Abstract
Background
Campylobacter jejuni is a common cause of human bacterial diarrhea in most parts of the world. Most C. jejuni infections are acquired from contaminated poultry, milk, and water. Due to health care costs and human suffering, it is important to identify all possible sources of infection. Unpasteurized milk has been associated with several outbreaks of C. jejuni infection. Campylobacter has been identified on fresh fruit, and other gastrointestinal pathogens such as Salmonella, E. coli O157:H7 and Cryptosporidium have been involved in fruit juice outbreaks. C. jejuni is sensitive to the acidic environment of fruit juice, but co-cultures with the amoeba, Acanthamoeba polyphaga, have previously been shown to protect C. jejuni at low pH.
Methods
To study the influence of A. polyphaga on the survival of C. jejuni in milk and juice, the bacteria were incubated in the two products at room temperature and at 4°C with the following treatments: A) C. jejuni preincubated with A. polyphaga before the addition of product, B) C. jejuni mixed with A. polyphaga after the addition of product, and C) C. jejuni in product without A. polyphaga. Bacterial survival was assessed by colony counts on blood agar plates.
Results
Co-culture with A. polyphaga prolonged the C. jejuni survival both in milk and juice. The effect of co-culture was most pronounced in juice stored at room temperature. On the other hand, A. polyphaga did not have any effect on C. jejuni survival during pasteurization of milk or orange juice, indicating that this is a good method for eliminating C. jejuni in these products.
Conclusion
Amoebae-associated C. jejuni in milk and juice might cause C. jejuni infections.
Campylobacter jejuni is a leading cause of bacterial, diarrheal disease worldwide (Citation1–Citation3). The majority of Campylobacter cases are sporadic, and although poultry is believed to be the main source of infection many other important sources, such as unpasteurized milk, water, and fresh fruit juice, have been identified (Citation4). Although uncommon, outbreaks mainly result from consumption of unpasteurized milk, poultry meat, and contaminated water (Citation5). C. jejuni outbreaks from unpasteurized milk are often the result of farmers serving visitors raw milk (Citation6, Citation7). There are also frequent outbreak reports from certain districts in the United States, where it is legal to sell unpasteurized milk for commercial purposes (Citation8. Due to healthcare costs and human suffering (Citation9–Citation11), it is important to identify all possible sources of infection. Recently, acidic fruit juices such as orange and apple juices have been found to be responsible for multiple outbreaks of Salmonella, E. coli O157:H7, and Cryptosporidium infections (Citation12–Citation14). The consumer's desire for a healthy lifestyle and their demand for fresh produce have with certainty contributed to a number of outbreaks involving unpasteurized juice (Citation15–Citation17). A study from Sobel et al. (Citation18) showed an association between children with diarrhea and consumption of freshly squeezed juice. Campylobacter has been shown to survive on fresh produce, and eating raw vegetables and fruit has been identified as a risk factor for Campylobacter infections (Citation19–Citation22). Little is known about Campylobacter contamination of fresh squeezed juice or juice products. In contrast to many other foodborne pathogens, C. jejuni is more sensitive to environmental conditions such as aerobic and acidic stress (Citation20, Citation23). On the other hand, we have previously shown that C. jejuni can survive and multiply within free-living amoebae of the genus Acanthamoeba and furthermore that co-cultures with Acanthamoeba polyphaga can protect C. jejuni from acid environments (Citation24–(Citation26). Acanthamoeba spp. are widespread in various environments, including water, and have been isolated from water distribution systems and potable water around the world (Citation27, Citation28). The use of potable water both in industry, when producing juice ready for immediate consumption (Citation29, and in households, when adding water to juice concentrate, would make it possible to find Acanthamoeba spp. in juice. In harsh conditions, Acanthamoeba trophozoites in their vegetative form can transform into a double-walled cyst that are highly resistant to chlorination, antimicrobials, and disinfectants, as well as to changes in pH and osmolarity. Their resistance against disinfection agents (Citation30, Citation31) and their ability to attach to various surfaces of different origin (Citation32–(Citation35) further promote acanthamoebae survival on equipment used for the production/preparation of food. In this study, we have investigated the protective effect of A. polyphaga on C. jejuni in milk and orange juice. In both products, we found significantly higher bacterial survival in co-cultures, compared to when C. jejuni was incubated alone.
