Abstract
Background
Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae is an emerging therapeutic challenge, especially in the treatment of urinary tract infections. Following an outbreak of CTX-M-15 Klebsiella pneumoniae in Uppsala, Sweden, an orphan drug trial on IgY chicken antibodies was undertaken in an attempt to eradicate faecal carriage of ESBL-producing K. pneumoniae and Escherichia coli.
Methods
Hens were immunised with epitopes from freeze-dried, whole-cell bacteria (ESBL-producing K. pneumoniae and E. coli) and recombinant proteins of two K. pneumoniae fimbriae subunits (fimH and mrkD). The egg yolks were processed according to good manufacturing practice and the product was stored at−20°C until used. Using an internal database from the outbreak and the regular laboratory database, faecal carriers were identified and recruited from May 2005 to December 2013. The participants were randomised in a placebo-controlled 1:1 manner.
Results
From 749 eligible patients, 327 (44%) had deceased, and only 91 (12%) were recruited and signed the informed consent. In the initial screening performed using the polymerase chain reaction, 24 participants were ESBL positive and subsequently randomised and treated with either the study drug or a placebo. The study was powered for 124 participants. Because of a very high dropout rate, the study was prematurely terminated. From the outbreak cohort (n=247), only eight patients were screened, and only one was positive with the outbreak strain in faeces.
Conclusions
The present study design, using IgY chicken antibodies for the eradication of ESBL-producing K. pneumonia and E. coli, was ineffective in reaching its goal due to high mortality and other factors resulting in a low inclusion rate. Spontaneous eradication of ESBL-producing bacteria was frequently observed in recruited participants, which is consistent with previous reports.
Extended-spectrum beta-lactamase (ESBL)-producing gram-negative rods are an increasing challenge for the healthcare sector. Among Enterobacteriaceae, Klebsiella pneumonia and Escherichia coli are the most common ESBL-producing bacteria and are of major concern because of treatment difficulties and dissemination in the healthcare system (Citation1). The number of healthy carriers of ESBL-producing bacteria is increasing dramatically (Citation2). The prevalence of ESBL faecal carriage in different parts of the world is based on regional data. In Europe, for instance, faecal carriage may be approximately 10% (predominantly with CTX-M-15) (Citation3). In Sweden, resistance to third-generation cephalosporins in 2012 was 4.4% for E. coli and 2.6% for K. pneumoniae (www.folkhalsomyndigheten.se, EARS-Net). International travelling contributes to a high risk for acquired ESBL genes to the gut flora (Citation4, Citation5), and consequently, the spreading of plasmid-borne resistance worldwide.
In the spring of 2005, a major outbreak of CTX-M-15-producing K. pneumoniae occurred at the Uppsala University Hospital (Citation6). Two hundred forty-seven patients (median age 78 years) were reported to be infected or colonised with this difficult-to-treat bacterium (Citation7). Considerable effort to combat this outbreak was undertaken and the outbreak was declared over in 2008. Various issues during the outbreak were studied, including hospital management, microbiology, infection control measures, and educational antibiotic interventions (Citation7, Citation8). Risk factors for blood stream infections with ESBL-producing Enterobacteriaceae are recent antibiotic therapy (i.e. beta lactam antibiotics), presence of comorbidities, previous invasive procedures and devices, and admission to long-term healthcare facilities (Citation9). A study on faecal carriage of ESBL enzymes revealed that 1) nearly 50% of the initial carriers were still positive 12 months later, 2) some of the carriers were transiently negative before 12 months, and 3) the ESBL genes were sometimes found in new species or strains during the observation period (Citation10). An indefinite carrier state is suggested and therefore viable alternatives to antibiotics that can eradicate resistant bacteria are urgently needed.
Thus, eradicating the colonisation of ESBL K. pneumoniae and E. coli from the gastrointestinal tract in carriers is an important target to accomplish to decrease the burden of antibiotic-resistant gram-negative bacteria. Oral immunotherapy with avian immunoglobulins (IgY) lacks the risk of resistance, and toxicity is low. The only precaution to consider is egg allergy. Human studies have shown that the number of lung infections with Pseudomonas aeruginosa in patients with cystic fibrosis can be reduced with IgY treatment by gargling (Citation11, Citation12). In a randomised, placebo-controlled study using IgY chicken antibodies for the treatment of gastroenteritis in children caused by rotavirus, stool output and oral rehydration solution were lower in the treatment group, along with a faster clearance of the virus infection, compared with the controls (Citation13). In the same study, no differences were found in duration of illness between the groups. IgY is an effective immunologic tool to influence unwanted microbes from colonising the alimentary tract of humans by adding its activity to the regular human immune system (Citation14).
