Abstract
Background
Human and animal influenza are inextricably linked. In particular, the pig is uniquely important as a mixing vessel for genetic reassortment of influenza viruses, leading to emergence of novel strains which may cause human pandemics. Significant reduction in transmission of influenza viruses from humans, and other animals, to swine may therefore be crucial for preventing future influenza pandemics. This study investigated the presence of the 2009 pandemic influenza A/H1N1 virus, A(H1N1)pdm09, in Nigerian and Ghanaian pigs, and also determined levels of acceptance of preventive measures which could significantly reduce the transmission of this virus from humans to pigs.
Methods
Nasal swab specimens from 125 pigs in Ibadan, Nigeria, and Kumasi, Ghana, were tested for the presence of influenza A/California/04/2009 (H1N1) by quantitative antigen-detection ELISA. A semi-structured questionnaire was also administered to pig handlers in the two study areas and responses were analyzed to evaluate their compliance with seven measures for preventing human-to-swine transmission of influenza viruses.
Results
The virus was detected among pigs in the two cities, with prevalence of 8% in Ibadan and 10% in Kumasi. Levels of compliance of pig handlers with relevant preventive measures were also found to be mostly below 25 and 40% in Ibadan and Kumasi, respectively.
Conclusion
Detection of influenza A(H1N1)pdm09 among pigs tested suggests the possibility of human-to-swine transmission, which may proceed even more rapidly, considering the very poor acceptance of basic preventive measures observed in this study. This is also the first report on detection of influenza A(H1N1)pdm09 in Ghanaian pigs. We recommend improvement on personal hygiene among pig handlers, enforcement of sick leave particularly during the first few days of influenza-like illnesses, and training of pig handlers on recognition of influenza-like signs in humans and pigs. These could be crucial for prevention of future influenza pandemics.
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
Human and animal influenza are inextricably linked. The pig has receptors for both avian and mammalian influenza viruses, and is uniquely important as a mixing vessel for genetic reassortment and evolution of influenza viruses (Citation1, Citation2). This role has been identified as being consistently important for the generation of novel influenza viruses with pandemic potentials (Citation2, Citation3). Thus, reducing exposure of pigs to influenza viruses from humans and other animal species could be crucial for prevention of future influenza pandemics.
Influenza epidemics and pandemics constitute significant global health problems. Seasonal influenza epidemics have been reported to cause significant morbidity and loss of man-hours (Citation4, Citation5), and previous pandemics, including the 2009 influenza A/H1N1 pandemic, resulted in high mortality and socioeconomic losses (Citation6). Although Southeast Asia was considered as the major influenza epicenter (Citation7), events in recent years indicate that other influenza epicenters may be evolving (Citation2). This could be because factors which led to the emergence of Southeast Asia as an influenza epicenter, such as close contact between humans, pigs, and ducks, are now seen in many developing countries, including those in sub-Saharan Africa (Citation2, Citation8) (Citation9).
Thus, reduction of reverse zoonotic transmission of influenza viruses (particularly human-to-swine transmission) and monitoring of influenza viruses circulating in pigs, especially in swine production facilities, could be key to preventing evolution of novel strains which could cause future influenza pandemics. Because humans may transmit far more influenza viruses to swine than swine transmitting to humans (Citation10), such monitoring is even more important in West African swine production facilities, where little or no attention is given to farm biosecurity and hygiene of pig handlers (Citation8, Citation9).
West Africa is the main pig meat producer in Africa, and Nigeria has the highest population of pigs in West Africa (Citation11). The pig populations of Nigeria and Ghana increased significantly from about 5 million and 0.3 million in 2002 to more than 6 million and 0.5 million in recent years, respectively (Citation12, Citation13). In rural communities of West Africa, including Nigeria and Ghana, pigs are commonly reared under the extensive (range) system. However, the number of intensive commercial swine production facilities has increased rapidly in recent years, especially in cities such as Ibadan and Lagos in Nigeria, and Kumasi in Ghana. Herd size in such facilities varies widely, typically ranging between 20 and 500. Large farms, in which more pigs are reared, also exist. Common breeds include Large White, Landrace, and Duroc.