Materials and methods
Bacterial and amoebal cultures
The C. jejuni strain CCUG 11284 and the A. polyphaga strain (Linc Ap-1) were used in all experiments. CCUG 11284 is a wild-type strain that was originally isolated from bovine feces. Before each experiment, bacteria were grown on conventional blood agar plates (Columbia agar II containing 8% vol/vol whole horse blood) at 42°C for 20 h in a microaerobic environment, using a CampyGen gas generating system (CN0025A; Oxoid Ltd., Basingstoke, UK) and a BBL GasPak system (BD, Franklin Lakes, NJ). Bacterial cells were harvested and diluted in a peptone-yeast extract-glucose (PYG) medium and used as stock solution for all treatments. The stock solution was striven to obtain a concentration of approximately 107 CFU/ml, as were detected by plate counting. A. polyphaga stock cultures were maintained in PYG medium at 27°C in 75 cm2 culture flasks (Sarstedt, Nürnbrecht, Germany), as described by Axelsson-Olsson et al. (Citation24). For the experiments, A. polyphaga were seeded into 12-well culture plates (Fischer Scientific GTF AB, Switzerland) in PYG medium (1 ml/well) and incubated at 27°C for 24 h, until the trophozoites formed confluent layers at the bottom of the wells. Commercially available milk with a pH of 6.4 (protein 3.4 g, sugar 5 g, fat 1.5 g, Ca 120 mg, vitamin A 25 µg, vitamin D 0.38 µg) and orange juice with a pH of 3.9 (protein 0.7 g, sugar 18 g, fat <0.5 g, Na 0.003 g, vitamin C 30 mg) were used for all experiments. Bolton Broth selective supplement (SR0208; Oxoid Ltd., Basingstoke, UK) was added to the products to inhibit growth of other bacteria than C. jejuni.
Survival of C. jejuni cells co-incubated with A. polyphaga in milk and orange juice stored at room temperature and 4°C
To mimic the conditions of storage in the fridge or at the bench, experiments were incubated at room temperature and 4°C. To test whether the presence of amoeba in two different beverage products, milk and orange juice, influenced the survival of C. jejuni, the following three treatments were used: C. jejuni preincubated with A. polyphaga before the addition of product (treatment A), C. jejuni mixed with A. polyphaga after the addition of product (treatment B), and C. jejuni in product without A. polyphaga (treatment C).
For treatment A, 12-well plates with confluent A. polyphaga layers in PYG medium were inoculated with 100 µl of the C. jejuni stock solution, generating a concentration of 106 CFU/ml and a multiplicity of infection (MOI) of one bacteria per amoeba, in each well. Before inoculation with C. jejuni, the medium in all wells were gently removed and replaced with 1 ml fresh PYG medium. The plates were incubated for 3 h at 32°C to allow the bacterial cells to attach to and invade amoebae, and thereafter the PYG medium was gently removed and replaced by 2 ml of product. This resulted in an approximately 2-log decrease in bacterial concentration. For treatment B, plates with confluent A. polyphaga were prepared by gently removing the PYG medium and replacing it with 2 ml of product. For the control treatment (treatment C), plates without amoebae were prepared with 2 ml product. After the addition of product, the plates for treatment B and C were inoculated with 100 µl of the C. jejuni stock solution generating a concentration of 5×105 CFU/ml and an MOI of one bacteria per amoeba in treatment B, in each well. Three plates (treatments A–C) were incubated at room temperature and at 4°C, respectively. All plates were incubated in an aerobic environment and each treatment was done in triplicate wells, resulting in three similar wells for each temperature, treatment and product. From each well, a 100-µl sample was taken at time zero (the addition of product) and at 3, 6, 18, 24, and 48 h. All samples were 10-fold serially diluted in PYG medium and spread on blood agar plates for colony counting. Three independent experiments were performed on separate occasions. To make sure that the pH level was not affected, the pH level of the fluid in each well was measured after 48 h, when experiments were completed. Compared to initial pH (milk: 6.41 and juice: 3.89) only a small increase in pH was observed (milk: 0.4 and juice: 0.1).
