Abstract
Background
Malaria transmission is perennial in the Assam–Arunachal Pradesh interstate border areas in the Sonitpur district of Assam, India. A yearlong study was carried out on the incidence of symptomatic and asymptomatic malaria and the role of asymptomatic malaria carriers in persistent transmission of the disease. The relationships between malaria incidence and weather parameters were also investigated.
Methods
Active and mass blood surveys were conducted on a monthly basis in Bengenajuli, Sapairaumari Pathar, and Nigam villages near the Assam–Arunachal Pradesh border. Epidemiological indices were estimated for malaria-positive cases. Multiple linear regression between monthly malaria incidence and monthly average temperature, and relative humidity along with monthly total rainfall was carried out. The known malaria vectors collected in CDC light traps were identified and recorded.
Results
Slide positivity rate (SPR) and Plasmodium falciparum percent (Pf%) for symptomatic malaria were 26.1 and 79.8, respectively. Prevalence of malaria vectors was observed throughout the year with varying density. Anopheles philippinensis/nivipes and A. annularis were predominant among the seven known vector species recorded currently. Asymptomatic parasitemia was detected throughout the year with SPR ranging from 4.8 to 5.3. Monthly rainfall with 1-month lag had the highest correlation (r=0.92) with SPR. The relationship between SPR and weather factors was established as SPR=−114.22+0.58 T min+1.38 RH+0.03 RF (R 2=0.89; p=0.00).
Conclusion
Low and relatively constant levels of asymptomatic parasitemia was present in the study area. High malaria vector density and presence of asymptomatic malaria parasite carriers were responsible for persistent malaria transmission in the region. This study concludes that passive detection and prompt treatment of asymptomatic carriers is essential for preventing persistent disease transmission. Rainfall along with some other weather variables may be used for predicting the malaria epidemics in the region. The predictive information could be useful to target resources more effectively.
Malaria is the most prevalent vector-borne disease and a major cause of morbidity and mortality (Citation1). The northeastern states of India are endemic to malaria and its transmission is perennial. The state of Assam in northeastern India with 2.6% of the national population contributes more than 5% of the total malaria cases in the country annually. The state is highly receptive to malaria transmission because of heavy rainfall, high humidity, and warmer climate for most of the year. Plasmodium falciparum accounts for >60% of cases, whereas the rest are attributed to P. vivax (Citation2). The interstate border areas in the region are prone to high incidence of malaria due to poor socioeconomic conditions and frequent movement of people through forested areas. In recent years, deforestation along the border areas has led to ecological changes affecting the behavior of malaria vectors and the pattern of disease transmission (Citation3, Citation4). Perennial transmission of malaria in Assam is attributed to the activity of efficient vectors such as Anopheles minimus (perennial species), A. dirus (monsoon species) and A. fluviatilis (winter species).
Asymptomatic parasitemia, which refers to the presence of malaria parasites in the blood with no symptoms, provides a reservoir for transmission and may be a precursor to symptomatic malaria. Although asymptomatic parasitemia has been detected in the population, its seasonal prevalence and role in persistent malaria transmission are not well understood in the region. Malaria transmission dynamics and distribution in the region are largely dependent on a broad spectrum of biotic and abiotic factors, mainly temperature, relative humidity (RH), and rainfall (Citation5, Citation6). Weather affects malaria incidence through its effects on vector population dynamics and development of the malaria parasite inside the vector mosquito (Citation7, (Citation8). Climate has been regarded as a major driving force in malaria transmission; hence, climate variables could be used for predicting and early warning of malaria epidemics (Citation8, (Citation9).
The present study was carried out to investigate the prevalence of asymptomatic malaria parasitemia and its role in persistent transmission along the Assam–Arunachal Pradesh border area. Malaria incidences in the study area were correlated with the weather factors.
Methods
Study area
The study was carried out in three villages, namely Bengenajuli, Sapairaumari Pathar, and Nigam in Sonitpur district (92° 20′ E–93° 45′ E and 26° 20′ N–27° 05′ N) near the Assam–Arunachal Pradesh border 2004–2005. The villages were surrounded by dense forests and had a total human population of about 10,383 individuals. The study period was grouped into pre-monsoon (March–May), monsoon (June–August), post-monsoon (September–November), and winter (December–February) seasons.