The aim of this study was to determine whether IgY chicken antibodies could be effective in eradicating faecal carriage of ESBL-producing K. pneumoniae and E. coli.
Methods
Study design and recruitment
This study was a randomised, placebo-controlled, parallel-group, phase II, orphan drug study of anti-ESBL IgY for the eradication of ESBL-producing K. pneumoniae and E. coli in faecal carriers. The study was approved by the Regional Ethical Committee (DNR 2011/170/1) and the Medical Products Agency in Sweden (Eudract 2009-011446). The study design is outlined in .
Fig. 1. Study flow chart of the screening and randomisation process, treatment, and follow-up for the participants in the study.
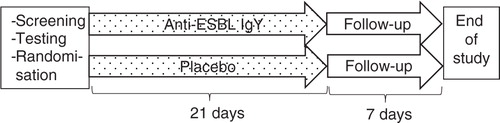
Two hundred forty-seven patients colonised or infected with CTX-M-producing K. pneumonia during the hospital outbreak during 2005 to 2007 were registered in an internal database at the Department of Microbiology, Uppsala University Hospital, and formed the base for this study. To increase the inclusion rate, patients found to be colonised or infected with ESBL-producing K. pneumonia or E. coli at Uppsala University Hospital between 2008 and 2013, and Falun Hospital between 2012 and 2013 were also added. The screening procedure and typing of ESBL-producing strains were carried out as previously described (Citation6). ESBL-producing K. pneumonia and E. coli isolates were reported to the research team. Written information and contact data were sent by mail to patients of age ≥18 years. Individuals who did not reply spontaneously were contacted by phone at least two times on separate occasions. After informed consent, patients were screened for colonisation with ESBL-producing K. pneumonia or E. coli, and, if positive, randomised to the study drug or placebo at a 1:1 ratio.
Preparation of study drug
Briefly, IgY antibodies against ESBL-producing K. pneumoniae and E. coli were developed as follows: recombinant variants of the two fimbrial K. pneumoniae subunits mrkD and fimH were developed by detailed instructions in the investigator's brochure. A combination of recombinant proteins was used for immunisation of one group of hens and a combination of ESBL K. pneumoniae and E. coli bacteria was used for the immunisation of another group of hens. The immunogens were prepared together with Freund's adjuvant. Egg yolk antibodies are actively transferred from the hen to the egg yolk where they are found in high concentration (Citation15). The preparation was carefully mixed and used for intramuscular immunisation. Equal amounts of eggs from the two immunisation groups were used for production of the active drug. Eggs from immunised hens were visually inspected and defective eggs were excluded from further processing. The remaining eggs were then washed in 70% ethanol and dried before the egg yolk was separated and weighed: the egg yolk was diluted with purified water (Citation16); the lipoproteins were sedimented; and the supernatant decanted, filtered, and finally dispensed in high-density polyethylene bottles with a polypropylene screw cap (70 mL in each) and labelled.
The specificity of the anti-ESBL IgY antibodies against the two antigen pairs, that is, the fimbrial proteins (mrkD and fimH) and ESBL-producing E. coli/K. pneumoniae was determined by an enzyme-linked immunosorbent assay (ELISA). There was a high specific binding of anti-ESBL IgY in IgY batches, whereas the placebo batches showed very low activity. The minimal amount of antibodies required for clinical effect was not established. Thus, an exact lower limit for the acceptance criterion has not been established, but samples tested with ELISA from the IgY batches showed 11.6–15.1 BNU/mL (Limit, >5 Bio Unit/mL), regardless of storage conditions (i.e. <−15°C up to 12 months, from +2 to 8°C up to 12 months, +25°C up to 1 week, thawing up to three times after freezing in <−15°C for 3 months). Procedures to eliminate the risk of bacterial contamination of the product were secured according to Medical Products Agency regulations. The egg yolk products were frozen at −20°C in bottles until used. The egg yolk contains two parts: low-density lipoproteins and water-soluble proteins. Approximately 30% of the water-soluble proteins comprise IgY. One egg yolk is made up of approximately 100 mg IgY. Of that amount, up to 10% are reported to form specific antibodies (Citation17). Protein concentration in the water-soluble part in all batches was calculated to 1.8 mg/mL as determined by the Bradford assay. Thus, the assumption for the IgY concentration was calculated to be 0.18 mg/mL.
