Abstract
The human population is growing, requiring more space for food production, and needing more animals to feed it. Emerging infectious diseases are increasing, causing losses in both human and animal lives, as well as large costs to society. Many factors are contributing to disease emergence, including climate change, globalization and urbanization, and most of these factors are to some extent caused by humans. Pathogens may be more or less prone to emergence in themselves, and rapidly mutating viruses are more common among the emerging pathogens. The climate-sensitive vector-borne diseases are likely to be emerging due to climate changes and environmental changes, such as increased irrigation. This review lists the factors within pathogens that make them prone to emergence, and the modes of transmission that are affected. The anthropogenic changes contributing to disease emergence are described, as well as how they directly and indirectly cause either increased numbers of susceptible or exposed individuals, or cause increased infectivity. Many actions may have multiple direct or indirect effects, and it may be difficult to assess what the consequences may be. In addition, most anthropogenic drivers are related to desired activities, such as logging, irrigation, trade, and travelling, which the society is requiring. It is important to research more about the indirect and direct effects of the different actions to understand both the benefits and the risks.
The earth today is populated by more than seven billion humans (Citation1), and we are affecting every part of the planet, directly or through worldwide pollution and climate changes (Citation2). Anthropogenic environmental changes threaten human health by causing food and water scarcity, increasing the risks for natural disasters and displacements of populations, and increasing the risks of infectious diseases (Citation3), which is the main focus of this review.
Historically, infectious diseases have had civilisation-altering consequences. During the Spanish flu pandemic in 1918–1920, an estimated 50–100 million humans worldwide succumbed to the infection (Citation4). When rinderpest spread to Eastern Africa in the nineteenth century, it caused massive death in livestock and the subsequent death by starvation of almost two-thirds of the East African Massai population (Citation5). The potato blight, a fungal disease, caused the Irish potato famine, reducing the Irish population by 25% either through starvation or migration (Citation6).
Because of improved living conditions and increased access to medications, the proportion of human deaths caused by infectious diseases has trended downwards over the last centuries, giving way to degenerative and lifestyle diseases (Citation7). However, history has previously witnessed spikes in morbidity and mortality, and this reduction may not be lasting. In Thailand, the number of deaths due to infections decreased to one-fifth from 1958 to 1997, after which it started increasing again, mainly due to the emergence of HIV (Citation8). Burden of disease is not equally distributed. Infections, including parasitic diseases, contribute to more than 20% of the global burden of disease (Citation9), but in Africa it is more than 70% (Citation10).
For infectious diseases considered tropical, such as malaria, socio-economic factors may be much more important than climate (Citation11). The effects of disease may also be a vicious circle where the diseases are poverty-promoting, making the poor even poorer, and in turn even more prone to diseases (Citation12). Arboviruses especially have a tendency to affect poor people disproportionally and cause long-lasting sequelae (Citation13), causing a burden for both families and societies. The effects of many diseases may also be directly incapacitating, which cause people lacking health care, to lay sick during the viraemic or parasitaemic phases, rendering them more prone to further vector bites and causing increased infection rates in the vectors.
Infectious diseases cause not only suffering and death, but also severe economic implications, which are not always immediately appreciated. The outbreak of foot-and-mouth disease in the UK in the beginning of the twenty-first century led to the culling of four million animals for the purpose of disease control, and cost the nation more than £3billion, not including losses from decreased tourism (Citation14). Economic losses may in addition be caused by secondary effects. Death of bats in North America, due to the infectious white-nose syndrome, caused by an emerging fungus, and other anthropogenic causes of death, may cause agricultural losses of at least US$3.7 billion per year (Citation15).
To estimate the importance of diseases, different measures can be used, such as morbidity and mortality. To measure both the impact of mortality, disease, and long-term sequelae of human disease, disability adjusted life years (DALY), have been established (Citation16, Citation17). The definition of one DALY is the loss of one healthy year of human life. In addition to these calculations, costs of illness for the public health sector, and losses to industry, tourism, and the agricultural sector can be estimated, although it may be more difficult to assess the costs of environmental impacts and loss of ecosystem services. To fully evaluate the economic and societal impact of a zoonotic disease, it is important to include all measurements (Citation18). The combined impact of zoonotic diseases on human health, animal health, and livelihoods make them especially costly. The World Bank (Citation19) estimates that direct costs of zoonotic outbreaks during the last century have exceeded US$20 billion, and US$200 billion in indirect costs.
The number of events of emerging infections has been increasing over the last 100 years (Citation20), although confounded by our better ability to detect disease and the upsurge in human emerging disease associated with HIV in the 1980s (Citation20). Emerging infectious diseases (EID) have been reviewed extensively during the last two decades, and it is now generally accepted that most drivers of emerging diseases are ecological, and the majority of these caused by anthropogenic influences. Some of these anthropogenic drivers are the increased travelling and transport of animals and goods; changes in ecosystems; deforestation and reforestation; altered land use; increased irrigation and creation of water dams and reservoirs; and urbanization (Citation21–Citation23).
In spite of the increased attention and all gained knowledge on EID, it may prove difficult to formulate policies on risk reduction. Part of this is due to lacking understanding of causality, trade-offs, and externalities of decisions. This paper aims to review existing literature on how human impacts are associated with disease emergence and transmission. The purpose of this analytic review is to provide a framework for evaluating the risks that anthropogenic ecosystem changes may have on disease transmission and dynamics.
