Abstract
Background
Toxoplasmosis is a prevalent protozoan infection with a complex lifecycle and wide profile of risk factors. The impact of congenital infection is well documented; however, there is increasing evidence of a much broader range of potential health outcomes and the need to improve our understanding of the transmission patterns and infection sources in the overall population. This study examined the epidemiology of toxoplasmosis in distinct community types from a highly endemic area of Chile.
Methods
A cross-sectional serosurvey was carried out in households from urban slums, rural villages, and farms which included collection of blood samples, as well as data on sociodemographic, behavioral, and spatial variables. Blood samples were analyzed for the presence of T. gondii-specific IgG antibodies. Avidity index was obtained for IgG-positive samples. Mixed-effects regression modeling was used to identify associations with relevant risk factors.
Results
Crude seroprevalence was 55.9% (95% CI: 52.6–59.1%) with no difference by community type. Results are indicative of early exposure to the parasite, including 40% of 13- to 17-year olds who were already seropositive. Sociodemographic factors associated with seropositivity included age, occupations, and income. However, sex modified the effect of occupation as well as of income. Practices associated with increased seropositivity were consumption of sheep and locally produced vegetables as well as cleaning household barns or sheds. Boiling water for household use was a protective factor. Living on a sloped terrain without vegetation was a protective factor, while living in an area with high flow accumulation index was a risk factor.
Conclusions
Seroprevalence of infection was high in both rural and urban slum communities with unique risk factor profiles for each community type. Findings highlight the role of the household and the community environment as influential factors in the epidemiology of the infection. Increasing awareness is needed at the health care and public health levels to establish disease burden and options for suitable control programs.
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
Toxoplasmosis is a widely prevalent protozoan infection caused by Toxoplasma gondii (Citation1). Acute infections in people are often asymptomatic or can present as flu-like illness. Toxoplasmosis is estimated to be the second and fourth highest cause of foodborne illness–related deaths and hospitalizations, respectively, in the United States (Citation2). However, due to the potentially serious implications of T. gondii infection on the fetus, the majority of research has examined vertical transmission from mother to fetus. Fetal infections may result in miscarriages, persistent ocular disease, and a variety of long-term neurological defects (Citation3). Even in asymptomatic cases, T. gondii is not cleared from the body and remains in the nervous and muscle tissue as dormant cysts (Citation1). Several studies have examined the possible link between the cysts and neurological sequelae such as delayed reaction time and risk of schizophrenia (Citation4, Citation5). Ocular lesions resulting from toxoplasmosis has been reported as a consequence of postnatal infections with estimates of 21,000 cases annually in the United States and occurrence of particularly severe manifestations in Brazil (Citation6, Citation7). Chronic infections may reactivate and lead to toxoplasmic encephalitis in immunocompromised populations, making an underlying toxoplasmosis infection an important consideration for people with HIV and/or autoimmune disorders (Citation8).
T. gondii has a complex lifecycle, with many opportunities to infect humans. The maintenance hosts of T. gondii are cats, who harbor the protozoa in their intestines and expel it through their feces in the form of sporozoites living within oocysts. The oocyst persists in both soil and water, which serve as a path of infection to incidental hosts (Citation9, Citation10). Consumption of contaminated water has been implicated in several outbreaks (Citation10–Citation12). Direct contact with oocyst-contaminated soil can occur through gardening and the consumption of contaminated fruits and vegetables; these risk factors can be mitigated through appropriate hand protection and thorough washing, respectively (Citation9, Citation13). While cat ownership itself has not been shown to increase the probability of infection, oocyst-associated infections may be acquired while cleaning the litter box of a shedding pet cat (Citation14).