Although previous reports have shown evidence of human-to-swine transmission of influenza A/H3N2 viruses in Nigeria and Ghana (Citation9, Citation14), such reports are either very scarce (in Nigeria) or completely unavailable (in Ghana) for influenza A/H1N1 viruses. In this study, we investigated the presence of a human strain of influenza A/H1N1 virus (A/California/04/2009) among intensively-raised pigs in Nigeria and Ghana. This strain, referred to as influenza A(H1N1)pdm09 (Citation15), was responsible for the 2009 influenza pandemic. In addition, it is now known to circulate in humans as a seasonal influenza virus (Citation15–Citation17). We also determined the levels of acceptance of key preventive measures which could significantly reduce the transmission of this virus from humans to pigs.
Materials and methods
Study locations, sampling method, specimen collection
The study was conducted between January 2014 and March 2015 at the Centre for Control and Prevention of Zoonoses, University of Ibadan, Nigeria. Specimens were obtained from the Municipal Abattoir, Bodija (Lat.7.435064°N, Long. 3.914304°E), and Teaching and Research Farm, University of Ibadan (Lat.7.453265°N, Long.3.895982°E), both in Ibadan, Oyo State, South West Nigeria and also from Kumasi Abattoir Company Limited (Lat.6.65953°N, Long.01.60414°W), Kumasi, Ashanti Region, Ghana. is a map showing Ibadan, Nigeria, and Kumasi, Ghana, within the West African subregion. Stratified random sampling technique was used to select apparently healthy Landrace and Duroc pigs which were raised intensively in the study areas. Sample size was obtained using the formula:
Fig. 1 Map of Africa showing Nigeria and Ghana. (Inset) Locations of Ibadan, Nigeria, and Kumasi, Ghana.
where N is the sample size, Z is the Z statistic for a level of confidence, P is the estimated prevalence; and d is the precision or margin of error (Citation18).
Nasal swabs were collected from a total of 125 pigs from locations in the two study areas from January to March 2014. This consisted of 75 pigs in Ibadan and 50 pigs in Kumasi. Swabs were obtained by inserting a sterile swab into the nares, allowing it to stay for a few seconds and slowly withdrawing it using a rotating movement down the side of the nares. One swab was used for both nares. The swab was immediately inserted into a labeled 2 mL cryo-vial containing virus transport medium, which was screw-capped after the applicator stick had been broken off. Virus transport medium was prepared by adding 10 mL penicillin–streptomycin and 0.4 mL fungizone to 0% D-MEM (Citation19). These were immediately transferred into an ice pack before transporting to the laboratory for storage at −80°C.
Administration of questionnaire for assessment of compliance with preventive measures
A semi-structured questionnaire was administered to pig handlers in the two study areas, after obtaining their informed consent, to obtain information on farm biosecurity, routine farm practices, pig handlers’ health, awareness about swine influenza, and other relevant details. Participants included workers in commercial pig farms and pig slaughter houses. These categories of people usually have regular contact with live pigs, and are commonly referred to as ‘pig handlers’. Responses were then analyzed to determine their levels of compliance with seven measures which could significantly reduce human-to-swine transmission of influenza viruses. These included: measures relating to pig farm administrative policies, such as enforcement of sick leave for pig handlers and controlled entrance of visitors to pig pens; measures relating to pig handlers hygiene, such as regular hand washing with soap or detergent; and those relating to awareness of pig handlers about influenza, such as training of pig handlers on recognition of signs of influenza in humans and pigs (Citation20–Citation22).
Influenza virus detection and estimation of virus concentration in samples
Viruses were detected from clinical specimens by Quantitative Solid Phase Antigen-capture Sandwich ELISA using anti-hemagglutinin protein monoclonal antibody. Previous studies have reported the development and use of Antigen-capture ELISA for detection, typing, and subtyping of influenza viruses (Citation23–Citation25). Kits, which are highly specific for influenza A(H1N1)pdm09 virus, were obtained from Sino Biological Inc., Collegeville, USA and used in line with manufacturer's instructions. Mouse anti-2009 influenza H1N1 HA monoclonal antibody, diluted before coating to a working concentration of 2.0 µg/mL in coating buffer (0.05 M NaHCO3 pH 9.6), was used as the capture antibody, whereas rabbit anti-2009 influenza H1N1 HA polyclonal antibody conjugated to horseradish peroxidase (HRP), diluted before use in antibody dilution buffer to a working concentration of 2.0 µg/ml, was used as the detection antibody. The standard antigen was a recombinant influenza H1N1 (A/California/04/2009) HA, using a high standard of 780 pg/ml. Each well of a 96-well microplate was coated with 100 µL of diluted capture antibody. The plates were sealed and incubated overnight at 4°C.