Emerging diseases
Definitions of EID vary, including: a disease which incidence in humans has been increasing; a disease which has a tendency to spread geographically, cause an increased incidence, or infect a new species or new populations; or, a disease spreading within any host population (Citation24–Citation26). Pathogens may also be considered emerging, for example, antimicrobial resistant bacteria. These definitions can be similarly applied to wildlife and plant diseases (Citation27, Citation28), in both terrestrial and marine ecosystems (Citation29). There can also be an apparent emergence of newly discovered or previously underdiagnosed diseases (Citation24, Citation26) (Citation30).
Taylor et al. (Citation31) found that viruses and protozoa had the highest proportions of emerging pathogens. Zoonotic pathogens were found twice as likely to be emerging as non-zoonotic, but this was only seen in some taxa (bacteria and fungi). The host jump occurring in zoonotic infection can either cause an establishment of the pathogen in the new population with subsequent spread, or there may be recurrent events of transmission from a reservoir to the new host, after which no further transmission occurs, or there is a limited small outbreak (Citation32). The dominance of zoonotic infections among emerging health threats has also been demonstrated among recent events of public health importance in the Americas, where 70% of the events were caused by zoonotic agents (Citation33).
Some areas of the world, ‘hot spots’, have a tendency to have more events of EID (Citation20, Citation34). These often have a rapid intensification of agricultural systems, especially of livestock keeping, and increasing interactions between animals, humans, and ecosystems, often caused by rapidly changing habits and practices within societies (Citation18, Citation35). Equally important from a public health point of view may be the ‘cold spots’, neglected locations where public health measurements are non-effective and diseases which are controlled elsewhere still flourish (Citation18) and may constitute a disease reservoir for future re-emergence.
Especially small-scale or backyard farmers may be disproportionally affected by the negative impacts of EID (Citation36). Emerging diseases, such as highly pathogenic avian influenza, can lead to industry decline or restructuring with negative effects on small-scale producers and value chain actors (Citation37).
McMichael (Citation38) proposed five categories of promoters for emerging infections: land use and environmental changes; demographic changes; host conditions; human consumption behaviour; and other behaviours such as social and cultural interaction, sexual habits, and drug use. Apart from these, factors within the pathogen, such as the capacity to evolve through mutations, are important for disease emergence (Citation39).
Pathogens
Viruses
The EID that have received most publicity during the last decades have been viruses. Notable examples have been HIV, SARS, and Ebola. It is estimated that 44% of the diseases considered emerging in humans are viral (Citation31).
RNA viruses are prone to emergence because of their rapid replication and high mutation rates, with around one misreading per replication, and large viral populations (Citation40, Citation41). However, the increased evolutionary pressure of having to adapt to both invertebrate and vertebrate hosts creates a lower rate of mutation in vector-borne viruses, and most of their mutations are synonymous (Citation42).
Apart from point mutations, viruses can evolve through recombination events, especially among segmented viruses. The reassortment that occurs in influenza viruses is one example of this whereby influenza viruses create new combinations of genes. Single-stranded viruses may also recombine when different viral strains circulate in the same area, and occasionally infect the same cell, as in the example of Japanese encephalitis virus (Citation43, Citation44). However, in spite of the increased tendency for recombinations among segmented viruses, single-stranded RNA viruses seem to be overrepresented among emerging pathogens (Citation32).
Bacteria
Bacteria and rickettsia constitute 38% of human pathogens, and 30% of the emerging pathogens in humans (Citation31). Because of public health breakdown or complacency, many bacterial diseases have been re-emerging, such as cholera and plague in India (Citation45). One of the most alarming phenomena in bacteria is the spread of antibiotic resistance. Although bacteria have a continuous evolution with mutations, they also have means of spreading their genetic material laterally between species through interchange of plasmids or integrons (Citation46–Citation49). This capacity to share genetic material is not a phenomenon restricted to antibiotic resistance but an efficient way of handling different adverse environmental circumstances in nature as well (Citation49, Citation50). In the same manner, lateral transfer may occur of virulence genes (Citation48), and integration of toxin gene elements from phages seems to commonly occur in Escherichia coli, although the toxins are not always expressed to the same amount (Citation26).
Most studies seem to show that the acquisition of antimicrobial resistance genes in bacteria do cause a comparative disadvantage compared with non-resistant bacteria in the absence of antibiotics, but studies of some genes have shown no difference, or even the opposite. A longer evolution together with a resistance gene may lower the costs for the bacteria (Citation51).
Fungi
Fungal infections are emerging not only among plants, where they have long been an important cause of losses, but also among fishes, corals, amphibians, bats, and humans (Citation52). In fact, fungal infections are contributing to the majority of extinction events that are known to have been caused by infectious diseases (Citation52, Citation53). This may be because fungi may effectively infect 100% of a population, before it is killed by the high mortality. Many fungi further have the possibility to persist as free-living spores (Citation52).
In addition to the fungi that directly infect humans and animals, fungi that produce toxins can cause disease indirectly. Fumonisins and aflatoxins are toxins produced by different moulds, mainly Fusarium and Aspergillus species, and the growth of these fungi is promoted by climatic circumstances and bad storage conditions (Citation54, Citation55). The toxins have severe health impacts on humans and animals, and the costs of diseases and of the condemned crops are high (Citation56, Citation57). Climate changes are likely to affect the impact further (Citation58).
Parasites
Even though part of the increased reports of parasitic disease may be due to previous underreporting, the incidence does seem to be increasing. Large parts of the industrialized countries have managed to reduce the burden of many parasites, whereas in many countries multiple chronic infections are common (Citation59). Most helminthic infections (95%) are zoonotic, and protozoal infections in humans, both zoonotic and non-zoonotic, are likely to be emerging (Citation31). An emerging problem in parasites is increasing resistance, which cause many drugs to be ineffective (Citation60).