Pasteurization of C. jejuni cells co-incubated with A. polyphaga in milk and orange juice
For pasteurization experiments, the same settings were used, as described above for treatments A, B, and C. Directly after the addition of product, samples of 100 µl were taken from the different treatments, A, B, and C and added to tubes containing 500 µl of milk or juice. The tubes were gently shaken at 1,400 rpm (MS2 Minishaker IKA®, Germany) and then incubated in a water bath (Heto DT Hetotherm, Denmark). Incubation conditions for milk tubes were 72–74°C for 15 sec (equivalent to Swedish low pasteurization guidelines). Incubation conditions for juice tubes were 85°C for 15 sec (equivalent to Swedish pasteurization guidelines). After heating, the sample tubes were put on ice and 100-µl samples were spread on blood agar for colony counting. All experiments were done in triplicates, resulting in three similar wells for each treatment and product.
Statistical analysis
For each well and each time point, a measure of C. jejuni cell survival was calculated by dividing the bacterial concentration of the sample (estimated from colony counts) by the bacterial concentration of that well at time 0 h (the addition of product). Statistical analysis was performed using Kruskal–Wallis test with Dunn's multiple comparison test. Data were analyzed using GraphPad Prism version 6 and p<0.05 were considered significant.
Results
Survival of C. jejuni co-incubated with A. polyphaga in milk or orange juice at room temperature
The experimental setup included three different treatments (A, B, and C); see Materials and Methods section. In milk, the highest C. jejuni survival was seen in treatment A, where bacteria were pre-incubated with amoebae before addition of milk (2.8%, 18 h; 3.8%, 24 h; 0.8%, 48 h; ; a). Treatment B showed 0.05% survival at 18 h and reached a fraction of 6.7×10−5of the inoculum at 48 h (equivalent to 14 CFU/ml; ). After 3 h, the survival of C. jejuni without amoebae (treatment C) decreased more rapidly compared to co-cultures (treatments A and B), and the fraction of the inoculum surviving after 18 h was only 3.5×10−5 (equivalent to 6 CFU/ml; ). No bacteria could be detected after 24 h. At 18–48 h, treatment A had significantly higher bacterial survival than treatment C (Kruskal–Wallis test with Dunn's multiple comparison test and Bonferroni correction for multiple tests; 18 h, p=0.0003; 24 h, p<0.0001; 48 h, p=0.0007; a).
Fig. 1. Survival of C. jejuni co-incubated with A. polyphaga in milk (a) or orange juice (b) at room temperature after 0 h, 3 h, 6 h, 24 h, and 48 h. Data are based on three independent experiments with C. jejuni treated in three different ways treatment A (dots), C. jejuni preincubated with A. polyphaga before the addition of product; treatment B (squares), C. jejuni inoculated to A. polyphaga after the addition of product; and treatment C (triangles), C. jejuni in product without A. polyphaga. To use the log10 scale, a constant 1 had to be added to all cfu values to manage zeros. Means±SEM (missing data points: one out of nine replicates for: milk treatment B at 0 h, milk treatment A at 3 h and juice treatment B at 3 h).