Within an incidental host, cysts form and reproduce asexually in the muscle and nervous tissue. The consumption of this tissue can transmit the infection, which is considered the most common source of infection (Citation15), due in part to the wide range of incidental hosts – nearly all mammals and birds (Citation16). Not all meat poses the same risk; pork, lamb, and wild game have been identified as particularly high-risk items, and properly cooked meat, frozen meats, and processed meats have considerably lower risk (Citation13). If a cat consumes the cysts, the dormant T. gondii reactivate in the intestine of the cat and reproduce sexually to form oocysts (Citation16).
Beyond individual behavioral risks, broader geographic trends have also been shown to affect seroprevalence in humans and other species. Seasonality may modulate infection rate, with increased incidence of diagnosis in the winter months, corresponding with infection occurring in autumn (Citation17, Citation18). Other studies have shown that higher rainfall is associated with an increased incidence of toxoplasmosis (Citation19). Both harsh cold and dry conditions are deleterious to the survival of the oocysts in the environment, lowering the risk of transmission (Citation20). Differences in seropositivity have also been noted between rural and urban populations, with rural communities often having higher seroprevalence than urban communities (Citation21, Citation22). Yet urban slums have occasionally been shown to have a higher seroprevalence than rural populations, suggesting drivers of infection are more complex than a simple urban/rural divide and may be confounded by additional factors (Citation23).
Toxoplasmosis is endemic in Chile. A national study in 1994 involving approximately 76,000 individuals yielded a prevalence of 37%, with greater prevalence in the more southern regions of the country, which have higher rainfall (Citation24). This study aims to describe the seroepidemiology of toxoplasmosis at a more refined scale with consideration of individual factors as well as households, community, and local spatial factors.
Methods
Study area and population
Survey data and samples were obtained from a larger study examining the epidemiology of other zoonoses in the Los Rios Region of Chile (Citation25, Citation26). Los Rios (39°15′S–40°66′S, 71°55′E–73°74′E) has a temperate rainforest climate with average annual precipitation of 259 cm, and average temperatures of 17°C in the summer and 8°C in the winter. As part of the larger study, a total of 12 communities were enrolled: four urban slums, four rural villages, and four farm areas. For the purposes of this study, urban slums are defined as informal settlements in the outskirts of a major city with substandard housing. Rural villages are defined as community settlements away from major cities where households are clustered together. Farm communities were defined as small family farms located in a specific rural locality. Between August 2010 and March 2012, study staff enrolled 934 participants in the serosurvey part of the study across 433 households.
Survey data collection
A survey collecting information on living conditions, demography, and food practices was performed at the time of household enrollment. The demographic and behavioral variables consisted of age, sex, education (uneducated, grade school, high school, postsecondary), occupation (in a home, in an office/city, or outdoors/other), whether the individual worked in a garden, whether the individual cleaned barns or sheds, and whether the individual drained flooded land.
Living condition variables considered monthly family income (scaled as income in Chilean pesos/100,000, where a value of 1=US$160), number of cats owned, having a vegetable garden, household water source (open water, pipes/communal faucet, or wells), and frequency of disinfecting drinking water by boiling (never, occasionally, often, or always). Frequency of consumption and source of acquisition were asked about meats and vegetables. Frequency of consumption considered responses of never, rarely, 1–2 times per week, frequently throughout the week, and daily. The food source was categorized as directly from a household (own house or from a family or neighbor), from a community-based vendor (local butcher, purchase from a different farm in the community, open market, mobile vendors), from a supermarket, or none (no consumption). The study protocol was approved by the University of Minnesota's Institutional Review Board (No. 0903M62042) and Austral University's Human and Ethics Committee (No. 01/09).
