Abstract
Porcine rubulavirus-La Piedad-Michoacan-Mexico virus (PorPV-LPMV) was identified as the causative agent of a viral disease that emerged spontaneously in Mexican swine in the 1980s. Since the report of the initial outbreak of the disease, only one full-length genome from a strain isolated in 1984 (PorPV-LPMV/1984) has been sequenced; sequence data are scarce from other isolates. The genetic variation of this virus that has spread throughout the main endemic region of Mexico is almost a complete mystery. The development of molecular techniques for improved diagnostics and to investigate the persistence, molecular epidemiology, and the possible reservoirs of PorPV are needed. Together, this will provide greater knowledge regarding the molecular genetic changes and useful data to establish new strategies in the control of this virus in Mexico.
Anew porcine viral disease was recognised in the early 1980s in Mexico with swine displaying respiratory, reproductive, and central nervous system (CNS) disorders. Porcine rubulavirus, the causative virus, was identified to be a new member of the family Paramyxoviridae, genus Rubulavirus, and was initially named La Piedad-Michoacan-Mexico virus (LPMV) after the town in which the virus was first isolated (Citation1, Citation2). It was later renamed P. rubulavirus (PorPV-LPMV) (Citation3). As a part of the lesions during viral infection, the eyes showed a uni- or bilateral corneal opacity and a blue colour. This fact was easy to recognise by veterinarians and producers, and the colloquial name of (Enfermedad del ojo azul) ‘blue eye disease’ was coined.
PorPV infection is still considered a devastating and economically important disease affecting the swine industry (Citation4, Citation5). Currently, the disease is endemic in pigs in central parts of Mexico while it remains unreported in other countries (Citation5). This review aims to describe observations and data from various fields (aetiology, evolution, pathogenesis, immunology, viral persistence, and diagnosis) that may explain the PorPV infection, its origin, and molecular epidemiology.
Virion characteristics
The Paramyxoviridae family is divided into the subfamilies Paramyxovirinae (genera: Rubulavirus, prototype: Mumps virus, MuV; Avulavirus, prototype: Newcastle disease virus, ND; Respirovirus (prototype: Sendai virus); Henipavirus, prototype: Hendra virus, HeV; Morbillivirus, prototype: Measles virus, MeV) (Citation3); and Pneumovirinae.
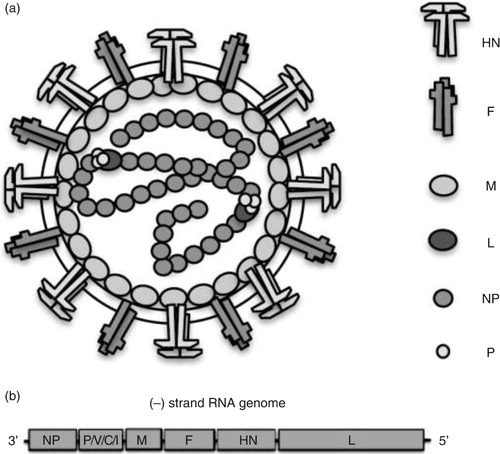
Similar to other Rubulavirus, the PorPV virion (a) consist of a lipid bilayer or envelope outer surface derived from the plasma membrane of the host cell. Into this bilayer, two glycoproteins, the fusion (F) and haemagglutinin/neuraminidase (HN), are inserted (Citation6, Citation7). Positioned just under the envelope is the matrix (M) protein associated with part of the glycoproteins and with the nucleocapsid. The nucleocapsid is present as a tightly packed structure. Two proteins are associated with the nucleocapsid core: the phosphoprotein (P) and the large (L) protein. The nucleocapsid consists of a non-segmented negative-sense, single-stranded RNA (approximately 15,000 nucleotides), surrounded by the nucleoprotein (NP). The genomic structure of the PorPV contains a single promoter in the untranslated 3'region, which is followed by NP, P, M, F, HN, and L Open reading frame (ORF) (b). The P gene has the capacity to encode four possible polypeptides, P, V, C, and I, from the same gene via RNA editing and alternative initiation of translation; that is, the addition of one or two G residues at the editing site allows expression of the I or P protein, respectively (Citation8–Citation10). All viruses belonging to the families in the order Mononegavirales require that the virion contain its own RNA-dependent RNA polymerase, as the cells lack such enzyme, thus these viruses direct the synthesis of mRNA at the start of the infectious cycle. Several viruses of this order can produce defective interfering (DI) particles including PorPV (Citation11, Citation12), measles virus (Citation13, (Citation14) and vesicular stomatitis virus (Citation15). The presence of DI particles also appears to be a common feature in persistent paramyxovirus infection in vitro (Citation11). DI particles have been implicated in persistent infection, but the exact mechanism is not clear.
