehrlichia ewingii infection and exposure rates in dogs from the southcentral United States. Vet Parasitol. 2010; 172: 355–60. [PubMed Abstract].
- Canadian guidelines for the treatment of parasites in dogs and cats. Canadian Parasitology Expert Panel for companion animals. 2009; 1–31. Available from: http://www.wormsandgermsblog.com/uploads/file/CPEP guidelines ENGLISH.pdf [cited 27 February 2014]..
- Vandem Brook T. More cities importing pound puppies. 2003; USA TODAY. Available from: http://usatoday30.usatoday.com/news/nation/2003-01-30-dogs-usat_x.htm [cited 27 February 2014]..
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, etal. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999; 37: 2631–8. [PubMed Abstract] [PubMed CentralFull Text].