The eggs were hatched at OVA Production AB, Sweden. Cobra Biologics AB, Sweden, purified the antibodies. The production of IgY followed good manufacturing practice standards and was under strict regulatory control. The placebo solution was purified from eggs laid by non-immunised hens.
Administration of study drug
The daily peroral dosage was set to 70 mL that was to be taken orally in equally divided doses twice daily (35 mL in the morning and 35 mL in the evening). Before intake of the IgY solution, sodium hydrogen carbonate (Na2HCO3) was administered to increase gastric pH. Na2HCO3 (2.09 g) was delivered in bags with effervescent granules (Samarin, Cederroth AB, Sweden) that should be dissolved in a glass of (approximately 150 mL) cold water. Intake of food or beverages 1 h before and after the dose was to be avoided.
Outcome analysis
According to the protocol, the primary endpoint was the eradication of faecal carriage with ESBL-producing K. pneumoniae or E. coli, or both, on day 21 (the last treatment day). The secondary endpoints were the proportion of participants found to be ESBL negative in faeces at different time points during the treatment and day 28 as well as morbidity, safety and tolerability. Complementarily, the enrolment group was studied regarding time from the first positive ESBL test to the screening test.
Statistics
The sample size was based on the following assumptions: two-sided type I error rate of 5% and power 85%; treatment allocation ratio of 1:1; eradication proportion in the test arm was 45%; and eradication proportion in the control group was 20%. Under these assumptions, 124 eligible participants were required (62 per group). For statistical calculations on the duration of carriage, paired t-tests and chi-square tests were performed. p-Values <0.05 were deemed significant.
Results
Recruitment process and duration of ESBL carriage
In total, 749 individuals were recorded for the recruitment process. Only 24 (12%) remained for randomisation to either active or placebo IgY treatment. There were several reasons for missing patients, including death (44%), lost to follow-up (15%), failure to meet with the study criteria (SC, 5%), and high threshold to participate (HTTP, i.e. high age, disabled, transportation problems, and unwillingness to participate, 24%). For details, see . Thus, no statistical analysis could be performed because of the low inclusion rate.
Fig. 2. Flow chart of the number of participants who did not complete the study until randomisation. LFU: lost to follow-up; SC: failure to meet with the study criteria; HTTP: high threshold to participate.
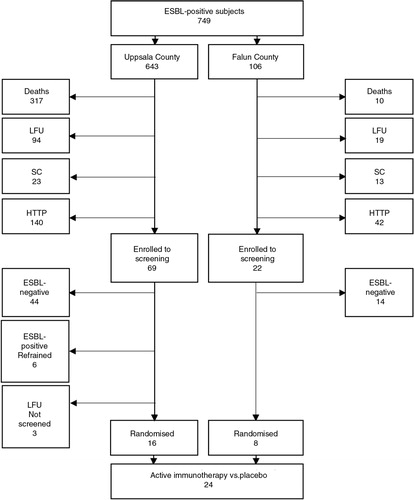
Detectable faecal carriage of the ESBL gene was found in 34% (30/88) of the enrolled participants. The median time from the first ESBL-positive test to the screening test was shorter in the group with detectable ESBL genes compared with the group with undetectable genes (7 months vs. 12.5 months, p=0.029). Only one male participant with detectable faecal carriage was found to stem from the original K. pneumoniae outbreak ().
Table 1 ESBL carriage: age, sex, strains, and the time interval between samples from the first and second positive test at the screening test of the enrolment group
Safety and tolerability
The proportion of participants who reported related adverse events was similar between the active 58% (7/12) and the placebo group 42% (5/12). The reported mild adverse events were diarrhoea (n=4), fatigue (n=2), loose stools, nausea, flatulence, and abdominal pain (n=1, respectively). Nausea and vomiting (n=1, respectively) were reported as severe adverse events from one participant from the active group, who did not complete the treatment course. One severe, non-related severe adverse event was reported from one participant, who became ill from diabetes ketoacidosis.