Prions
In the analysis by Taylor et al. (Citation31) on human pathogens, the causal agent of bovine spongiform encephalopathy was the only listed prion, classified as both zoonotic and emerging. There are, however, other infections of importance among animals. Chronic wasting disease in cervids is spreading in North America and affects cervid populations, but is believed to have low zoonotic potential (Citation61). New strains of atypical scrapie in sheep and the detection of other new transmissible spongiform encephalopathies have also caused increased concerns, both for emergence within animal populations as well as for their possible zoonotic implications (Citation62).
Routes of transmission
Infections transmitted directly between individuals are dependent on the contact rate between susceptible and infectious people, and thus subsequently on the population density and the mixing of populations. Direct transmission of zoonotic diseases requires contact between animal hosts and humans, as in the case of rabies transmission, but transmission can also occur in the other direction. Close contact increases risk of transmission from pets or livestock to their owners, and the growing demands for exotic pets (Citation63) with subsequent increased trade further increases risk for introduction of new pathogens. Food- and water-borne pathogens are the major contribution to the billions of annual diarrhoea cases that occur (Citation18). Increases in food-borne transmission may be an effect of the difficulties in handling the manure from animal production safely, as this can be a source of many zoonotic pathogens (Citation64). This is an issue both for small-scale farming where there may be no systems to handle manure at all, and in industrialized systems where the sheer amount of manure produced daily causes management problems. In addition, increasing water scarcity and water pollution in the future (Citation65) may cause increased risks for decreased food safety.
Vector-borne diseases constitute around 23% of the infections considered emerging (Citation20). Although arboviruses can be transmitted by a wide range of arthropods, mosquitoes are the most important from a veterinary and medical point of view and may have been parasitizing on mammalian blood for 100 million years (Citation66). Disease from vector-borne pathogens often occurs as spillover events, as the pathogens generally circulate between reservoir hosts and the invertebrate vectors without causing apparent disease. However, many vectors are not specific in their requirements of their feeding hosts and may feed on other animals. These opportunistic, oligophilic vectors can thus transfer a pathogen from a reservoir host to animals or humans where disease occurs. Often these new incidental hosts are less capable of amplifying the pathogen and are epidemiological dead ends.
The complex nature of vector-borne transmission makes it difficult to predict how changes will affect the incidence. Temperature affects both the longevity, the incubation period within the vector, abundance, behaviour, and the reproduction cycles of the mosquito and thus warmer climates may lead both to increased transmission as well as reduced, when the lifespan of the mosquito is reduced below the time required for the virus to replicate (Citation67). The essence is that any factors that contribute to shorter incubation periods, increased mosquito abundance, increased proportion of suitable hosts, or increased vector survival will increase the disease transmission.
The opportunistic behaviour of many vectors can cause them to change their feeding according to the host availability, and even mosquitoes with a strong preferences for humans will feed on other hosts if they are abundant enough (Citation68). Presence of multiple species can, in theory, have both a diluting effect, where the feeding on other species decreases the proportion of vectors feeding on the target species for a disease, and an amplifying effect where the access to multiple feeding hosts cause an increased abundance of vectors (Citation69). The dilution effect of other animals has been used in zooprophylaxis, when a species, often cattle, is used to divert mosquitoes away from another species, but this does not work if the vector abundance is increased (Citation68).
Pathogen dynamics
The concept of Susceptible-Infected–Removed (SIR) has been used to model infectious diseases since it was proposed in the 1920s. The model is, however, simplified, and for more appropriate modelling it may be necessary to include a category of exposed and latently infected (Citation70).
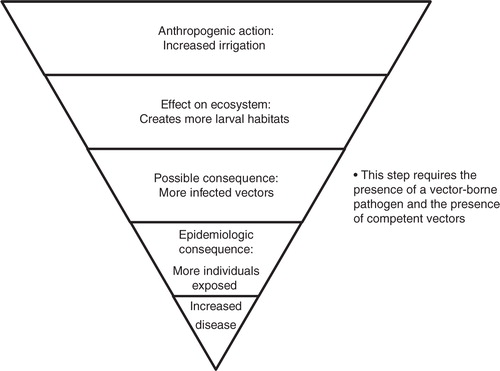
Generally, the spread of infectious diseases is promoted by all factors that increase the contact rate, especially between susceptible and infected individuals; create more susceptible individuals; and increase the time of infectiousness (Citation71). Actions causing the opposite will thus reduce the spread. Often there are multiple steps before an action taken by humans converts into increased risk for disease, which may cause a delayed increase of incidence (). Because the disease dynamics of SIR is essential and basic to epidemiology of humans, animals, and plants, all factors proposed by the literature are listed here according to their effect on these categories. Thus, for the purpose of this framework, the factors: 1) increasing the number of susceptible individuals, 2) increasing the risks of exposure, and 3) increasing how infectious the infected individual is, are considered factors increasing the risks for disease emergence.