Origin and evolution
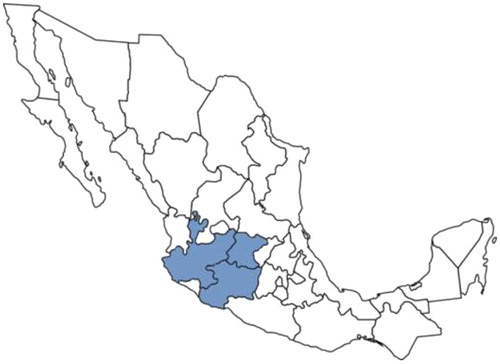
Pigs are the only animals known to be affected clinically by PorPV under natural conditions. PorPV infection remains endemic in Mexico with a seroprevalence ranging from 9 to 23.7% and is thus a major problem in the region (). The disease has been serologically diagnosed in at least 16 states. In this respect, an important point is the difference of antigenic variants that have been identified in unvaccinated swine from the endemic region in Mexico (Guanajuato, Jalisco, Michoacan, and Estado de Mexico) (Citation4). These findings suggest that antigenically different PorPV variants have spread into the swine population, imposing challenges to diagnostic and vaccination efforts.
The source of PorPV that is responsible for the disease outbreaks in Mexico has been suggested to be subclinically infected and/or persistently infected pigs (Citation16, Citation17). However, since the first recognised outbreak in the 1980s, sporadic outbreaks have continued to occur, and the specific source of many of these outbreaks remains unknown.
Molecular studies of PorPV, with an emphasis on proteins of the replicative complex, suggest that this virus has existed as a separate species for a long time in nature and that it could have been transmitted from a natural wildlife reservoir to domesticated pigs (Citation10, Citation18) (Citation19). Early sequence analysis showed the relationship of PorPV to the MuV and the Simian virus 5 (SV5) (Citation7–Citation10, Citation18) (Citation19), with an amino acid identity of approximately 40%. Recent phylogenetic analyses comparing the completed genome sequence and the genetic organisation indicate that PorPV is more closely related to Mapuera virus (MprPV) than to other members of the genus Rubulavirus, suggesting that PorPV may possibly originate from bats (Citation20). In addition, a serological analysis in 108 non-hematophagous bats from the Central Pacific coast of Mexico showed the presence of antibodies against PorPV in one insectivorous bat (Rhogeessa parvula major). However, because only one bat was seropositive, the authors suggested that bats do not play a role in the epidemiology of PorPV in this area (Citation21). Nevertheless, because frugivorous bats are considered to be the natural host of not only MprPV but also of other related paramyxoviruses (HeV, NiV, MenPV, and TioPV) (Citation20, Citation22–Citation29), it has been suggested that bats could be the original natural host of the PorPV strain responsible for the disease outbreak in pigs (Citation20). Our preliminary data show that PorPV can be isolated from bat tissue further supporting the notion that bats are one possible reservoir for PorPV in nature [unpublished data (Citation22)]. Furthermore, other natural reservoirs are, of course, also possible. The findings of potential natural reservoirs will be important for our understanding of its ecology, evolution, and mechanism of cross-species transmission, as has been reported for other emerging paramyxoviruses (Hendra in 1994, Menangle in 1997, Nipah in 1999, and Tioman viruses in 2001) (Citation23, Citation26–Citation29).
During the last 10 years, the evolution of PorPV has been investigated mainly through phylogenetic analyses of the HN protein and studies of the clinical histories and experimental infections using different isolates. Currently, several strains of PorPV have been isolated, and their HN gene has been sequenced; hence, the evolution of PorPV has so far solely been based on the HN gene. Recent studies of different PorPV isolates (PorPV-PAC-7/2002, PorPV-PAC-8/2002, and PorPV-PAC-9/2002) have identified changes in the HN structure, and it has been suggested that these mutations are associated with an increased neurovirulence of PorPV. The amino acid sequence comparisons and phylogenetic analyses of the HN protein have revealed three genetically different lineages: Group 1 is composed of PorPV-LPMV/1984 and PorPV-PAC-4/1993 and is characterised exclusively by the presence of neurological signs in piglets; Group 2 is composed of PorPV-PAC-2/1990, PorPV-PAC-3/1992, and PorPV-CI, II, III, IV and is characterised by neurological signs, high mortality in piglets and older pigs (3–4 months), and lesions in the reproductive tract of adult pigs; and Group 3 is composed of PorPV-PAC-6/2001, PorPV-PAC-7/2002, PorPV-PAC-8/2002, and PorPV-PAC-9/2003 and is associated with clinical signs of neurological involvement in adult animals and in commercial fattening lines (Citation30–Citation32).