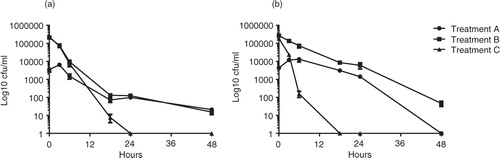
Table 1 Mean fraction of the C. jejuni inoculum surviving after 18 h, 24 h, and 48 h for treatments A, B, and C when incubated in milk and juice at room temperature (RT) and at 4°C
C. jejuni incubated in juice showed similar trends as in milk albeit with more pronounced differences between co-cultures and bacteria incubated alone. The highest C. jejuni survival was seen in treatment A (88.4%, 18 h; 36.9%, 24 h; 0%, 48 h; ; b) and the lowest survival was found in treatment C, C. jejuni without amoebae. After 3 h, the survival of C. jejuni incubated without amoebae decreased rapidly compared to co-cultures (treatments A and B) and no viable bacteria could be detected after 18 h (, b). The relative survival of C. jejuni in treatment B (4.1%, 18 h; 2.0%, 24 h; 0.02%, 48 h; ) was lower than in treatment A but higher than in C. However, due to the higher start concentration in treatment B, compared to treatment A, the bacterial concentration in the juice was still 45 CFU/ml at 48 h. Statistically significant differences in survival were seen between: A and B (Kruskal–Wallis test with Dunn's multiple comparison test and Bonferroni correction for multiple tests: 18 h, p=0.0428; 24 h, p=0.0428; 48 h, p=0.0029; A and C: 18 h, p<0.0001; 24 h, p<0.0001; B and C: 18 h, p=0.0428; 24 h, p=0.0428; 48 h, p=0.0029; b; ).
Survival of C. jejuni co-incubated with A. polyphaga in milk and orange juice at 4°C
At 4°C, C. jejuni generally survived better in both milk and juice and the differences were less pronounced between co-cultures and bacteria incubated alone. In milk, the highest C. jejuni survival was seen in treatment A for time point up to 24 h (90.3%, 18 h; 34.3%, 24 h; ; a). At these time points, treatment C (24.1%, 18 h; 20.1%, 24 h; ) gave a higher survival than treatment B (19.2%, 18 h; 18.2%, 24 h; ). At 48 h, the highest C. jejuni survival was seen in treatment C (3.3% ), and the survival in treatments A and B were 0.4 and 0.6%, respectively (). Statistically significant differences in survival were seen at 18 h between: A and B as well as A and C (Kruskal–Wallis test with Dunn's multiple comparison test and Bonferroni correction for multiple tests: A and B:18 h, p=0.001; A and C: 18 h, p=0.0045; a).
Fig. 2. Survival of C. jejuni co-incubated with A. polyphaga in milk (a) or orange juice (b) at 4°C after 0 h, 3 h, 6 h, 24 h, and 48 h. Data are based on three independent experiments with C. jejuni treated in three different ways treatment A (dots), C. jejuni preincubated with A. polyphaga before the addition of product; treatment B (squares), C. jejuni inoculated to A. polyphaga after the addition of product; and treatment C (triangles), C. jejuni in product without A. polyphaga. To use the log10 scale, a constant 1 had to be added to all cfu values to manage zeros. Means±SEM (missing data points: one out of nine replicates for juice treatment C at 18 h).
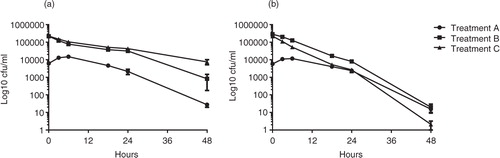
When C. jejuni were incubated in juice at 4°C, the differences seen between co-cultures (treatments A and B) and C. jejuni incubated without amoebae (treatment C), were similar to those seen in juice at room temperature, although the bacteria in treatment C survived longer compared to incubation at room temperature. The highest C. jejuni survival was seen in treatment A: 90.6%, 18 h; 56.7%, 24 h; 0.35%, 48 h; ) and the lowest survival was found in treatment C: 3.3%, 18 h; 2.0%, 24 h; 0.00074%; 1 CFU/ml; 48 h; ). The survival of C. jejuni in treatment B: 7.4%, 18 h; 3.4%, 24 h; 0.01%, equivalent to 18 CFU/ml; 48 h, ) was lower than A but higher than C. Statistically significant differences in survival were seen at 18–24 h between A and B, and 18–48 h between A and C (Kruskal–Wallis test with Dunn's multiple comparison test and Bonferroni correction for multiple tests; A and B: 18 h, p=0.0125; 24 h, p=0.005; A and C: 18 h, p=0.0001; 24 h, p=0.0002; 48 h, p=0.0489; b).