Each well was aspirated, washed thrice with at least 300 µL wash buffer, and blotted against clean paper towels. Blocking buffer (300 µL) was added to each well and plates were incubated at room temperature for 1 h. After repeating the washing step, 100 µL of sample or standards was added to each well, as appropriate. Wells A1 to G1 and A12 to G12 were used for standards, whereas wells H1 and H12 served as blanks. The plates were then sealed and incubated for 2 h at room temperature. Washing was repeated and 100 µL of diluted detection antibody was added to each well. Plates were again sealed and incubated for 1 h at room temperature. Washing was again repeated and 200 µL of substrate solution was added to each well. After incubating plates in the dark at room temperature for 20–40 min, 50 µL of stop solution was added to each well. Plates were then gently tapped to ensure thorough mixing. The optical density of each well was determined immediately using a microplate reader (IRE 96, SFRI, Saint Jean d'Illac, France) at a wavelength of 450 nm.
The mean absorbance for each set of duplicate standards, controls, and samples was entered into Microsoft Excel sheet (Microsoft Office, 2007) and the mean zero standard absorbance was subtracted from each absorbance reading. Using GraphPad Prism 6.04 (GraphPad Software, San Diego, CA, USA), a standard curve was constructed by plotting the mean absorbance for each standard on the y-axis against the concentration on the x-axis. In line with manufacturer's protocols (Citation26) and other well-established protocols (Citation27, Citation28) concentrations of the samples were then estimated from the standard curve.
Results
Six of the seventy-five and five of the fifty pigs tested in Ibadan and Kumasi respectively were positive for influenza A/California/04/2009 (H1N1) virus. Thus, prevalence of this human strain of influenza virus among pigs in Ibadan and Kumasi during this study was 8.0 and 10.0%, respectively. reveals the virus concentrations of positive samples. These were extrapolated from the standard curve generated, and they ranged from 202 to 948 pg/mL for Ibadan and from 238 to 484 pg/mL for Kumasi.
Table 1 Extrapolated virus concentrations of positive samples
Analysis of questionnaires administered to 41 and 8 pig handlers in Ibadan and Kumasi, respectively, revealed that 17.1 and 25.0% of pig handlers lived within 5–10 km of a pig or poultry farm, whereas 22 and 25% actually resided on their farm premises in Ibadan and Kumasi, respectively. In addition, levels of compliance with key measures for prevention of interspecies transmission of influenza viruses were statistically similar (P >0.05) but generally very low in the two study areas (mostly below 25 and 40% in Ibadan and Kumasi, respectively). These findings are summarized in and details of analysis of these data by paired t-test, using GraphPad QuickCalcs (GraphPad Software, San Diego, CA, USA), are presented as Supplementary File. We also compared the rate of detection of influenza A/H1N1 in pigs in the two study areas to the levels of awareness of their handlers about swine influenza. This is presented in .
Fig. 2 Relationship between awareness of pig handlers about swine influenza and prevalence of influenza A(H1N1)pdm09 viruses in pigs.