As mentioned, until now, only one complete genome of PorPV has been sequenced; and only a few individual genes have been sequenced from other isolates. However, preliminary molecular studies based on the genetic similarities of the encoded proteins (NP, P, M, F, HN, and L) and phylogenetic analysis suggest that a new generation of circulating virus has begun to emerge in nature during the last 10 years with a pronounced attenuation grade [unpublished data (Citation33)].
Clinical studies and pathogenesis
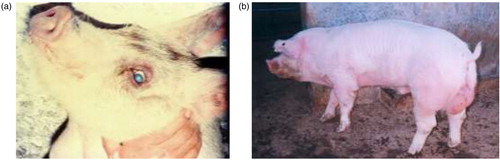
PorPV infection is characterised by respiratory distress and progressive neurological signs and uni- or bilateral corneal opacity (a). Piglets are most susceptible to infection, showing high morbidity and mortality. The piglets often die within 2–7 days after the appearance of clinical signs. The clinical signs in adult pigs have mainly been associated with the reproductive organs (b) and adult pigs generally have a lower mortality than piglets (Citation1, Citation2) (Citation17, Citation34) (Citation35). Atypical outbreaks, including neurological signs in fattening and adult pigs, have been documented between 2000 and 2003 with alterations in the clinical behaviour and increased virulence of PorPV. This change in behaviour was presumed to be associated with mutations in the HN as mentioned earlier (Citation31).
Fig. 3. (a) Corneal opacity in natural infected piglet (‘blue eye’). (b) Testicular lesion observed in boars naturally infected by porcine rubulavirus.
In infected PK-15 cells, the distribution of the HN and NP proteins indicates that the mechanisms of PorPV replication are similar to those of other members of the Rubulavirus genus (Citation36). The pathogenesis of PorPV deends on a positive organ tropism, associated with the receptors of sialic acid-expressing cells and viral adhesion proteins. The virus recognises neuraminic 5 Acid α-2,3 galactose (Neu5Ac α-2,3Gal) oligosaccharides, which are required for the infection process. These receptors are modified during maturation of porcine tissues in relation to the age of the pigs. There is high expression of Neu5Ac α-2,3Gal in CNS and in respiratory tissues of newborn pigs, principally in olfactory bulb, hippocampus, brain cortex, cerebellum, and medulla oblongata with less expression in urogenital tissues. The expression of specific oligosaccharide Neu5Ac α-2,3Gal appears to be related with the severity of PorPV infection (Citation37, Citation38). It has been suggested, based on experimental intratracheal or intranasal exposure by either instillation or aerosol, that these are effective routes of infection and result in clinical signs and lesions similar to those observed in natural PorPV infection (Citation17).
A serial study of PorPV distribution in experimentally infected pigs has shown that the severity of the disease is age-related and is most severe in piglets less than 3 days of age with high mortality at 8 days post-infection (PI), whereas only 30% of piglets infected at an age of 17 days were affected. The viral distribution showed a dual mode of spread to the brain. After primary propagation in the respiratory tract and tonsils, the virus spread throughout the brain via the trigeminal and olfactory nerves combined with a low-level viraemia and passage through the immature blood–brain barrier. The virus showed to be very localised and the excretion occurred mainly via the respiratory tract and urine (Citation39).
Recent studies on the pathogenesis and distribution in the respiratory tract of experimentally infected pigs (6 weeks old) by PorPV-PAC-3/1992 (a low-virulence strain) showed virus excretion from nasal fluid up to 23 days PI and from the respiratory system up to 28 days PI. The distribution in tonsil and lymph nodes showed high viral loads. The main microscopic lesions in the lungs were interstitial pneumonia and hyperplasia of the associated lymphoid tissue (Citation40). Similar to these results, the presence of antigen was demonstrated by immunofluorescence (IF) in the head of the epididymis of experimentally infected boars at 15, 30, 45, or 70 days PI (Citation34). Also viral mRNA was identified by reverse-transcriptase polymerase chain reaction (RT-PCR) in semen samples 5 and 48 days PI and in testis and epididymis between 64 and 142 days PI. However, no infectious virus was detected in the semen of three of the nine infected pigs (Citation41). In other studies of experimental inoculation of young hybrid boars, inflammation and oedema of the testis and epididymis was shown 15 days after inoculation. Boars sacrificed 80 days after infection showed fibrosis and granuloma formation in the epididymis as well as testicular atrophy (Citation34).
Immunological aspects
All viruses of the Paramyxoviridae family are extremely infectious. Because of their common labile structure, this family is dependent on transmission by the close association of hosts. Infections occur through aerosols; direct physical contact between hosts is not required (Citation42).