Epidemiological studies
Blood smears were collected on a monthly basis during March 2004–February 2005 separately from persons having fever or other symptoms of malaria, as well as from persons having no symptoms of malaria. A total of 4,300 and 7,699 blood smears were collected from persons with and without malaria symptoms, respectively. Blood smears were stained with Giemsa for microscopical examination for malaria parasitemia. Epidemiological indices, namely slide positivity rate (SPR), slide falciparum rate (SfR), P. falciparum percent (Pf%), annual blood examination rate (ABER), and annual parasitic index (API) were calculated separately for symptomatic and asymptomatic malaria parasitemia. The participants were informed about the study and consent was obtained.
Entomological studies
Monthly collection of adult mosquitoes was carried out in houses using battery-operated CDC miniature light traps during 1800–0600 hours. The collected mosquitoes were identified using standard keys and per-trap night densities of known vectors were estimated (Citation10, Citation11).
Meteorological data
The data on monthly mean maximum (T max) and minimum temperature (T min), monthly mean RH, and monthly total rainfall (RF) were obtained from the North Eastern Regional Institute of Water and Land Management (NERIWALM), Tezpur, Assam.
Data analysis
Chi-square test was used for sex, age, and season-wise analysis of malaria incidence. The relationship between SPR and meteorological parameters was established by Pearson correlation and stepwise multiple linear regression.
Results
The SPR for symptomatic malaria was 26.1 and the API was 108.1. The monthly parasitic index (MPI) was lowest in January (1.9) and highest in July (20.3). SPR was high during the months of June (39.1) and July (38.2), while low in March (10.6) and April (10.9). SfR and Pf% in the study area were 20.8 and 79.8, respectively. SfR was the lowest in March (6.6) and the highest in June (32.6) while Pf% ranged from 62.5 (March) to 84.1 (September). Sex-wise distribution of malaria cases () indicated that SPR was statistically higher in males (27.5) than females (24.5) (χ 2=4.8; p=0.03). However, the females were observed to have higher Pf% (82.2) than the males (77.9). The SfR in males and females were 21.4 and 20.1, respectively. Age-wise analysis of malaria incidence () showed that the SPR had significant variation among the age groups (χ 2=70.7; p<0.001) and was found to be high in the 10–14 (33.5) and 5–9 year age groups (30.6). Low SPR was observed in the age groups of 0–11 months (15.5) and 12–23 months (17.8), whereas moderate rates of malaria incidence was recorded in >14 year (SPR=20.9) and 2–4 year age group (SPR=24.4). SPR and SfR for asymptomatic malaria were 5.0 and 3.6, respectively (). SPR was highest during August (5.3), while lowest in April (4.8). However, SfR was found to be highest in November (4.0) while lowest in March (3.3). Pf% ranged from 66.7 (March) to 76.0 (November). API was 36.9, while MPI ranged from 2.4 (November) to 3.6 (August). Season-wise analysis of asymptomatic malaria revealed that there was no significant change in SPR over the seasons in a year (χ 2=0.1; p=0.99).
Table 1 Sex-wise distribution of symptomatic malaria cases in the study area
Table 2 Age-wise distribution of symptomatic malaria cases in the study villages
Table 3 Season-wise distribution of asymptomatic malaria cases in the study villages
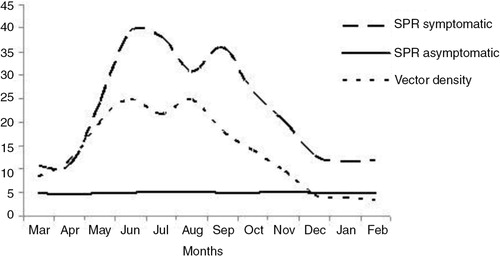
Anopheles philippinensis/nivipes and A. annularis were the predominant species of known malaria vectors observed in the light trap collections. The other vectors recorded were A. minimus, A. culicifacies s.l., A. fluviatilis s.l., A. dirus s.l. and A. varuna. The mean per-trap night density of known malaria vectors was 13.7. Vector density was high during June and August (per-trap night density=99.0), while low in February (per-trap night density=14.1). The change in symptomatic malaria incidence pattern was observed to follow the seasonal change in a known malaria vector population ().