Discussion
The aim of this study was to study whether the use of IgY chicken antibodies could be effective in eradicating ESBL K. pneumoniae and ESBL E. coli from human faecal carriers. However, because of a high dropout rate, the study was discontinued before completion. The high number of deceased and those with HTTP because of disability and transportation issues made up the major proportion of the study participants. Thus, leaving the study process was the main cause of terminating the study. This fact mirrors that the patient group, which was the target group in the present study, had a high degree of comorbidities and high age that are known to predispose death and disability (Citation9). Most related side effects were mild among the treated participants. However, in one case, treatment had to be interrupted because of intensive nausea and vomiting.
This study provides some information about the duration of carriage of ESBL-producing bacteria. Only one-third of the participants screened for persistent faecal carriage in our study were ESBL positive, indicating a high proportion of spontaneous eradication. The median interval between the initial detection of ESBL genes and the faecal screening performed within this study was significantly shorter in the ESBL-positive group than in the ESBL-negative group. These results are consistent with previous studies on colonisation with ESBL-producing Enterobacteriaceae. The median time for carriage has been reported to be 6.6 months and might be prolonged in some patients (Citation18). However, carriage can also be transiently negative over time, indicating that persistence of ESBL-producing bacteria is sometimes overlooked with conventional screening methods (Citation10).
Fimbriae are important components for bacterial adhesion to the host cells. Thus, IgY specifically binding to these antigens and others from the bacteria may have the ability to prevent these ESBL-producing bacteria from colonising the gastrointestinal tract (Citation19). ESBL genes are not linked to specific antigens but may exert their effect on the specific bacteria in an unknown manner. The hypothesis is that specific IgY exerts its effect locally in the intestine by decreasing adherence of the pathogen to the gut epithelium. Another theoretical mechanism is that IgY binds to specific antigens on the bacterial surface, which prevents bacteria from interacting with different epitopes in the gut environment to survive.
This study reveals no information on IgY concentrations in faeces. For correlation studies, IgY concentrations could be analysed along with determinants of polymerase chain reaction -positivity of ESBL genes. However, because of pharmacokinetic difficulties to establish a steady-state concentration in this particular fluid with the current dosing used, we decided not to attempt it. Instead, we propose to develop a gut model in which IgY binding can be studied. Analysing antibody activity in stool samples has inherent technical challenges. The stomach contains pepsin. Digestion of immunoglobulins with pepsin normally produces one F(ab′)2 fragment and numerous small peptides of the Fc portion. Secondary anti-IgG antibodies are primarily directed against the conserved Fc portions of the antibody. If the antibodies are digested by pepsin, the F(ab′)2 fragment could still bind the bacteria, but because of the limited reactivity between the F(ab′)2 fragment and the secondary antibody, the F(ab′)2 fragment will give a very weak signal in ELISA assays. It has recently been shown that specific IgY enhances phagocytosis of Pseudomonas bacteria and that this process was independent of Fc-mediated mechanisms (Citation20). Such an observation suggests that the F(ab′)2 fragment could also have an antibacterial effect.
Oral administration of egg components is considered safe in that they are part of the normal food intake. Furthermore, immunotherapy with IgY does not have the risk of resistance, and toxicity is low. The only precaution to consider is egg allergy. The stability of IgY is shown high in vitro in temperatures below 40°C and in acidity at pH>4 (Citation21), indicating that IgY activity remains after passage through the ventricle. As an extra protection against acidity, sodium hydrogen bicarbonate was used.
At the time of the initial idea of this study, we were in the midst of a turbulent hospital care situation, that is, we were treating a large number of patients infected or colonised with ESBL-producing K. pneumoniae. The hospital's structure, care process, and economic status were all put under tremendous stress. The timeline for the development of this study was severely delayed because of different manufacturer and regulatory regulations. Thus, we propose that further studies on IgY chicken antibodies should be done in a preclinical setting to better understand the mechanisms of action. Human studies of this kind may be performed as a long-term investigation on discovered carriers of ESBL genes in faeces. Such an ongoing study process may be suitable to intensify in an outbreak situation. For now, increasing the inclusion rate to a reasonable level in such a study should be done in medium- to high-prevalent regions.