Studies have shown that during paramyxovirus infection, antibodies are produced against internal and external proteins. The reported immune response to PorPV in experimentally infected adult pigs indicates an immunodominance of specific antibodies against the HN protein, although antibodies against the M and NP proteins have also been demonstrated (Citation43). PorPV induce a durable humoral immune response in pigs that have recovered from an acute PorPV infection, providing lifelong protection (Citation17). In this respect, it is important to mention that antibodies to the HN and F proteins are vital for eliciting a virus-neutralising response (Citation44).
It has been shown that in adult experimentally infected pigs an increase of the CD2+ T-cells during the initial phases of infection is seen, whereas the proportion of B-cell lymphocytes is reduced at 4 weeks PI. The CD4+ T-cells were reduced 3 weeks PI and the CD4–CD8+ and CD4 + CD8+ T-cell proportions increased at 4 weeks PI (Citation43). In 5- and 17-day-old piglets, a relative increase of CD2+ and CD8+ has been observed (Citation45).
The changes induced in the T-cell population during acute and persistent infections with PorPV have been investigated in six 17-day-old Vietnamese pot-bellied piglets. Mild signs characteristic of PorPV infection, such as sneezing, coughing, and slight conjunctivitis were observed 7–10 days PI. A piglet that died 11 days after inoculation showed moderate pneumonia and encephalitis at necropsy. The surviving piglets recovered from the acute infection around 13 days PI and survived until euthanised at day 277 PI. Macroscopic lesions could not be observed in either the convalescent or non-infected pigs (Citation46).
In another experimental infection study, it was shown that infected piglets (3 days old) died 6–8 days after infection, whereas 60% of the piglets infected at an age of 17 days survived (Citation39). All infected animals showed high levels of CD8+, CD4+, and CD2+ T-lymphocytes 10 days PI (Citation46). Studies on the SV5 virus, a paramyxovirus phylogenetically related to PorPV, have showed that CD8+ and CD2+ may have a role in the virus clearance (Citation47, Citation48). Interestingly, there were high levels of both CD4+ and CD8+ T-cells during the observation period (250 days PI). Possibly this may be ‘double-positive’ T-lymphocytes produced by stimulation with recall antigen (Citation46, Citation49).
Field studies on viral persistence
Paramyxovirus are known for their capacity to establish persistent infection in vitro and in vivo. Persistent infection can be defined as a prolonged existence of a virus in an infected host as opposed to that normally expected in an acute infection. The exact mechanism of viral persistence is not known, however, several members of the Paramyxoviridae family suggested that DI particles or viral variants (Citation11, Citation12) (Citation44, Citation50–Citation52) may play a roll establishing a low-grade persistent infections. Persistence requires that the virus must change to a non-lytic phenotype or infect cells that do not support a lytic infection (Citation44). For example, Sendai virus can infect olfactory neurons and establish long-term persistence in the nerve tissue for at least 168 days PI (Citation51). SV5 can establish a persistent infection in murine fibroblastic cells by remaining inactive in cytoplasmic inclusion bodies, from which the virus may be reactivated (Citation52). In relation to this, it has been shown that in cell culture PorPV indeed can establish persistent infection, which is associated with DI particles and subgenomic RNA (Citation11, Citation12). The expression of the P and L proteins has been reported to be decreased and could subsequently be of importance for the persistent state (Citation11, Citation53). The changes in the expression of viral protein levels could be associated with a reduction of the relative amount of mRNA of the P and L protein genes related to a shift in editing of the P gene (Citation11. PorPV can also persist in the CNS of experimentally infected pigs. Although RNA for the NP and P gene was detected at 53 days PI, after immune suppression, RNA of these genes could also be detected in lung samples (Citation16).
The persistence of the viral genome of PorPV in naturally infected pigs has also been investigated 13 months after exposure to the virus (Citation54). Viral mRNA specific for the NP or P genes were detected in the CNS, tonsil, salivary gland, lung, and pancreas, which may indicate that the expression of the NP and P proteins is maintained during persistent PorPV infection in convalescent pigs. Additionally, viral RNA was also detected in sentinel pigs housed together with persistently infected but clinically recovered pigs. The sentinel pigs can be infected without any clinical signs; this suggests that the PorPV could have been transmitted by direct physical contact or virus produced as defective particles during the persistent stage (Citation11, Citation12) (Citation54). One can thus speculate whether the age of sentinel pigs may be a decisive condition when a reactivation of PorPV in convalescent pigs is expected. This is a novel finding of significance to the epidemiology and pathogenesis of PorPV (Citation54). Similarly, mRNA of the NP gene was detected in the lymph nodes (parotid, submaxillary, cervical, and mesenteric) and pancreas of experimentally infected pigs at 277 days PI, whereas mRNA of the P gene was detected in all tissues including the epididymis (Citation46). The persistence of viral mRNA in lymphoid, nervous, respiratory, and genitourinary tissues may explain the high level of Neu5Ac α-2,3Gal receptors specific to PorPV (Citation37, Citation38). The presence of viral mRNA in the epididymis tissue suggested that PorPV can be transmitted via infected semen as a potential source of virus that can infect sows (Citation46, Citation55). In fact, it has been shown that it is possible to isolate PorPV from semen (5–48 days), testicles, and epipidymis (64–142 days) from experimentally infected boars (Citation41).