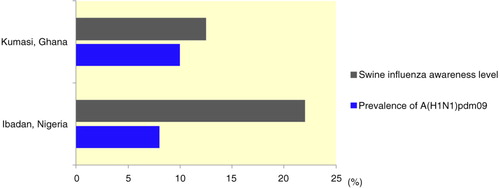
Table 2 Levels of acceptance of key measures for prevention of reverse zoonotic transmission of influenza viruses
Discussion
During this study, influenza A(H1N1)pdm09 was detected among pigs in the two countries, with prevalence of 8 and 10% in Ibadan and Kumasi, respectively. Although we recognize the need for additional studies, which are ongoing, these results suggest human-to-swine transmission of this virus, and the prevalence obtained may be indicative of the rate of human-to-swine spread of the virus in these two West African cities. Furthermore, because pigs serve as the ‘mixing vessels’ in which reassortment between different co-infecting influenza viruses take place (Citation29), such reverse transmission may be key to the emergence of influenza diversity in swine. This is even more important as previous studies by some of the authors of the present paper have shown that different types of influenza viruses, including human strains such as influenza A/Brisbane/59/2007 (H3N2), co-circulate among Nigerian and Ghanaian pigs (Citation9, Citation30). In addition, previous sero-epidemiological studies in Nigeria also revealed evidence of transmission of human influenza A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2) and influenza B/Shanghai/361/2002-like viruses to pigs (Citation14, Citation31). Thus, there is a high possibility of reassortment of the strain detected in this study with other co-circulating influenza viruses.
These findings are even more important when considered with current situations and practices in Nigerian and Ghanaian swine production facilities. For instance, from the responses of pig handlers in the present study, levels of compliance with seven key measures for prevention of reverse zoonotic transmission of influenza viruses to pigs were found, not only to be comparable in these two West African countries, but were also appallingly very low. Such low-level compliance could enhance reverse zoonotic transmission of influenza viruses (Citation21, Citation22).
Furthermore, to illustrate the effect of this low-level compliance, we compared the prevalence of influenza A(H1N1)pdm09 in pigs in the two study areas to the levels of awareness of their handlers about swine influenza. This was done to show the significance of one of these preventive measures (training of pig handlers on recognition of signs of influenza in humans and pigs). Results obtained, shown in , revealed that the prevalence of influenza A(H1N1)pdm09 was higher among Ghanaian pigs, which were reared by handlers who had relatively lower levels of awareness about swine influenza (12.5% of respondents in Ghana compared to 22.0% of respondents in Nigeria). In addition, many pig handlers in this study lived either on or within 5–10 km of a pig or poultry farm, and spent a very long time with these pigs in their pens. All these factors are akin to those which defined traditional influenza epicenters in Southeast Asia (Citation2, Citation7).
Considering the peculiarities of pig production facilities in West Africa, the following recommendations would greatly reduce reverse zoonotic transmission of the pandemic influenza virus and help prevent future influenza pandemics. To start with, personal hygiene of pig farm workers should be encouraged. Pig handlers should cover their nose and mouth with a tissue when coughing or sneezing. The tissue should be properly disposed in the trash bin after use. Hands should be washed with water and soap or detergent. If these are not available, an alcohol-based hand rub could be used (Citation32).
Second, sick leave should be enforced for pig handlers. Mandatory sick leave for pig handlers is important for reduction of exposure of handlers to pigs. Considering several socioeconomic factors which come into play in developing countries, such sick leave could be limited to the onset of clinical signs of influenza, a time during which virus shedding is expected to peak. However, adequate personal hygiene measures should be adhered to.
In addition, adequate attention should be given to enforcement of farm biosecurity measures. Such measures include risk assessment check for visitors, policy on farm dressing (such as use of clean boots and protective clothes by animal handlers), hand washing before and after handling animals, restriction on sharing of equipment and tools between farms, and policy relating to movement of vehicles in and out of the farm.
Finally, training of live pig handlers to recognize influenza-like symptoms in humans is very important. Pig handlers should also be trained to recognize signs of influenza in pigs. Influenza-like symptoms in humans include fever, cough, sore throat, runny or stuffy nose, body aches, headache, chills, fatigue, and sometimes vomiting or diarrhea (Citation33). The signs in pigs include nasal discharge, coughing, fever, dyspnea, and conjunctivitis (Citation19, Citation34).
Conclusion
This study has provided antigenic evidence of the presence of influenza A(H1N1)pdm09 virus among swine populations in Ibadan and Kumasi. Although we recognize the need for additional virological and molecular studies, which are ongoing, these preliminary results suggest human-to-swine transmission of this virus in these two socioeconomically important countries. In addition, this is the first report on detection of influenza A/H1N1 virus among pigs in Ghana. We also investigated and found that the levels of acceptance of key measures for preventing human-to-swine transmission of influenza viruses were very low. Premised on these findings, we have also provided some recommendations for prevention of human-to-swine transmission of influenza viruses in the subregion. These include improvement on personal hygiene among pig handlers; observance of sick leave especially during the first few days of influenza-like illnesses, during which virus shedding is expected to peak; improvement on farm biosecurity; and training of pig farm workers on recognition of influenza-like signs in humans and pigs. These would significantly limit reverse zoonotic transmission, thereby reducing the possibility of generations of novel reassortments among these pigs. This could be vital for prevention of future influenza pandemics.