Survival of C. jejuni co-incubated with A. polyphaga at pasteurization temperatures
A. polyphaga did not show any protective effect on C. jejuni when heated to recommended pasteurization temperatures, neither in milk nor in orange juice (data not shown). Growth was totally inhibited in all samples from treatments A, B, and C. Results were based on three independent experiments. Each experiment included three similar wells for each treatment and product.
Discussion
Campylobacter causes approximately 200,000 human infections annually in the European Union, with sometimes very severe chronic complications such as Guillain–Barre syndrome (GBS) (Citation36, Citation37). In this study, we investigated the protective effect of the free-living amoeba A. polyphaga on C. jejuni survival in milk and orange juice. It is well known that drinking unpasteurized milk is a risk for acquiring C. jejuni infections (Citation38; however, the consumption of unpasteurized milk and milk products (Citation39, Citation40) as well as unpasteurized juices is common in many countries (Citation12. The acidic pH in orange juice has been considered lethal to C. jejuni (Citation23, Citation41), and therefore orange juices have not been considered a risk factor for acquiring campylobacteriosis. On the other hand, C. jejuni seem well adapted to survive the acidic milieu of the human stomach as well as disinfection with acid in poultry stables (Citation42, (Citation43). We have previously shown an increased acid tolerance of C. jejuni CCUG 11284 in co-cultures with A. polyphaga (Citation26. Free-living amoebae are common inhabitants of potable water plumbing systems (Citation44, Citation45) and fruit squeezing machines have surfaces that are difficult to clean, with possible formation of biofilms inhabited by amoebae as a result (Citation32, (Citation34–Citation46). Hence, such systems could provide entry ports where the presence of amoeba could increase the C. jejuni survival and cause infections.
As differences in bacterial survival were observed in a previous study depending on weather the bacteria were added to A. polyphaga before (treatment A) or after addition of acidified medium (treatment B), we evaluated the effect of these two treatments in juice and milk (Citation26). The effect of temperature was studied by incubation at room temperature and 4°C. We found significantly higher bacterial survival in co-cultures compared to when C. jejuni were incubated alone (treatment C). In both products and at both temperatures, the highest survival was found in co-cultures where C. jejuni were added to A. polyphaga before the addition of the product (treatment A). The effect of co-culture was most pronounced in juice stored at room temperature, as no C. jejuni survival was detected after 18 h in cultures with C. jejuni alone. Also milk stored at room temperature and juice stored at 4°C showed significantly higher bacterial survival in co-cultures, compared to when C. jejuni were incubated alone. However, in milk stored at 4°C the bacterial survival at 24 h was not significantly affected by co-culture with amoebae and at 48 h, C. jejuni incubated alone (treatment C) actually survived better than in co-culture. This is consistent with previous studies reporting good survival of Campylobacter in refrigerated milk (Citation41, Citation47). In our previous study assessing C. jejuni survival in an acidified medium, we found the highest bacterial survival in co-cultures where C. jejuni were added after the addition of acidic media. In that study we found that the acid milieu triggered C. jejuni motility and uptake into the amoebae. However, the products tested here are more complex than a defined bacterial growth medium and other constituent of milk and juice may likely have affected the results. In the majority of cases, the concentration of amoebae in juice or milk is most likely very low. This was not studied by us, and hence possible C. jejuni protection from amoebae present in low concentrations needs to be evaluated in the future. High concentrations of amoebae could be present in contaminated water or by growth of amoebae in beverages stored at room temperature for a longer period of time. In all experiments the survival curves for C. jejuni in treatment A were characterized by an increase in bacterial concentration at the beginning of the experiments (3 and 6 h). This increase might be explained by extracellular C. jejuni residing in the adherent trophozoite layer. When the medium was changed from PYG to milk or juice these bacteria did probably gradually disperse into the liquid creating a transient increase in bacterial concentration. Together, our results suggest that A. polyphaga can prolong C. jejuni survival both in milk and juice.