Diagnosis
Because of the uncontrolled production system practiced in the endemic region of Mexico and the poor sanitary conditions, outbreaks of PorPV infection often appear as a component of co-infections with other agents, making PorPV difficult to diagnose. Clinical symptoms, necropsy findings, and histopathological changes can often provide an insight into the aetiology of the disease. Currently different methods have been used for the diagnosis of PorPV infection, including serological tests and virus isolation (Citation40, Citation41) (Citation46, Citation56) (Citation57). The virus has been shown to be able to grow in many different cells including pig kidney cells with typical syncytial formation, demonstrating fusion activity (Citation1). Virus isolation, electron microscopy, direct immunofluorescence, and classical RT-PCR have been used to detect virus or viral RNA of PorPV for various research purposes (Citation9, Citation16).
The most common serological method for diagnosis is hemagglutination inhibition test. Other serological techniques frequently used are indirect fluorescent antibody, serum-virus neutralisation (Citation40, Citation41) (Citation46, Citation57) and a blocking ELISA (Citation46, Citation54) (Citation57). Classical PCR technologies have been used for different research purposes (Citation16, Citation41) (Citation46), and a real-time PCR (qRT-PCR) method specific to PorPV has been developed for PorPV to study epidemiology aspects of the disease (Citation58, Citation59). This qRT-PCR is based on the detection of viral RNA in clinical samples. The P gene was used in the standardisation of this test because this gene is highly expressed (Citation12), because of its sequence availability (Citation9) and because the genetic variations of this gene among different isolates were assumed to be limited. The TaqMan® assay was shown to be more sensitive than a conventional nested RT-PCR. Detection of all current known variants of the PorPV was achieved (Citation58), which was an important point to increase the knowledge of molecular epidemiology and continue with studies of various features of PorPV infection. The detection of PorPV is a diagnostic challenge because the concentration of viral RNA in tissue samples is very limited (Citation39). The implementation of highly sensitive assays that yield results quickly will be of great assistance in improving the control strategies of PorPV infection in Mexico. Real-time PCR also has numerous advantages over the classical PCR and the procedure limits possible cross-contamination (Citation60).
Conclusion
P. rubulavirus (PorPV) was discovered in the early 1980s and is the causative agent of a disease in pigs that is considered to be one of the most economically important diseases in the pig production system in Mexico (Citation3). The virus can establish viral persistence, which could have an important effect on the pathogenesis of PorPV because of the risk of reactivation of the virus from persistently infected pigs and spread/infection of the virus to new susceptible pigs. During the last 10 years, the evolution of PorPV has been investigated mainly through phylogenetic analyses of the HN protein, studies of the clinical histories, and experimental infections using different isolates. However, research on new isolates obtained from clinical cases of infected swine and phylogenetic analysis indicated that three different genetic variants of PorPV had spread in the swine population and that a new generation of circulating virus with a pronounced attenuation has begun to emerge. The knowledge of the presence of different virus variants in nature, associated with a possible wildlife reservoir of PorPV can provide greater knowledge regarding the molecular genetic changes and useful data to establish new strategies in the control of this virus in Mexico.
Conflicts of interest and funding
The authors declare no conflict of interest. Financial assistance was provided by SLU, University; CENID-Microbiología Animal, INIFAP and CONACyT, Grant 2003-023.
Notes
This review is based on the doctoral thesis ‘Studies of the Molecular Genetics and Epidemiology of Porcine rubulavirus Infection’ by Julieta Sandra Cuevas-Romero 2015, published by Acta Universitatis agriculturae Sueciae, SLU. ISBN 978-91-576-8301-4.
References
- Moreno-López J, Correa-Girón P, Martínez A, Ericsson A. Characterization of a paramyxovirus isolated from the brain of a piglet in Mexico. Arch Virol. 1986; 91: 221–31.
- Stephano HA, Gay GM, Ramírez TC. Encephalomyelitis, reproductive failure and corneal opacity (blue eyes) in pigs, associated with a paramyxovirus infection. Vet Rec. 1988; 122: 6–10.