Spatial and climate data
Satellite images from WorldView-2 were obtained through Spatial Solutions, Inc. (Bend, OR, USA). The images covered a 2-km buffer around the outermost sampled households in each of the 12 sites, taken during the time of sampling and without cloud cover and with a pan-sharpen resolution of 50 cm. The images were digitized as previously described (Citation26, Citation27) to produce shapefiles. These distinct shapefiles were then classified into 11 basic land-cover types such as wetlands, crop fields, brush, bushes, forests, and barren land. Dominant land type was derived from the land-cover data by categorizing similar land-cover types and calculating which category had the highest presence in 100-, 250-, and 500-m buffers. Elevation was derived from the NASA Shuttle Radar Topography Mission with cell resolution of 70–90 m and used to produce a flow accumulation index by comparing the elevation of a particular point with the elevation of surrounding points. Climate data were obtained from NASA. Rainfall estimates were obtained from Tropical Rainfall Measuring Mission in 3-h increments, compiled with 3B42 algorithm version 7, and surface temperature estimates were obtained through Global Land Data Assimilation System in 3-h increments. A daily average was computed in each grid using all dates during the survey and 70 days before and after the survey for both temperature and precipitation.
Laboratory analysis
Human serum was analyzed for the presence of Toxoplasma IgG antibodies using an enzyme immunoassay (EIA) (Toxoplasma IgG EIA, Bio-Rad Laboratories, Redmond, WA, USA) according to the manufacturer's instructions. Briefly, serum samples were diluted to a ratio of 1:51, and after combination with the reagents and the subsequent half-hour incubations at room temperature, their absorbance was measured at 405 nm. Samples were run in duplicate. According to the manufacturer's recommended cutoffs, a sample with an index value <0.9 was considered negative, ≥0.9 and <1.1 as equivocal, and ≥1.1 as positive. For analysis, a sample was considered positive if both results were positive to maximize specificity. Avidity index was measured for all samples classified as IgG positive using a commercial kit (VIR-ELISA Toxo-IgG Avidity, Viro-Immun, GmbH, Oberursel, Germany). Following the manufacturer's instructions, results were classified as low avidity (index <35%), equivocal (index 35–40%), and high avidity (index >40%).
Data analysis
Descriptive statistics were used to characterize study participants from each community type as well as distribution of avidity values among IgG-positive individuals. Crude univariable, as well as age-adjusted, analysis on the outcome describing serostatus was evaluated using binary logistic regression with random effects for community. Analyses for separate multivariable mixed-effects regression models predicting seropositivity were carried out for each community type (urban slum, rural villages, and farm communities). We used the lme4 package in R for all analyses (Citation28). Model selection was based on minimizing the Akaike's information criterion while manually assessing for biologically meaningful confounders and effect modification. Results were reported as odds ratios (OR) and 95% confidence intervals. Statistical significance was set at p<0.05.
Results
Study population characteristics
The study included 268 subjects from urban communities, 303 from rural villages, and 363 from farm communities. Community types were markedly different in sociodemographic characteristics and living conditions (). A greater proportion of participants from urban communities were female, younger, and high school graduates, while participants from farm community had a higher income and a greater proportion having attended college. The most common occupations were associated with the domestic environment (i.e. homemaker, students, and retirees), although outdoor-associated occupations (i.e. agriculture, forestry, and construction) were also common in farm communities. Working in the garden was more common among participants from farm communities, and cleaning barns was more common among urban slum participants.
Table 1 Sociodemographic characteristics and living conditions of study participants by community type, Los Rios Region, Chile, 2010–2012
Farm communities were more likely to obtain water from an open source; however, the greatest proportion of households using containers to store household water corresponded to urban communities. Approximately 50% of the households reported to own cats with no difference by community type (p=0.088). A large proportion of the participants reported to consume vegetables obtained from the community. Locally produced meat was more likely to be consumed in farm communities. Urban communities tended to be located in areas with a higher flow accumulation index and with a higher abundance of tree/shrub cover and wetlands ().