Fig. 1. Incidence patterns of symptomatic and asymptomatic malaria in relation to known malaria vector density in the study area.
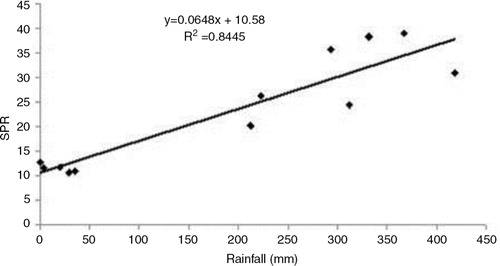
The meteorological data indicated that the monthly mean maximum temperature (T max) during the study period ranged from 23.0 (January) to 36.5°C (August), while monthly mean minimum temperature (T min) ranged from 8.0 (December) to 24.5°C (August), respectively. The range of monthly mean RH was 81.6 (February) to 90.3% (June), while the highest monthly total rainfall was recorded in July (418.6 mm). Multiple linear regression between malaria SPR and weather variables indicated that malaria incidence correlates with weather during the preceding month. Although, all four weather parameters were significantly correlated with SPR (p<0.05), the total rainfall was found to have the highest correlation (r=0.92). The relationship between monthly total rainfall and SPR was derived as: SPR=10.58+0.06 RF (R 2=0.84) (). The correlation with RH (r=0.88) was slightly higher than that with minimum temperature (r=0.88). Maximum temperature was found to have least correlation with malaria incidence (r=0.67) and hence the regression relationship was established using rainfall, RH and minimum temperature as, SPR=−114.22+0.58 T min+1.38 RH+0.03 RF (R 2=0.89; p=0.00).
Discussion
The studies on symptomatic and asymptomatic malaria patients in the selected villages along the Assam–Arunachal Pradesh border were carried out in a yearlong survey. Sex-wise analysis of symptomatic malaria indicated that the incidences of symptomatic malaria were higher among males, but the percentage of P. falciparum infections was higher in females. The earlier studies conducted in the region have also indicated higher incidence rates among males (Citation12). Children in the age group of 5–14 were found to be highly vulnerable to malaria with high P. falciparum percentage. Similar observations have also been reported in a recent study conducted in the region (Citation13). These areas are exposed to intense and constant malaria transmission all year round; hence, the population living for a long time tends to develop high levels of immunity against malaria (Citation14). In endemic areas, progressive acquisition of immunity with age is observed, which results in decreased malaria incidence with increasing age (Citation15, Citation16). Studies have suggested that the time to acquire clinical immunity against malaria depends on the intensity of transmission, interventions used, and genetic diversity of malaria parasite (Citation15, (Citation16).
Monthly observations on asymptomatic parasitemia were carried out for the first time in this region of the country. Asymptomatic parasitemia was detected among the population throughout the year with SPR ranging from 4.8 to 5.3. The majority of asymptomatic parasitemia was due to P. falciparum (71.3%). Many studies conducted in the region suggest that P. falciparum has been a dominant malaria parasite while other species contribute comparatively less to the malaria episodes Citation2–(Citation4, Citation12) (Citation13). The current study has reported some little known malaria vectors such as A. annularis and A. philippinensis/nivipes in high density; however, the well-established malaria vectors such as A. minimus s.l and A. dirus s.l were reported in very less numbers. These findings suggest that previously considered secondary vectors may be supporting malaria transmission. Previous studies conducted on identification of malaria vectors indicated that A. annularis and A. philippinensis/nivipes are most likely involved in malaria transmission in many parts of northeastern India (Citation17, (Citation18). A. culicifacies and A. varuna were recorded in very less density. A. culicifacies has been regarded a major vector of malaria in many parts of India, whereas A. varuna is a vector of local importance and is implicated in a few malaria endemic areas (Citation19, (Citation20). The periods of high vector density (June–August) corresponded to the periods of high malaria incidence (June–September).