- Wang LF, Collins PL, Fouchier RAM, Kurath G, Lamb RA, Randall RE, etal., King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Paramyxoviridae. Virus taxonomy: classification and nomenclature of viruses. Ninth Report of the International Committee on Taxonomy of Viruses . 2012; San Diego, CA: Elsevier Academic Press. 640–53.
- Kirkland PD, Stephano A. Straw B, Zimmerman J, D'Allaire S, Taylor D. Paramyxoviruses: rubulavirus, Menangle, and Nipah virus infections. Diseases of swine . 2006; Ames, IA: Blackwell. 455–67. 9th ed.
- Escobar-López AC, Rivera-Benítez JF, Castillo-Juárez H, Ramírez-Mendoza H, Trujillo-Ortega ME, Sánchez-Betancourt JI. Identification of antigenic variants of the porcine rubulavirus in sera of field swine and their sero prevalence. Transbound Emerg Dis. 2012; 59: 416–20.
- Sundqvist A, Berg M, Hernández-Jáuregui P, Linné T, Moreno-López J. The structural proteins of a porcine paramyxovirus (LPMV). J Gen Virol. 1990; 71: 609–13.
- Sundqvist A, Berg M., Moreno-López J, Linné T. The haemagglutinin-neuraminidase glycoprotein of the porcine paramyxovirus LPMV: comparison with other paramyxoviruses revealed the closest relationship to simian virus 5 and mumps virus. Arch Virol. 1992; 122: 331–40.
- Berg M, Sundqvist A, Moreno-López J, Linné T. Identification of the porcine paramyxovirus LPMV matrix protein gene: comparative sequence analysis with other paramyxoviruses. J Gen Virol. 1991; 72: 1045–50.
- Berg M, Hjertner B, Moreno-López J, Linné T. The P gene of the porcine paramyxovirus LPMV encodes three possible polypeptides P, V and C: the P protein mRNA is edited. J Gen Virol. 1992; 73: 1195–200.
- Berg M, Bergvall AC, Svenda M, Sundqvist A, Moreno-López J, Linné T. Analysis of the fusion protein gene of the porcine rubulavirus LPMV: comparative analysis of paramyxovirus F proteins. Virus Genes. 1997; 14: 55–61.
- Hjertner B, Linné T, Moreno-López J. Establishment and characterisation of a porcine rubulavirus (LPMV) persistent infection in porcine kidney cells. Acta Vet Scand. 1997; 38: 213–24. [PubMed Abstract].
- Hjertner B, Wiman AC, Svenda M, Berg M, Moreno-López J, Linné T. Multiple factors including subgenomic RNAs and reduced viral protein expression are associated with a persistent infection by porcine rubulavirus (LPMV). Arch Virol. 1998; 143: 425–39.
- Guido ReG. Kingsbury DW. Deletion mutants of paramyxoviruses. The paramyxoviruses . 1991; New York: Plenum Press. 275–98.
- Schneider-Schaulies J, Niewiesk S, Schneider-Schaulies S, TerMeulen V. Measles virus in the CNS: the role of viral and host factors for the establishment and maintenance a persistent infection. J Neuro Virol. 1999; 5: 613–22.
- Sreevalsan T. Homologous viral interference: induction by RNA from defective particles of vesicular stomatitis virus. Science. 1970; 169: 991–2.
- Wiman AC, Hjertner B, Linné T, Herron B, Allan G, McNeilly F, etal. Porcine rubulavirus LPMV RNA persists in the central nervous system of pigs after recovery from acute infection. J Neuro Virol. 1998; 4: 545–52.
- Stephano HA. Morilla A, Yoon K, Zimmerman J. Blue eye disease: clinical signs and lesions. Trends in emerging viral infections of swine . 2002; Ames, IA: University Press. 47–50.
- Svenda M, Berg M, Moreno-López J, Linné T. Analysis of the large (L) protein gene of the porcine rubulavirus LPMV: identification of possible functional domains. Virus Res. 1997; 48: 57–70.
- Svenda M, Hjertner B, Linné T, Berg M. Both the P and V proteins of the porcine rubulavirus LPMV interact with the NP protein via their respective C-terminal unique parts. Virus Res. 2002; 83: 31–41.
- Wang LF, Hansson E, Yu M, Chua KB, Mathe N, Crameri G, etal. Full-length genome sequence and genetic relationship of two paramyxoviruses isolated from bat and pigs in the Americas. Arch Virol. 2007; 152: 1259–71.
- Salas-Rojas M, Sánchez-Hernández C, Romero-Almaráz ML, Schnell GD, Schmid RK, Aguilar-Setién A. Prevalence of rabies and LPM paramyxovirus antibody in non-hematophagous bats captured in the Central Pacific coast of Mexico. Trans R Soc Trop Med Hyg. 2004; 98: 577–84.