Factors increasing the number of susceptible individuals
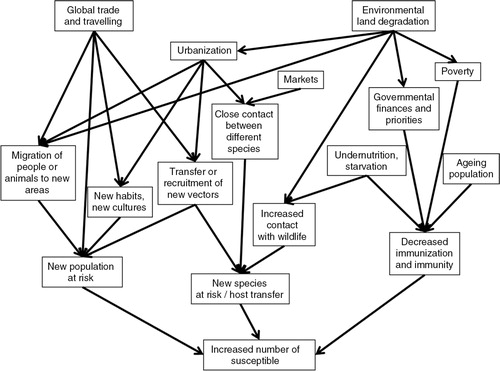
A new population can become at risk for an infection if a new pathogen is transferred to a previously uninfected area. This both can occur over a distance where a pathogen is brought by anthropogenic means, with an infected individual, in a vector, or in contaminated products, and it can be a slow progression into neighbouring areas, by animal, human, or vector movements; or through trade. Cultural exchange may also cause a population to adopt new habits and acquire new risks.
A new population can also become at risk if naïve individuals are moved into an area where a pathogen exists, which occurs during migration of people and animals, due to disasters or political instability; or through encroachment into pristine areas, in order to expand agriculture or exploit natural resources. Furthermore, the number of susceptible individuals can be increased if the existing population in an area where a pathogen exists are increasingly immunosuppressed. This may occur in well-developed countries with increasing proportions of ageing citizens and increasing obesity-related diseases, and advanced medicine with subsequent iatrogenic immunosuppression; or in poorer nations where vaccination programmes cannot be supported and large parts are immunocompromised due to undernutrition or chronic infections (Citation72). It is also possible that a new species constitutes the new population at risk, if a pathogen makes a host jump. The risk for a host jump is increased by all factors that force different species into contact with each other. The changes in land use and social drivers leading up to these changes may however be complex. The framework showing factors contributing to increased susceptible populations is shown in .
Factors increasing the risk of exposure
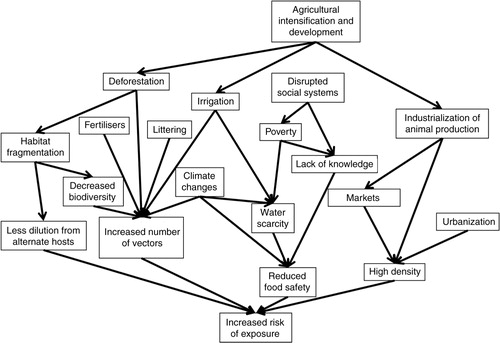
A major factor in the risk of exposure to a pathogen already in place is the pattern of interaction between individuals, which depends on the population density and behaviour. Increasing urbanization, as well as intensified animal keeping, increases exposure. For vector-borne pathogens, the risk of exposure is dependent on the abundance of vectors, as well as the likelihood that these will feed on the appropriate host. Because of the variety of vector habitats and the adaptability of vector species, it is difficult to exhaustively list all factors that may contribute to increases. Pathogens causing infections through food and water are likely to be influenced by social factors, and by climate changes. A framework showing factors contributing to increased exposure is shown in .
Factors increasing infectiousness
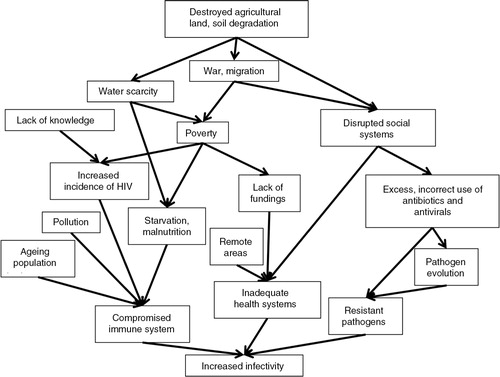
How infectious an individual is following an infection, and for how long time, is dependent on factors in the infected individual, on the pathogen, and the possibility in veterinary and medical care to cure the infection. A framework showing factors contributing to increased infectivity is shown in .
Global drivers of disease emergence
The manner in which anthropogenic activities affect the pathogen dynamics is not always evident and may have several steps. It is also necessary to remember that, because of the stochastic nature, the same scenario might not occur at two occasions, even though circumstances are apparently similar. If a pathogen is dependent on a vector or a reservoir, the pattern may become more complex.
Most changes done to existing ecosystems are done deliberately, often desired for economic or other reasons. It must be remembered that many drivers of disease sometimes are associated with decreased spread of other diseases, or bring other benefits. In fact, many suggested drivers of disease are promoted by governments and society because of their clearly visible and desired positive effects on livelihoods and economies.
Globalization
Although globalization brings along opportunities for knowledge transfer, cultural and scientific exchanges, and rapid aid responses, the increasing globalization has also been suggested to be a reason for increased transfer of pathogens into new areas. Historically, major transition periods when people travelled, and a mixing of populations were achieved, have been followed by large disease outbreaks and spread of pathogens (Citation73). This has been especially marked when travel is accompanied by large-scale societal dislocation as is the case for wars, and colonialization. In addition to human travels, millions of animals are transported annually, both legally and illegally, and only a minor portion is subject to disease control (Citation74). This may also affect wildlife, and trade with exotic and pet animals is most likely one of the causes behind the global spread of amphibian chytridiomycosis (Citation22). Moreover, pathogens do not necessarily need to be transported within a host but can also be transported in, for example, ballast water, in the example of cholera (Citation75).
In summary, globalization has both desired and undesired effects. On the one hand, it brings new pathogens and vectors to previously naïve populations or vice versa and facilitates rapid worldwide dissemination of diseases. On the other hand, it is essential for today's trade and economies and highly desired by the part of the world's population with economic means for travelling.