The present results indicated that cases of asymptomatic parasitemia and vector activity were present in the study area throughout the year. Increased prevalence of asymptomatic carriers of P. falciparum has been associated with high transmission of malaria throughout the malaria endemic countries (Citation21). The asymptomatic carriers remain an infection source for vector mosquitoes for a longer period than the symptomatic carriers although the rates of infection are lower in the former (Citation22). The individuals with asymptomatic parasitemia do not seek medical attention and may become gametocyte carriers and contribute to the persistent malaria transmission (Citation23). Non-clearance of parasites from blood due to under-dosage of antimalarial drugs along with repeated infections has been found to result in the development of immunity and asymptomatic carriers (Citation24).
In addition to the abundance of vector mosquitoes and the presence of parasites, malaria transmission is affected by climate (Citation25). The development rates and survival of malaria parasites and mosquito vectors is affected by temperature, whereas rainfall affects the availability of breeding habitats. Hence, increased temperature and rainfall to an optimum level have synergistic effects on malaria incidence (Citation26). The results indicated that weather corresponding to a 1-month lag period was more correlated with SPR (R 2=0.89) than corresponding to the same month (R 2=0.82) or a 2-month lag period (R 2=0.80). Monthly rainfall was found to be the most important factor contributing to malaria incidence. This is in agreement with the studies in Dehradun (India) where rainfall and minimum temperature showed positive correlations with malaria incidence with a 1-month time lag, whereas the correlation was less with 0-, 2-, and 3-month time-lags (Citation27). In the present study, heavy rainfall (311.9 mm) received in April resulted in the doubling of SPR during April–May (10.9–24.3). Similarly, dry weather in November resulted in a reduction of SPR from 20.3 to 12.7 during November–December. A study conducted in Sri Lanka showed that malaria follows rainfall with a few months delay in which a high seasonal rainfall peak is followed by a high seasonal malaria peak (Citation7). The present results suggest that the mean temperature ranged from 17.4 to 30.5°C and the lag period following rainfall was 1 month. Furthermore, minimum temperature and RH were also found to have a significant influence on malaria incidence.
Current findings indicate that asymptomatic malaria cases are prevalent in the region and may serve as epicenters for initiation of malaria epidemics during the favorable season. In conclusion, the study emphasizes that targeted efforts are needed for diagnosis and treatment of asymptomatic malaria carriers. Indoor residual sprays need to applied and the use of bed nets impregnated with insecticide need to be popularized to reduce man–mosquito contact. Mass blood surveys to detect asymptomatic carriers should be conducted followed by treatment with proper doses of recommended antimalarial drugs.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
Principal author thanks Dr. Reji Gopalakrishnan for his help in preparation of manuscript.
References
- Alemu A, Abebe G, Tsegaye W, Golassa L. Climatic variables and malaria transmission dynamics in Jimma town, South West Ethiopia. Parasit Vectors. 2011; 4: 30. [PubMed Abstract] [PubMed CentralFull Text].
- Dev V, Dash AP, Khound K. High-risk areas of malaria and prioritizing interventions in Assam. Curr Sci. 2006; 90: 32–6.
- Nath MJ, Bora A, Talukdar PK, Das NG, Dhiman S, Baruah I, etal. A longitudinal study of malaria associated with deforestation in Sonitpur district of Assam, India. Geocarto Int. 2012; 27: 79–88.
- Dev V, Phookan S, Sharma VP, Anand SP. Physiographic and entomologic risk factors of malaria in Assam, India. Am J Trop Med Hyg. 2004; 71: 451–6. [PubMed Abstract].
- Thomson AJ. Climate indices, rainfall onset and retreat, and malaria in Nigeria. J Vect Borne Dis. 2010; 47: 193–203.
- Dhiman RC, Pahwa S, Dash AP. Climate change and malaria in India: interplay between temperatures and mosquitoes. Reg Health Forum. 2008; 12: 27–31.
- Briet OJT, Vounatsou P, Gunawardena DM, Galappaththy GNL, Amerasinghe P. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008; 7: 77. [PubMed Abstract] [PubMed CentralFull Text].