Conflict of interest and funding
Authors have declared that no competing interests exist. Support for this work was received through a grant from the John D. and Catherine T. MacArthur Foundation to the Center for Control and Prevention of Zoonoses (CCPZ), University of Ibadan, Nigeria (Grant number 97944).
Supplementary Material
Download PDF (26.8 KB)Acknowledgements
We thank the management and members of staff of Municipal Abattoir, Bodija, Ibadan, Nigeria; Piggery Unit, Teaching and Research Farms, University of Ibadan, Nigeria; and Kumasi Abattoir Company, Kumasi, Ghana, for their cooperation during this study.
Notes
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
References
- Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, etal. Genetic reassortment of avian, swine, and human Influenza A viruses in American pigs. J Virol. 1999; 73: 8851–6. [PubMed Abstract] [PubMed CentralFull Text].
- Ma W, Kahn RE, Richt JA. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J Mol Genet Med. 2009; 3: 158–66.
- World Health Organization (WHO). Implications of H5N1 infections in pigs in China. 2004. Avian Influenza Update. Available from: http://www.who.int/csr/don/2004_08_25/en/ [cited 25 October 2015]..
- RamjeeSingh D, Wright AS, McDavid H. An intense influenza pandemic – possible subtype of H5N1 its: implications for Jamaica. West Indian Med J. 2010; 59: 76–81. [PubMed Abstract].
- World Health Organization (WHO). . Influenza (Seasonal). Fact Sheet No 211. 2014. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/ [cited 27 October 2015]..
- Tempia S, Walaza S, Viboud C, Cohen AL, Madhi SA, Venter M, etal. Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting – South Africa, 1998–2009. Clin Infect Dis. 2014; 58: 1241–9.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56: 152–79.
- Adeola OA, Adeniji JA. Prevalence of antibodies to influenza viruses among handlers of live pigs at three locations in Ibadan, Nigeria. Vet Ital. 2010; 46: 147–53.
- Adeola OA, Olugasa BO, Emikpe BO. Antigenic detection of human strain of influenza virus A(H3N2) in Swine populations at three locations in Nigeria and Ghana during the dry early months of 2014. Zoonoses Public Health. 2015. doi: http://dx.doi.org/10.1111/zph.12210. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26094828 [cited 29 November 2015]..
- Nelson MI, Vincent AL. Reverse zoonosis of influenza to swine: new perspectives on the human–animal interface. Trends Microbiol. 2015; 23: 142–53.
- Economic Communities of West African States/Sahel and West Africa Club- Organisation for Economic Co-operation and Development (ECOWAS/SWAC-OECD). Livestock and regional market in the Sahel and West Africa: potentials and challenges. 2008. Available from: http://www.oecd.org/swac/publications/41848366.pdf [cited 30 November 2015]..
- Food and Agricultural Organization (FAO). Global Pig Numbers – World Hog Population, 2002. The Pig Site. 2003. Available from: http://www.thepigsite.com/articles/858/global-pig-numbers-world-hog-population-2002/ [cited 30 November 2015]..
- Adzitey F. Animal and meat production in Ghana-An overview. J, World's Poult Res. 2013; 3: 1–4.
- Adeola OA, Adeniji JA, Olugasa BO. Detection of haemagglutination–inhibiting antibodies against human H1 and H3 strains of influenza A viruses in pigs in Ibadan, Nigeria. Zoonoses Public Health. 2010; 57: e89–94.
- World Health Organization (WHO). Standardization of terminology of the pandemic A(H1N1)2009 virus. Influenza. 2011. Available from: http://www.who.int/influenza/gisrs_laboratory/terminology_ah1n1pdm09/en/ [cited 30 November 2015].