Deforestation and reforestation
Deforestation is often the result of the economically important logging industry; but it may also be a deliberate act to use a previously forested area for habitation, industry, or other purposes. It often brings human inhabitants into the deforested area needing food, and bringing their livestock. Deforestation often creates more larval habitats, increasing the number of vectors (Citation69). Because of the high diversity of vectors, there will always be some species with a preference for the larval habitats created. As most mosquitoes are opportunistic, they adapt to new hosts, and the introduction of a disease to a new area may cause other animals to become reservoirs. When mosquitoes adapt to new animals, there may in fact be an increased risk for viral transmission as the mosquitoes require prolonged time for probing and thereby salivate more (Citation76).
Reforestation of rural as well as suburban areas, which is popular not least for the cultural ecosystem services provided by forests close to urban habitations, brings along wildlife, but forests managed by humans often provide fragmented habitats and have less biodiversity compared with virgin forests (Citation77). Decreased biodiversity has several effects on disease transmission. The increased transmission of vector-borne pathogens is explained by the disappearance of hosts which are not amplifying the virus, thus reducing the dilution effects caused by non-reservoir animals being bitten by the arthropod vectors (Citation78–Citation80). Reforestation in North America has been associated with increased wildlife, although with less predators, and often dominated by wild animals that benefit from the fragmented habitats, such as mice, squirrels, and deer. Reduction in species that are non-competent hosts for Lyme disease, and increase in reservoirs caused Lyme disease to increase in association with increased reforestation (Citation81). However, reduced biodiversity is not always associated with increased disease, and hot spots for disease emergence often have rich biodiversity (Citation20, Citation80) (Citation82).
Irrigation and dams
Increased irrigation in agricultural areas has contributed to a large extent to the development and is necessary for 40% of global crop production (Citation9). The so-called green evolution in India, with increased irrigation, rice production, and livestock keeping, has been beneficial for food production, but the problems of vector-borne diseases have been increasing, as have non-infectious diseases due to increased obesity (Citation83). The association between increased irrigation and increased incidence of disease has been demonstrated for a number of vector-borne pathogens, such as Japanese encephalitis virus (JEV). The density of rice fields has been shown to be positively associated with the abundance of one of the main vectors for JEV, Culex tritaeniorhynchus (Citation84), and the incidence of Japanese encephalitis (Citation85). Thus, irrigation increases vector habitats and vector-borne and parasitic diseases, but is also a major driver of increased agricultural outputs and may thus also be beneficial for health.
Livestock intensification and extensification
There is an increasing demand for and production of animal products in developing countries, a trend known as the livestock revolution (Citation86). Intensification, with more animals kept in a more commercial, highly productive environment, has been ongoing for many years. In many developed countries, the demand for organic products and higher animal welfare is increasing as a counter-reaction, a process often referred to as extensification, with animals being kept more extensively and often producing less. Intensification can be associated with both increased and decreased risks. For example, increased indoor pig keeping did cause a reduction in Toxoplasma gondii, a neglected but important parasite, and free-ranging pig farms are showing a re-emergence of the infection (Citation59, Citation87). Similarly for cattle, zero-grazing systems may decrease transmission of some parasites (Citation60).
Intensified animal production causes high rates of contact between many individual animals, many of which are genetically similar, bred for other purposes than disease resistance, and kept in stressful environments. This increases the risks of spreading diseases, and high numbers of individuals potentially carrying a zoonotic pathogen increases the risk for a host jump (Citation64). Large amounts of manure and effluence cause problems of safe disposal and risks for contamination of crops and water (Citation60, Citation64) (Citation88). Even though efforts to increase biosecurity can be made, the requirements of intense animal keeping, such as high ventilation, also pose means of introductions of pathogens and vectors (Citation88.
More industrialized animal keeping causes an increasing segregation of the animals and their vectors from humans. This has actually been one of the proposed explanations for the eradication of malaria in many developed countries (Citation89). In Japan, the number of pigs produced has been increasing because of increased industrialized pig keeping, whereas there has been a decreasing number of pig farms. The incidence of JEV has not been following the increasing numbers of pigs, but instead has decreased with the number of pig farms (Citation90).
Extensive animal keeping, backyard farming, and mixed production systems have also been associated with disease risks. The outbreaks of highly pathogenic avian influenza in Southeast Asia have been demonstrated to be dependent on rice production, duck densities, and human population density (Citation91). In addition to the traditional backyard poultry keeping in poor rural or urban areas, there are increasing trends of keeping small flocks of poultry in middle- and high-income urban areas in many countries. In both cases, biosecurity measures and awareness of the importance thereof are often limited (Citation35, Citation92).
In summary, intensive livestock keeping is often promoted for economic reasons but the high density of animals causes high risk of disease transmission; it is also associated with increased risk of allergies, occupational diseases, and antimicrobial resistance. Extensive livestock keeping is often the only option for small holders but may also entail risks: increased exposures to pathogens in the environment, decreased biosecurity, and more interactions between species increase risks for pathogen jumps.
Population growth and urbanization
Population growth is to a large extent an effect of decreased childhood mortality, and improved living conditions and health care. More than 50% of the world's population live in urban areas, and this proportion keeps increasing (Citation1, Citation93). The reasons for migration from rural to urban areas vary, but it is common to migrate in the hope of better jobs or lifestyle. On average, people living in African cities are healthier than in the countryside (Citation94), but statistics are seldom based on subdivision and the health situation is often worse in poorer areas (Citation95).
Cities create ecosystems with higher temperatures and less seasonal changes (Citation78, Citation96). This elongates vector transmission seasons, increasing risk of vector-borne diseases. Population growth and urbanization causing increased densities of people have been associated with the evolution of Dengue virus, which prior to this development may have been of minor impact (Citation97). However, vectors are not always equally distributed in an urban area, but can occur in higher densities in lower income areas (Citation98).