- Cuevas-Romero S, Rivera-Benítez JF, Hernández-Baumgarten E, Guerrero JA, Santos-López G, etal. Detection and isolation of PorPR-like in Mexican bats, Proceedings of the 23rd IPVS Congress, Cancún, Mexico; 2014,Vol. 2; p. 245.
- Chant K, Chan R, Smith M, Dwyer DE, Kirkland P. Probable human infection with a newly described virus in the family Paramyxoviridae. The NSW Expert Group. Emerg Infect Dis. 1998; 4: 273–5.
- Philbey AW, Kirkland P, Ross AD, Davis RJ, Gleeson AB, Love RJ, etal. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg Infect Dis. 1998; 4: 269–71.
- Halpin K, Young PL, Field H, Mackenzie JS. Newly discovered viruses of flying foxes. Vet Microbiol. 1999; 68: 83–7.
- Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000; 81(Pt 8): 1927–32.
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, etal. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000; 288: 1432–5.
- Barr JA, Smith C, Marsh GA, Field H, Wang LF. Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs. J Gen Virol. 2012; 93: 2590–4.
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006; 19(3): 531–45.
- Reyes-Leyva J, Espinosa B, Hernández J, Zenteno R, Vallejo V, Hernández-Jáuregui P, etal. NeuAc alpha 2,3gal-glycoconjugate expression determines cell susceptibility to the porcine rubulavirus LPMV. Comp Biochem Physiol. 1997; 118: 327–32.
- Sánchez-Betancourt JI, Santos-López G, Alonso R, Doporto JM, Ramírez-Mendoza H, Mendoza S, etal. Molecular characterization of the hemagglutinin-neuraminidase gene of porcine rubulavirus isolates associated with neurological disorders in fattening and adult pigs. Res Vet Sci. 2008; 85: 356–67.
- Sánchez-Betancourt JI, Trujillo ME, Mendoza ES, Reyes-Leyva J, Alonso RA. Genetic and antigenic changes in porcine rubulavirus. Can J Vet Res. 2012; 76: 33–7.
- Cuevas-Romero JS. Studies of the molecular genetics and epidemiology of Porcine rubulavirus infection. Acta Universitatis Agriculturae. Sueciae, 2015:51 (Doctoral Thesis).
- Ramírez-Mendoza H, Hernández-Jáuregui P, Reyes-Leyva J, Zenteno E, Moreno-López J, Kennedy S. Lesions in the reproductive tract of boars experimentally infected with porcine rubulavirus. J Comp Pathol. 1997; 117: 237–52.
- Hernández-Jáuregui P, Ramírez Mendoza H, Mercado García C, Moreno-López J, Kennedy S. Experimental Porcine rubulavirus (La Piedad-Michoacan virus) infection in pregnant gilts. J Comp Pathol. 2004; 130: 1–6.
- Hernández-Jáuregui P, Yacoub A, Kennedy S, Curran B, Téllez C, Svenda M, etal. Uptake of porcine rubulavirus (LPMV) by PK-15 cells. Arch Med Res. 2001; 32: 400–9.
- Reyes-Leyva J, Santos G, Hernández J, Espinosa B, Borraz MT, Ramírez H, etal., Cea Bonilla A, del Arenal Mena IP, Riveros Rosas H, Vázquez Contreras E. Mecanismos moleculares de la patogenia viral: Estudios con el Rubulavirus porcino. Mensaje Bioquímico, Vol. XXVI . 2002; México City: Facultad de Medicina, Universidad Nacional Autónoma de México. 99–127.
- Vallejo V, Reyes Leyva J, Hernández J, Ramírez H, Delannoy P, Zenteno E. Differential expression of sialic acid on porcine organs during the maturation process. Comp Biochem Physiol. 2000; 126: 415–24.
- Allan GM, McNeilly F, Walker I, Linné T, Moreno-López J, Hernández P, etal. A sequential study of experimental porcine paramyxovirus (LPMV) infection of pigs: immunostaining of cryostat sections and virus isolation. J Vet Diagn Invest. 1996; 8: 405–13.
- Rivera-Benítez JF, Cuevas-Romero S, Pérez-Torres A, Reyes-Leyva J, Hernández J, Ramírez-Mendoza H. Respiratory disease in growing pigs after Porcine rubulavirus experimental infection. Virus Res. 2013; 176: 137–43.
- Rivera-Benítez JF, Martínez-Bautista R, Pérez-Torres A, García-Contreras AC, Reyes-Leyva J, Hernández J, etal. Persistence of Porcine rubulavirus in experimentally infected boars. Vet Microbiol. 2013; 162: 491–8.