- Hoshen MB, Morse AP. A weather-driven model of malaria transmission. Malar J. 2004; 3: 32. [PubMed Abstract] [PubMed CentralFull Text].
- Teklehaimanot HD, Lipsitch M, Teklehaimanot A, Schwartz J. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malar J. 2004; 3: 41. [PubMed Abstract] [PubMed CentralFull Text].
- Wattal BL, Kalra NL. Regionwise pictorial keys to the female Indian Anopheles. Bull Nat Soc India. 1961; 9: 85–138.
- Nagpal BN, Srivastava A, Saxena R, Ansari MA, Dash AP, Das SC. Pictorial identification key for Indian anophelines. 2005; Delhi, India: Malaria Research Centre (ICMR).
- Das NG, Talukdar PK, Kalita J, Srivastava RB. Malaria situation in forest fringed villages of Sonitpur district (Assam), India bordering Arunachal Pradesh during outbreak. J Vec Borne Diseases. 2007; 44: 213–18.
- Rabha B, Goswami D, Dhiman S, Das NG, Talukdar PK, Nath MJ, etal. A cross sectional investigation of malaria epidemiology among seven tea estates in Assam, India. J Parasit Dis. 2011; 36: 1–6. [PubMed Abstract] [PubMed CentralFull Text].
- Coura JR, Suárez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection-a review. Memoirs Inst Oswaldo de Cruz. 2006; 101: 229–37.
- Rogier C, Trape JF. Malaria attacks in children exposed to high transmission: who is protected?. Trans R Soc Trop Med Hyg. 1993; 87: 245–6. [PubMed Abstract].
- Dhiman S, Veer V. Culminating anti-malaria efforts at long lasting insecticidal net?. J Infect Public Health. 2014; 7: 457–64. [PubMed Abstract].
- Dhiman S, Bhola RK, Goswami D, Rabha B, Kumar D, Baruah I, etal. Polymerase chain reaction detection of human host preference and Plasmodium parasite infections in field collected potential malaria vectors. Pathog Glob Health. 2012; 106: 77–80.
- Dhiman S, Rabha B, Goswami D, Das NG, Baruah I, Bhola RK, etal. Insecticide resistance and human blood meal preference of Anopheles annularis in Assam Meghalaya border, Northeast India. J Vect Borne Dis. 2014; 51: 133–6.
- Sharma VP. Fighting malaria in India. Curr Sci. 1998; 75: 1127–40.
- Dash AP, Adak T, Raghavendra K, Singh OP. The biology and control of malaria vectors in India. Curr Sci. 2007; 92: 1571–8.
- Fugikaha E, Fornazari PA, Penhalber RSR, Lorenzetti A, Maroso RD, Amoras JT, etal. Molecular screening of Plasmodium sp. asymptomatic carriers among transfusion centers from Brazilian Amazon region. Rev Inst Med Trop Sao Paulo. 2007; 49: 1–4. [PubMed Abstract].
- Alves FP, Durlacher RR, Menezes MJ, Krieger H, da Silva LHP, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002; 66: 641–8. [PubMed Abstract].
- Cucunuba ZM, Guerra AP, Rahirant SJ, Rivera JA, Cortés LJ, Nicholls RS. Asymptomatic Plasmodium spp. infection in Tierralta, Colombia. Mem Inst Oswaldo Cruz. 2008; 103: 668–73. [PubMed Abstract].
- Das NG, Baruah I, Das SC. Situation of malaria in forest-fringed villages of North Lakhimpur district, Assam. Indian J Malariol. 2002; 39: 43–7. [PubMed Abstract].
- Usher PK. Modelling malaria transmission potential for climate change scenarios in West Africa and Europe. Earth Environ. 2010; 5: 40–65.
- Zhou G, Minakawa N, Githeko AK, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci USA. 2004; 101: 2375–80. [PubMed Abstract] [PubMed CentralFull Text].
- Devi NP, Jauhari RK. Climatic variables and malaria incidence in Dehradun, Uttaranchal, India. J Vect Borne Dis. 2006; 43: 21–8.