Increasing numbers of scavengers and pets may lead to increased transmission of zoonoses, such as echinococcosis (Citation99, Citation100). Although increased population densities and mixes of animals and humans may facilitate spread of diseases, urban agriculture also provides economic possibilities and provides animal products in cities.
Hunting and bushmeat
Hunting may have an impact on diseased risks through several mechanisms. Hunting increases the interface between humans and wildlife, may expose humans to wildlife vectors, and may have effects on biodiversity and cause decreases of disease reservoirs, as in the case of deer in North America, with subsequent decrease in Lyme disease (Citation101); or may increase reservoirs, if the predators are removed (Citation102).
The habits of bushmeat consumption are known risk factors for disease transmission. Bushmeat is an important source of food, and especially proteins, in areas such as the Congo basin. The handling and trade with bushmeat includes direct contact of multiple people in the value chain with the pathogens of the wild animals (Citation103) and the products are brought to an increasing urban market (Citation104) where outbreaks can be caused, such as the recent outbreaks of Ebola in Kampala, Uganda. Hunting is desired by people for the products and sporting activity, but inevitably increases the human–wildlife interface and changes the fauna and biodiversity in ecosystems.
Conclusions
In spite of the knowledge that exists on EID, there are still gaps in the understanding of ecosystem disease regulation and how human actions may affect disease indirectly and in the long term. A multidisciplinary approach is needed, both in research and in policymaking. It is necessary to understand that although humans are depending on nature's ecosystems for our wellbeing, the different priorities of people and cultures necessitate compromises and trade-offs to be done. Top-down interventions may be counterproductive if the incentives of the local populations are not fully understand, and control measures may be devastating for the public health if the disease epidemiology is not fully grasped. Thus, disease control and monitoring is no longer to be considered a science of medicine and epidemiology alone, but also must include the social, environmental, and economic values appreciated by people and societies.
Conflict of interest and funding
Dynamic Drivers of Disease in Africa: Ecosystems, livestock/wildlife, health and wellbeing: REF:NE/J001422/1 was partly funded with support from the Ecosystem Services for Poverty Alleviation Programme (ESPA). The ESPA programme is funded by the Department for International Development, the Economic and Social Research Council, and the Natural Environment Research Council. In addition, this work was financed by the Swedish Research Council and the Consultative Group on International Agricultural Research programme ‘Agriculture for Nutrition and Health’.
References
- Bloom DE. 7 billion and counting. Science. 2011; 333: 562–9.
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997; 277: 494–9.
- Myers SS, Patz JA. Emerging threats to human health from global environmental change. Annu Rev Environ Resour. 2009; 34: 223–52.
- Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002; 76: 105–15.
- AU-IBAR. The eradication of rinderpest from Africa: a great milestone. 2011
- Fraser EDG. Social vulnerability and ecological fragility: building bridges between social and natural sciences using the Irish Potato Famine as a case study. Conserv Ecol. 2003; 7: 9.
- Barrett R, Kuzawa CW, McDade T, Armelagos GJ. Emerging and re-emerging infectious diseases: the third epidemiologic transition. Annu Rev Anthropol. 1998; 27: 247–71.
- Aungkulanon S, McCannon M, Lertiendumrong J, Olsen SJ, Bundhamcharoen K. Infectious disease mortality rates, Thailand, 1958–2009. Emerg Infect Dis. 2012; 18: 1794–801.
- Patz JA, Confalonieri UEC, Amerasinghe FP, Chua KB, Daszak P, Hyatt AD. Hassan R, Scholes R, Ash N. Human health: ecosystem regulation of infectious diseases. 2005; Washington, DC: Island Press. 391–415. Millennium ecosystem assessment. Condition and Trends Working Group. Ecosystems and human well-being: current state and trends. Vol. 1: Findings of the Condition and Trends Working Group.
- Engels D, Savioli L. Reconsidering the underestimated burden caused by neglected tropical diseases. Trends Parasitol. 2006; 22: 363–6.
- Farmer P. Social inequalities and emerging infectious diseases. Emerg Infect Dis. 1996; 2: 259–69.
- Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009; 373: 1570–5.
- LaBeaud AD. Why arboviruses can be neglected tropical diseases. PLoS Negl Trop Dis. 2008; 2: e247.
- Thompson D, Muriel P, Russell D, Osborne P, Bromley A, Rowland M, etal. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev Sci Tech Int des Epizoot. 2002; 21: 675–85.
- Boyles JG, Cryan PM, McCracken GF, Kunz TH. Economic importance of bats in agriculture. Science. 2011; 332: 41–2.
- Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994; 72: 429–45. [PubMed Abstract] [PubMed CentralFull Text].
- Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis. 2007; 1: e114.
- McDermott J, Grace D. Fan S, Pandya-Lorch R. Agriculture-associated diseases: adapting agriculture to improve human health. Reshaping agriculture for nutrition and health. 2012; Washington, DC: IFPRI. 12–103.
- World Bank. People, pathogens, and our planet. Volume 1: towards a one health approach for controlling zoonotic diseases. Washington, DC: The World Bank; 2010.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, etal. Global trends in emerging infectious diseases. Nature. 2008; 451: 990–3.
- Morse SS, Schluederberg A. Emerging viruses: the evolution of viruses and viral diseases. J Infect Dis. 1990; 162: 1–7.
- Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001; 78: 103–16.
- Gould EA, Higgs S, Buckley A, Gritsun TS. Potential arbovirus emergence and implications for the United Kingdom. Emerg Infect Dis. 2006; 12: 549–55.