- World Health Organization (WHO). Pandemic (H1N1) 2009. Pandemic and Epidemic Diseases, Global Alert and Response (GAR). 2010. Available from: http://www.who.int/csr/disease/swineflu/en/ [cited 25 October 2015]..
- Centre for Disease Control and Prevention (CDC). The 2009 H1N1 pandemic: summary highlights, April 2009–April 2010. H1N1 Flu. 2010. Available from: http://www.cdc.gov/h1n1flu/cdcresponse.htm [cited 25 October 2015].
- Daniel WW. Biostatistics: a foundation for analysis in the health sciences. 7th ed. 1999; New York: Wiley.
- World Health Organization (WHO). Laboratory procedures. WHO manual on animal influenza diagnosis and surveillance. 2002; Geneva, Switzerland: WHO. 15–64.
- Centre for Disease Control and Prevention (CDC). Transmission of Influenza A Viruses between animals and people. Avian Influenza (Bird Flu). 2015. Available from: http://www.cdc.gov/flu/avianflu/virus-transmission.htm [cited 25 October 2015]..
- Brown IH, Chakraverty P, Harris PA, Alexander DJ. Disease outbreaks in pigs in Great Britain due to an influenza A virus of H1N2 subtype. Vet Rec. 1995; 1: 328–9.
- Australian Pork Industry Biosecurity Programme (APIBP). Farm Biosecurity. Australian Pork Industry Biosecurity Program, Version 1, p. 26. 2003. Available from: http://farmbiosecurity.com.au/wp-content/uploads/2013/01/Australian-Pork-Industry-Biosecurity-Program.pdf [cited 25 October 2015]..
- Lee BW, Bey RF, Baarsch MJ, Simonson RR. ELISA method for detection of Influenza A infection in Swine. J Vet Diagn Invest. 1993; 5: 510–15.
- Luo Q, Huang H, Zou W, Dan H, Guo X, Zhang A, etal. An indirect sandwich ELISA for the detection of avian influenza H5 subtype viruses using anti-hemagglutinin protein monoclonal antibody. Vet Microbiol. 2009; 137: 24–30.
- Matveeva VM, Koshemetov ZhK, Mamadaliev SM. The development and application of solid-phase ELISA for the identification of avian influenza virus and its subtypes H5 and N3 in different biological samples. Vestn Ross Akad Med Nauk. 2011; 3: 10–14.
- Sino Biologicals Incorporated. General ELISA Protocol. 2013. Available from: http://old.sinobiological.com/GENERAL-ELISA-PROTOCOL-a-5456.html [cited 25 October 2015]..
- Abcam Plc. Calculating and evaluating ELISA data, Immunoassay kits and reagents. 2015. Available from: http://www.abcam.com/protocols/calculating-and-evaluating-elisa-data [cited 25 October 2015]..
- Song F, Sun X, Wang X, Nai Y, Liu Z. Early diagnosis of tuberculous meningitis by an indirect ELISA protocol based on the detection of the antigen ESAT-6 in cerebrospinal fluid. Ir J Med Sci. 2014; 183: 85–8.
- Castrucci MR, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster RG. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993; 193: 503–6.
- Adeola OA, Adeniji JA, Olugasa BO. Isolation of Influenza A viruses from pigs in Ibadan, Nigeria. Vet Ital. 2009; 45: 383–90.
- Adeola OA, Adeniji JA. Detection of HI-antibodies to a circulating human Influenza B virus among live pigs in Ibadan, Nigeria. 849–51. 849–51. Proceedings of the XV International Congress on Animal Hygiene, Vienna, Austria, 3–7 July 2011. Vol 2.
- World Health Organization (WHO). WHO guidelines on hand hygiene in health care: a summary. 2009. Available from: http://www.who.int/gpsc/5may/tools/who_guidelines-handhygiene_summary.pdf [cited 27 October 2015]..
- Wright PF, Neumann G, Kawaoka Y, Knipe DM, Howley PM. Orthomyxoviruses. Fieid's virology . 2007; Philadelphia: Lippincott Williams and Wilkins. 1691–740. 5th ed.
- Easterday BC, Hinshaw VS, Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ. Swine influenza. Diseases of swine . 1992; Ames, IA: Iowa State Press. 349–5. 7th ed.