It has been shown that legionellae increase their thermal resistance when co-cultured with acanthamoebae, and that intracellular legionellae pneumophila can survive temperatures up to 80°C (Citation48). Acanthamoebae cysts alone have been shown to survive at temperatures up to 80°C and even up to 95°C for at least 10 min (Citation48–Citation50). We studied if C. jejuni in co-culture with A. polyphaga could survive heating to recommended pasteurization temperatures for milk (72–74°C) and juice (85°C). However, A. polyphaga did not have any effect on C. jejuni survival during pasteurization of milk or orange juice, confirming that this is a good method for eliminating C. jejuni in these products.
In conclusion, amoebae associated C. jejuni in milk and juice survived better than free bacteria both at room temperature and at 4°C, but A. polyphaga could not protect the bacteria from pasteurization.
Conflict of interest and funding
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank Dr. Alexis Avril for valuable comments regarding the statistical analysis. The study was financially supported by the Swedish Research Council FORMAS (grant numbers: 2220-8779-120, 221-2012-1442).
References
- Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. Human campylobacteriosis in developing countries. Emerg Infect Dis. 2002; 8: 237–44. [PubMed Abstract] [PubMed CentralFull Text].
- Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, etal. Incidence and trends of infection with pathogens transmitted commonly through food – Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. MMWR Morb Mortal Wkly Rep. 2014; 63: 328–32. [PubMed Abstract].
- ECDC. Annual epidemiological report 2014 – food- and waterborne diseases and zoonoses. 2014; Stockholm: European Centre for Disease Prevention and Control. 20–4.
- Domingues AR, Pires SM, Halasa T, Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012; 140: 970–81. [PubMed Abstract].
- Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol. 2011; 2: 200. [PubMed Abstract] [PubMed CentralFull Text].
- Evans MR, Roberts RJ, Ribeiro CD, Gardner D, Kembrey D. A milk-borne campylobacter outbreak following an educational farm visit. Epidemiol Infect. 1996; 117: 457–62. [PubMed Abstract] [PubMed CentralFull Text].
- Heuvelink AE, van Heerwaarden C, Zwartkruis-Nahuis A, Tilburg JJ, Bos MH, Heilmann FG, etal. Two outbreaks of campylobacteriosis associated with the consumption of raw cows’ milk. Int J Food Microbiol. 2009; 134: 70–4. [PubMed Abstract].
- Longenberger AH, Palumbo AJ, Chu AK, Moll ME, Weltman A, Ostroff SM. Campylobacter jejuni infections associated with unpasteurized milk – multiple States, 2012. Clin Infect Dis. 2013; 57: 263–6. [PubMed Abstract].
- Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. 2012; 75: 123–31. [PubMed Abstract].
- Walker RI. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine. 2005; 23: 3369–85. [PubMed Abstract].
- Peterson MC. Clinical aspects of Campylobacter jejuni infections in adults. West J Med. 1994; 161: 148–52. [PubMed Abstract] [PubMed CentralFull Text].
- Tribst AA, Sant'Ana Ade S, de Massaguer PR. Review: microbiological quality and safety of fruit juices–past, present and future perspectives. Crit Rev Microbiol. 2009; 35: 310–39. [PubMed Abstract].
- Vojdani JD, Beuchat LR, Tauxe RV. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J Food Prot. 2008; 71: 356–64. [PubMed Abstract].