- Lederberg J, Shope RE. Emerging infections: microbial threats to health in the United States. 1992; Washington, DC: National Academies Press.
- Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995; 1: 7–15.
- Schrag SJ, Wiener P. Emerging infectious disease: what are the relative roles of ecology and evolution?. Trends Ecol Evol. 1995; 10: 319–24.
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science. 2000; 287: 443–9.
- Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004; 19: 535–44.
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, etal. Emerging marine diseases – climate links and anthropogenic factors. Science. 1999; 285: 1505–10.
- Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nat Med. 2004; 10: S70–6.
- Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philos Trans R Soc B Biol Sci. 2000; 356: 983–9.
- Woolhouse MEJ, Haydon DT, Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol Evol. 2005; 20: 238–44.
- Schneider MC, Aguilera XP, Smith RM, Moynihan MJ, da Silva JB Jr., Aldighieri S, etal. Importance of animal/human health interface in potential Public Health Emergencies of International Concern in the Americas. Rev Panam Salud Pública. 2011; 29: 371–9.
- Grace D, Mutua F, Ochungo P, Kruska R, Jones K, Brierley L, etal. Mapping of poverty and likely zoonoses hotspots. 2012. Available from: https://cgspace.cgiar.org/handle/10568/21161 [cited 15 October 2015]..
- Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, etal. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013; 21: 8399–404.
- Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet. 2011; 377: 599–609.
- Otte J, Hinrichs J, Rushton J, Roland-Holst D, Zilberman D. Impacts of avian influenza virus on animal production in developing countries. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour. 2008; 3
- McMichael AJ. Environmental and social influences on emerging infectious diseases: past, present and future. Philos Trans R Soc London Ser B Biol Sci. 2004; 359: 1049–58.
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004; 430: 242–9.
- Holmes EC. The phylogeography of human viruses. Mol Ecol. 2004; 13: 745–56.
- Nichol ST, Arikawa J, Kawaoka Y. Emerging viral diseases. Proc Natl Acad Sci USA. 2000; 97: 12411–12.
- Jenkins GM, Rambaut A, Pybus OG, Holmes EC. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. J Mol Evol. 2002; 54: 156–65.
- Twiddy SS, Holmes EC. The extent of homologous recombination in members of the genus Flavivirus. J Gen Virol. 2003; 84: 429–40.
- Chuang C-K, Chen W-J. Experimental evidence that RNA recombination occurs in the Japanese encephalitis virus. Virology. 2009; 394: 286–97.
- Chugh TD. Emerging and re-emerging bacterial diseases in India. J Biosci. 2008; 33: 549–55.
- Lan R, Reeves PR. Gene transfer is a major factor in bacterial evolution. Mol Biol Evol. 1996; 13: 47–55.
- Lawrence JG. Gene transfer, speciation, and the evolution of bacterial genomes. Curr Opin Microbiol. 1999; 2: 519–23.
- Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000; 405: 299–304.
- Mazel D. Integrons: agents of bacterial evolution. Nat Rev Microbiol. 2006; 4: 608–20.
- Martínez JL. Bacterial pathogens: from natural ecosystems to human hosts. Environ Microbiol. 2012; 15: 325–33.
- Lenski RE. Bacterial evolution and the cost of antibiotic resistance. Int Microbiol. 2010; 1: 265–70.
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, etal. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012; 484: 186–94.
- Gurr S, Samalova M, Fisher M. The rise and rise of emerging infectious fungi challenges food security and ecosystem health. Fungal Biol Rev. 2011; 25: 181–8.
- Richard JL. Some major mycotoxins and their mycotoxicoses – an overview. Int J Food Microbiol. 2007; 119: 3–10.
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010; 31: 71–82.
- Wu F, Liu Y, Bhatnagar D. Cost-effectiveness of aflatoxin control methods: economic incentives. Toxin Rev. 2008; 27: 203–25.
- Wu F, Narrod C, Tiongco M, Liu Y. The health economics of aflatoxin: global burden of disease. 2011. IFPRI Project paper: http://www.ifpri.org/publication/health-economics-aflatoxin?print [cited 15 October 2015]..
- Tirado MC, Clarke R, Jaykus LA, McQuatters-Gollop A, Frank JM. Climate change and food safety: a review. Food Res Int. 2010; 43: 1745–65.
- Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Vet Parasitol. 2009; 163: 196–206.
- Donald AD. Parasites, animal production and sustainable development. Vet Parasitol. 1994; 54: 27–47.
- Saunders SE, Bartelt-Hunt SL, Bartz JC. Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerg Infect Dis. 2012; 18: 369–76.
- Manson J, Cancellotti E, Hart P, Bishop M, Barron R. The transmissible spongiform encephalopathies: emerging and declining epidemics. Biochem Soc Trans. 2006; 34: 1155–8.
- Cutler SJ, Fooks AR, Van Der Poel WHM. Public health threat of new, reemerging, and neglected zoonoses in the industrialized world. Emerg Infect Dis. 2010; 16: 1–7.
- Greger M. The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Crit Rev Microbiol. 2007; 33: 243–99.
- Rosegrant MW, Ringler C, Zhu T. Water for agriculture: maintaining food security under growing scarcity. Annu Rev Environ Resour. 2009; 34: 205–22.
- Eldridge BF, Marquardt WC, Kondratieff BC, Moore CG, Freier JE, Hagedorn HH, Black WCI V, etal. Mosquitoes, the Culicidae. 2005; San Diego, CA: Elsevier Academic Press. 95–111. Biology of disease vectors. 2nd ed.