- Black FL. Kingsbury DW. Epidemiology of paramyxoviridae. The paramyxoviruses . 1991; New York: Plenum Press. 509–36.
- Hernández J, Reyes-Leyva J, Zenteno R, Ramírez H, Hernandez-Jáuregui P, Zenteno E. Immunity to porcine rubulavirus infection in adult swine. Vet Immunol Immunopathol. 1998; 64: 367–81.
- Randall RE, Russell WC. Kingsbury DW. Paramyxovirus persistence: consequences for host and virus. The paramyxoviruses . 1991; New York: Plenum Press. 299–321.
- Rodríguez Ropón A, Hernández Jaúregui P, Sánchez Torres L, Favila Castillo L, Estrada Parra S, Moreno López J, etal. Apoptosis in lymph nodes and changes in lymphocyte subpopulations in peripheral blood of pigs infected with porcine rubulavirus. J Comp Pathol. 2003; 128(1): 1–8.
- Cuevas JS, Rodríguez-Ropón A, Kennedy S, Moreno-López J, Berg M, Hernández-Jáuregui P. Investigation of T-cell responses and viral mRNA persistence in lymph nodes of pigs infected with Porcine rubulavirus. Vet Immunol Immunopathol. 2009; 127: 148–52.
- Young DF, Randall RE, Hoyle JA, Souberbielle BE. Clearance of a persistent paramyxovirus infection is mediated by cellular immune responses but not by serum-neutralizing antibody. J Virol. 1990; 64: 5403–11. [PubMed Abstract] [PubMed CentralFull Text].
- Gray PM, Parks GD, Alexander-Miller MA. A novel CD8-independent high-avidity cytotoxic T-lymphocyte response directed against an epitope in the phosphoprotein of the paramyxovirus simian virus 5. J Virol. 2001; 75: 10065–72.
- Zuckerman FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996; 87: 500–12.
- Ahmed R, Morrison LA, Knipe DM. Fields BM, Knipe DM, Howley PM. Persistence of viruses. Fields virology . 1996; Philadelphia, PA: Lippincott-Raven. 219–49. 3rd ed.
- Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J Gen Virol. 1995; 76: 1251–4.
- Fearns R, Young DF, Randall RE. Evidence that the paramyxovirus simian virus 5 can establish quiescent infections by remaining inactive in cytoplasmic inclusion bodies. J Gen Virol. 1994; 75: 3525–39.
- Garcin D, De Melo M, Roux L, Kolakofsky D, Curran J. Presence of a truncated form of the Sendai virus P protein in a long-term persistent infection: implications for the maintenance of the persistent state. Virology. 1994; 15: 19–25.
- Cuevas-Romero S, Hernández-Baumgarten E, Kennedy S, Hernández-Jáuregui P, Berg M, Moreno-López J. Long-term RNA persistence of Porcine rubulavirus (PorPV-LPMV) after an outbreak of a natural infection: the detection of viral mRNA in sentinel pigs suggests viral transmission. Virus Res. 2014; 188: 155–61.
- Solís M, Ramírez-Mendoza H, Mercado C, Espinosa S, Vallejo V, Reyes Leyva J, etal. Semen alterations in porcine rubulavirus-infected boars are related to viral excretion and have implications for artificial insemination. Res Vet Sci. 2007; 83: 403–9.
- Mc Neilly F, Walker I, Allan GM, Foster JC, Linné T, Merza M, etal. A comparative study on the use of virus and antibody detection techniques for the diagnosis of La Piedad Michoacan paramyxovirus (LPMV) infection in pigs. J Vet Diagn Invest. 1997; 9: 3–9.
- Nordengrahn A, Svenda M, Moreno-López J, Bergvall A, Hernández P, McNeilly F, etal. Development of a blocking ELISA for screening antibodies to porcine rubulavirus, La Piedad Michoacán Virus. J Vet Diagn Invest. 1999; 11: 319–23.
- Rivera-Benítez JF, García-Contreras AC, Reyes-Leyva J, Hernández J, Sánchez-Betancourt JI, Ramírez-Mendoza H. Efficacy of quantitative RT-PCR for detection of the nucleoprotein gene from different porcine rubulavirus strains. Arch Virol. 2014; 158: 1849–56.
- Cuevas-Romero S, Blomström A, Alvarado IA, Hernández-Jáuregui P, Rivera-Benítez JF, Ramírez-Mendoza H, etal. Development of a real-time RT-PCR method for detection of porcine rubulavirus (PoRV-LPMV). J Virol Methods. 2013; 189: 1–6.
- Belák S, Thorén P. Molecular diagnosis of animal diseases: some experiences over the past decade. Expert Rev Mol Diagn. 2001; 1: 434–43.