- Burnett SL, Beuchat LR. Human pathogens associated with raw produce and unpasteurized juices, and difficulties in decontamination. J Ind Microbiol Biotechnol. 2000; 25: 281–7. [PubMed Abstract].
- Noel H, Hofhuis A, De Jonge R, Heuvelink AE, De Jong A, Heck ME, etal. Consumption of fresh fruit juice: how a healthy food practice caused a national outbreak of Salmonella Panama gastroenteritis. Foodborne Pathog Dis. 2010; 7: 375–81. [PubMed Abstract].
- Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, etal. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010; 12: 2385–97. [PubMed Abstract].
- Parish ME. Public health and nonpasteurized fruit juices. Crit Rev Microbiol. 1997; 23: 109–19. [PubMed Abstract].
- Sobel J, Gomes TA, Ramos RT, Hoekstra M, Rodrigue D, Rassi V, etal. Pathogen-specific risk factors and protective factors for acute diarrheal illness in children aged 12–59 months in Sao Paulo, Brazil. Clin Infect Dis. 2004; 38: 1545–51. [PubMed Abstract].
- Castillo A, Escartin EF. Survival of Campylobacter jejuni on sliced watermelon and papaya. J Food Prot. 1994; 57: 166–8.
- Karenlampi R, Hanninen ML. Survival of Campylobacter jejuni on various fresh produce. Int J Food Microbiol. 2004; 97: 187–95. [PubMed Abstract].
- Fullerton KE, Ingram LA, Jones TF, Anderson BJ, McCarthy PV, Hurd S, etal. Sporadic campylobacter infection in infants: a population-based surveillance case-control study. Pediatr Infect Dis J. 2007; 26: 19–24. [PubMed Abstract].
- Verhoeff-Bakkenes L, Jansen HA, in't Veld PH, Beumer RR, Zwietering MH, van Leusden FM. Consumption of raw vegetables and fruits: a risk factor for Campylobacter infections. Int J Food Microbiol. 2011; 144: 406–12. [PubMed Abstract].
- Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002; 74: 177–88. [PubMed Abstract].
- Axelsson-Olsson D, Waldenstrom J, Broman T, Olsen B, Holmberg M. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl Environ Microbiol. 2005; 71: 987–92. [PubMed Abstract] [PubMed CentralFull Text].
- Axelsson-Olsson D, Ellstrom P, Waldenstrom J, Haemig PD, Brudin L, Olsen B. Acanthamoeba-Campylobacter coculture as a novel method for enrichment of Campylobacter species. Appl Environ Microbiol. 2007; 73: 6864–9. [PubMed Abstract] [PubMed CentralFull Text].
- Axelsson-Olsson D, Svensson L, Olofsson J, Salomon P, Waldenstrom J, Ellstrom P, etal. Increase in acid tolerance of Campylobacter jejuni through coincubation with amoebae. Appl Environ Microbiol. 2010; 76: 4194–200. [PubMed Abstract] [PubMed CentralFull Text].
- Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012; 5: 6. [PubMed Abstract] [PubMed CentralFull Text].
- Thomas JM, Ashbolt NJ. Do free-living amoebae in treated drinking water systems present an emerging health risk?. Environ Sci Technol. 2011; 45: 860–9. [PubMed Abstract].
- Averbeck M, Schieberle P. Characterisation of the key aroma compounds in a freshly reconstituted orange juice from concentrate. Eur Food Res Technol. 2009; 229: 611–22.
- Coulon C, Collignon A, McDonnell G, Thomas V. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J Clin Microbiol. 2010; 48: 2689–97. [PubMed Abstract] [PubMed CentralFull Text].
- King CH, Shotts EB Jr., Wooley RE, Porter KG. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol. 1988; 54: 3023–33. [PubMed Abstract] [PubMed CentralFull Text].
- Thomas V, Bouchez T, Nicolas V, Robert S, Loret JF, Levi Y. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J Appl Microbiol. 2004; 97: 950–63. [PubMed Abstract].