- Kramer LD, Ebel GD, Chambers TJ, Monath TP. Dynamics of flavivirus infection in mosquitoes. 2003; San Diego, CA: Academic Press Inc.. 187–232. Flaviviruses: pathogenesis and immunity.
- Service MW. Agricultural development and arthropod-borne diseases: a review. Rev Saude Publica. 1991; 25: 165–78.
- Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000; 30: 1395–406.
- Wearing HJ, Rohani P, Keeling MJ. Appropriate models for the management of infectious diseases. PLoS Med. 2005; 2: e174.
- Petney TN. Environmental, cultural and social changes and their influence on parasite infections. Int J Parasitol. 2001; 31: 919–32.
- Cohen ML. Changing patterns of infectious disease. Nature. 2000; 406: 762–7.
- McMichael AJ. Population, environment, disease, and survival: past patterns, uncertain futures. Lancet. 2002; 359: 1145–8.
- Marano N, Arguin PM, Pappaioanou M. Impact of globalization and animal trade on infectious disease ecology. Emerg Infect Dis. 2007; 13: 1807–9.
- Wilson ME. Travel and the emergence of infectious diseases. Emerg Infect Dis. 1995; 1: 39–46.
- Ribeiro JMC. Blood-feeding in mosquitoes: probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex). Med Vet Entomol. 2000; 14: 142–8.
- Perfecto I, Vandermeer J, Hanson P, Cartín V. Arthropod biodiversity loss and the transformation of a tropical agro-ecosystem. Biodivers Conserv. 1997; 6: 935–45.
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007; 22: 95–102.
- Schmidt KA, Ostfeld RS. Biodiversity and the dilution effect in disease ecology. Ecology. 2001; 82: 609–19.
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, etal. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010; 468: 647–52.
- Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Sci. 1993; 260: 1610.
- Salkeld DJ, Padgett KA, Jones JH. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013; 16: 679–86.
- Sarkar A, Aronson KJ, Patil S, Hugar LB, vanLoon GW. Emerging health risks associated with modern agriculture practices: a comprehensive study in India. Environ Res. 2012; 115: 37–50.
- Richards EE, Masuoka P, Brett-Major D, Smith M, Klein TA, Kim HC, etal. The relationship between mosquito abundance and rice field density in the Republic of Korea. Int J Health Geogr. 2010; 9: 32.
- Impoinvil DE, Solomon T, Schluter WW, Rayamajhi A, Bichha RP, Shakya G, etal. The spatial heterogeneity between Japanese encephalitis incidence distribution and environmental variables in Nepal. PLoS One. 2011; 6: e22192.
- Hall DC, Ehui S, Delgado C. The livestock revolution, food safety, and small-scale farmers: why they matter to us all. J Agric Environ Ethics. 2004; 17: 425–44.
- Kijlstra A, Eissen OA, Cornelissen J, Munniksma K, Eijck I, Kortbeek T. Toxoplasma gondii infection in animal-friendly pig production systems. Invest Ophthalmol Vis Sci. 2004; 45: 3165–9.
- Leibler JH, Otte J, Roland-Holst D, Pfeiffer DU, Soares Magalhaes R, Rushton J, etal. Industrial food animal production and global health risks: exploring the ecosystems and economics of avian influenza. Ecohealth. 2009; 6: 58–70.
- Shaman J. Strategies for controlling the epizootic amplification of arboviruses. J Med Entomol. 2011; 48: 1189–96.
- Oya A, Kurane I. Japanese encephalitis for a reference to international travelers. J Travel Med. 2007; 14: 259–68.
- Gilbert M, Xiao X, Pfeiffer DU, Epprecht M, Boles S, Czarnecki C, etal. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc Natl Acad Sci USA. 2008; 105: 4769–74.
- Karabozhilova I, Wieland B, Alonso S, Salonen L, Häsler B, Hasler B. Backyard chicken keeping in the Greater London Urban Area: welfare status, biosecurity and disease control issues. Br Poult Sci. 2012; 53: 421–30.
- Satterthwaite D, McGranahan G, Tacoli C. Urbanization and its implications for food and farming. Philos Trans R Soc B Biol Sci. 2010; 365: 2809–20.
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Tropical infectious diseases: urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005; 3: 81–90.
- Moore M, Gould P, Keary BS. Global urbanization and impact on health. Int J Hyg Environ Health. 2003; 206: 269–78.
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 2006; 21: 186–91.
- Zanotto PM, Gould EA, Gao GF, Harvey PH, Holmes EC. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc Natl Acad Sci USA. 1996; 93: 548–53.
- LaDeau SL, Leisnham PT, Biehler D, Bodner D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int J Env Res Public Health. 2013; 10: 1505–26.
- Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004; 20: 77–84.
- Deplazes P, van Knapen F, Schweiger A, Overgaauw PAM. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet Parasitol. 2011; 182: 41–53.
- Barbour AG. Fall and rise of Lyme disease and other Ixodes tick-borne infections in North America and Europe. Br Med Bull. 1998; 54: 647–58.
- Ostfeld RS, Holt RD. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front Ecol Environ. 2004; 2: 13–20.
- Karesh WB, Cook RA, Bennett EL, Newcomb J. Wildlife trade and global disease emergence. Emerg Infect Dis. 2005; 11: 1000–2.
- Wilkie DS, Carpenter JF. Bushmeat hunting in the Congo Basin: an assessment of impacts and options for mitigation. Biodivers Conserv. 1999; 8: 927–55.