In summary, the present study of anti-ESBL IgY for the eradication of ESBL K. pneumoniae and E. coli in faecal carriers was prematurely discontinued because of high mortality and other factors resulting in a low inclusion rate. Spontaneous eradication of ESBL-producing bacteria was frequently observed in recruited participants, which is consistent with previous reports.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
Grants from Uppsala Bio-X and the Swedish Society of Medicine were gratefully received. The staff at the Uppsala Clinical Research Center (UCR), the Clinical Chemical and Microbiology Laboratory, and the Department of Infectious Diseases at Uppsala University Hospital and Falun Hospital have contributed with skilful help. Special gratitude to Elin Nilsson, PhD, who initially designed the study and Malin Vretblad-Plesse, study nurse, and Marie Selbrand, secretary, for the carefulness regarding the patients care, the data monitoring, the skilful set-up of the study, and the administrative issues.
References
- Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008; 8: 159–66.
- Valverde A, Coque TM, Sanchez-Moreno MP, Rollan A, Baquero F, Canton R. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J Clin Microbiol. 2004; 42: 4769–75.
- Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013; 26: 744–58.
- Tangden T, Cars O, Melhus A, Lowdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010; 54: 3564–8.
- Tham J, Odenholt I, Walder M, Brolund A, Ahl J, Melander E. Extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers’ diarrhoea. Scand J Infect Dis. 2010; 42: 275–80.
- Lytsy B, Sandegren L, Tano E, Torell E, Andersson DI, Melhus A. The first major extended-spectrum beta-lactamase outbreak in Scandinavia was caused by clonal spread of a multiresistant Klebsiella pneumoniae producing CTX-M-15. APMIS. 2008; 116: 302–8.
- Ransjo U, Lytsy B, Melhus A, Aspevall O, Artinger C, Eriksson BM, etal. Hospital outbreak control requires joint efforts from hospital management, microbiology and infection control. J Hosp Infect. 2010; 76: 26–31.
- Tangden T, Eriksson BM, Melhus A, Svennblad B, Cars O. Radical reduction of cephalosporin use at a tertiary hospital after educational antibiotic intervention during an outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2011; 66: 1161–7.
- Trecarichi EM, Cauda R, Tumbarello M. Detecting risk and predicting patient mortality in patients with extended-spectrum beta-lactamase-producing Enterobacteriaceae bloodstream infections. Future Microbiol. 2012; 7: 1173–89.
- Titelman E, Hasan CM, Iversen A, Nauclér P, Kais M, Kalin M, etal. Faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae is common 12 months after infection and is related to strain factors. Clin Microbiol Infect. 2014; 20: O508–15.
- Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol. 2003; 35: 433–40.
- Nilsson E, Larsson A, Olesen HV, Wejaker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol. 2008; 43: 892–9.
- Sarker SA, Casswall TH, Juneja LR, Hoq E, Hossain I, Fuchs GJ, etal. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J Pediatr Gastroenterol Nutr. 2001; 32: 19–25.
- Rahman S, Van Nguyen S, Icatlo FC Jr, Umeda K, Kodama Y. Oral passive IgY-based immunotherapeutics: a novel solution for prevention and treatment of alimentary tract diseases. Hum Vaccin Immunother. 2013; 9: 1039–48.
- Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today. 1995; 16: 392–8.
- Akita EM, Nakai S. Immunoglobulins from egg-yolk – isolation and purification. J Food Sci. 1992; 57: 629–34.
- Akita EM, Li-Chan EC. Isolation of bovine immunoglobulin G subclasses from milk, colostrum, and whey using immobilized egg yolk antibodies. J Dairy Sci. 1998; 81: 54–63.
- Birgand G, Armand-Lefevre L, Lolom I, Ruppe E, Andremont A, Lucet JC. Duration of colonization by extended-spectrum beta-lactamase-producing Enterobacteriaceae after hospital discharge. Am J Infect Contr. 2013; 41: 443–7.
- Cook SR, Maiti PK, DeVinney R, Allen-Vercoe E, Bach SJ, McAllister TA. Avian- and mammalian-derived antibodies against adherence-associated proteins inhibit host cell colonization by Escherichia coli O157:H7. J Appl Microbiol. 2007; 103: 1206–19.
- Thomsen K, Christophersen L, Bjarnsholt T, Jensen PO, Moser C, Hoiby N. Anti-Pseudomonas aeruginosa IgY antibodies induce specific bacterial aggregation and internalization in human polymorphonuclear neutrophils. Infect Immun. 2015; 83: 2686–93.
- Shin JH, Yang M, Nam SW, Kim JT, Myung NH, Bang WG, etal. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2002; 9: 1061–6. [PubMed Abstract] [PubMed CentralFull Text].