- Vaerewijck MJ, Sabbe K, Van Hende J, Bare J, Houf K. Sampling strategy, occurrence and diversity of free-living protozoa in domestic refrigerators. J Appl Microb. 2010; 109: 1566–78.
- Erdem AK, Sanli-Yürüdü NO, Arslan-Aydoğdu EÖ, Dogruoz N, Zeybek Z, Türetgen I, etal. Quantitative microbiological analysis of biofilm communities from the surfaces of different cooling tower materials. IUFS J Biol. 2010; 67: 9–16.
- Doğruöz N, Minnos B, Ilhan-Sungur E, Çotuk A. Biofilm formation on copper and galvanized steel surfaces in a cooling-water system. IUFS J Biol. 2009; 68: 105–11.
- Vucic S, Kiernan MC, Cornblath DR. Guillain-Barre syndrome: an update. J Clin Neurosci. 2009; 16: 733–41. [PubMed Abstract].
- Curtis D, Hill A, Wilcock A, Charlebois S. Foodborne and waterborne pathogenic bacteria in selected Organisation for Economic Cooperation and Development (OECD) countries. J Food Sci. 2014; 79: R1871–6. [PubMed Abstract].
- Committee on Infectious Diseases, Committee on Nutrition, American Academy of Pediatrics. Consumption of raw or unpasteurized milk and milk products by pregnant women and children. Pediatrics. 2014; 133: 175–9.
- Hegarty H, O'Sullivan MB, Buckley J, Foley-Nolan C. Continued raw milk consumption on farms: why?. Commun Dis Public Health. 2002; 5: 151–6. [PubMed Abstract].
- Macdonald LE, Brett J, Kelton D, Majowicz SE, Snedeker K, Sargeant JM. A systematic review and meta-analysis of the effects of pasteurization on milk vitamins, and evidence for raw milk consumption and other health-related outcomes. J Food Prot. 2011; 74: 1814–32. [PubMed Abstract].
- Blaser MJ, Hardesty HL, Powers B, Wang WL. Survival of Campylobacter fetus subsp. jejuni in biological milieus. J Clin Microbiol. 1980; 11: 309–13. [PubMed Abstract] [PubMed CentralFull Text].
- Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988; 157: 472–9. [PubMed Abstract].
- Pattison M. Practical intervention strategies for Campylobacter. Symp Ser Soc Appl Microbiol. 2001; 30: 121S–5S. [PubMed Abstract].
- Wang H, Edwards MA, Falkinham JO3rd, Pruden A. Probiotic approach to pathogen control in premise plumbing systems?. A review. Environ Sci Technol. 2013; 47: 10117–28. [PubMed Abstract].
- Delafont V, Brouke A, Bouchon D, Moulin L, Hechard Y. Microbiome of free-living amoebae isolated from drinking water. Water Res. 2013; 47: 6958–65. [PubMed Abstract].
- Sospedra I, Rubert J, Soriano JM, Mañes J. Incidence of microorganisms from fresh orange juice processed by squeezing machines. Food Contr. 2012; 23: 282–5.
- Doyle MP, Roman DJ. Prevalence and survival of Campylobacter jejuni in unpasteurized milk. Appl Environ Microbiol. 1982; 44: 1154–8. [PubMed Abstract] [PubMed CentralFull Text].
- Storey MV, Winiecka-Krusnell J, Ashbolt NJ, Stenstrom TA. The efficacy of heat and chlorine treatment against thermotolerant Acanthamoebae and Legionellae. Scand J Infect Dis. 2004; 36: 656–62. [PubMed Abstract].
- Ahearn DG, Gabriel MM. Contact lenses, disinfectants, and Acanthamoeba keratitis. Adv Appl Microbiol. 1997; 43: 35–56. [PubMed Abstract].
- Chang CW, Lu LW, Kuo CL, Hung NT. Density of environmental Acanthamoeba and their responses to superheating disinfection. Parasitol Res. 2013; 112: 3687–96. [PubMed Abstract].