Seroprevalence and univariable analyses
A total of 522 of 934 subjects were seropositive for T. gondii-specific antibodies, resulting in an overall seroprevalence of 55.9% (95% CI: 52.6–59.1%), the highest seroprevalence in people from farms (58.7%) and no significant difference between rural villages (54.8%, p=0.346) and urban slums (53.4%, p=0.213) (Supplementary Table 1). Variables statistically significantly associated with seropositivity in the univariable analysis included age, education level, working in the garden, cleaning barns, water source, boiling water, slopped terrain without vegetation, and land cover that corresponded to fields/low vegetation or wetland (Supplementary Table 1). A high avidity index in most of the IgG-positive individuals (517/522 = 99%) established past infections. The exceptions were two individuals with a low avidity index and three individuals with values that fell within the equivocal range.
Association between sociodemographic variables and seropositivity
Regression models for each community type were controlled for age, sex, and income (). Age was a statistically significant factor across all communities, with a 3% increase in the odds of seropositivity by year of age. Age-specific seroprevalence estimates showed that 40% of the children 13–17 years old had already been exposed to T. gondii. Furthermore, seroprevalence was as high as 80% among the age group of 73–77 years (). Income was an independent predictor of seropositivity in urban slum communities, where an increase per unit of scaled income was associated with 84% greater odds of seropositivity. In contrast, income was a protective factor in farm communities, but only among women (OR = 0.65). Effect modification by sex was also found in the association between occupation and seropositivity in urban slum communities. Occupations related to being in the domestic (OR = 5.63) or an outdoor (OR = 4.95) environment were associated with seropositivity compared with having an office or indoor type of occupation; however, this was observed only for men ().
Fig. 1 Age-specific seroprevalence of toxoplasmosis in 934 individuals (men and women) from urban slums, rural villages, and farms, Los Rios Region, Chile.
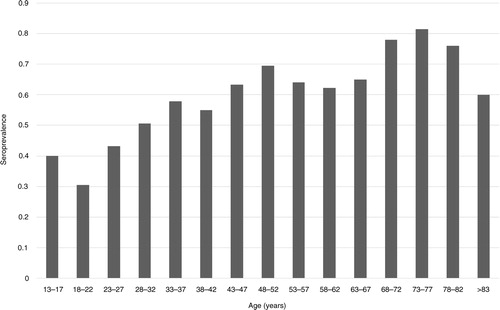
Table 2 Risk factors for Toxoplasma gondii seropositivity in people 13 years and older from Los Rios Region, Chile, obtained from multivariable regression modeling
Association between behavioral factors and seropositivity
The final model for farm communities identified that a person who cleaned barns had 73% greater odds of being seropositive than someone who did not clean barns. Always boiling water for household use was a significant protective factor (OR=0.33) in this community type. In the rural village final model, cleaning barns was also a risk factor (OR=1.75). Other significant risk factors in rural villages included obtaining vegetables from a household or a local community source (OR=2.19) instead of a supermarket and consumption of sheep (OR=7.96).
Association between spatial variables and seropositivity
In urban slum communities, living in a sloped terrain without vegetation was associated with a 7% lower OR of seropositivity. Although not statistically significant at p<0.05, there was evidence of a positive association between flow accumulation index and seropositivity (OR = 1.05, p=0.056) among rural village participants ().
Discussion
Findings document the high seroprevalence of toxoplasmosis in the study area of southern Chile (55%) and a significant risk of exposure that starts early in life resulting in close to 40% of children 13–17 years of age already displaying serologic evidence of past infection. The overall seroprevalence was higher than the 47% estimate obtained from a large-scale survey of 6,438 people carried out in a greater southern region of Chile in 1990 (Citation29) indicating that no improvements have been accomplished in terms of infection reduction and prevention programs. The seroprevalence was also higher than the estimates from other regions in Chile and consistent with a previous finding showing higher seroprevalences in southern regions compared with northern regions (Citation24). Although there are many reports of seroprevalence studies in the literature, the majority focuses on women of childbearing age or other specific populations such as blood donors or rural inhabitants. This makes comparison with other studies difficult, nevertheless, seroprevalence in Chile is similar to several others countries in Latin America and Europe (Citation30) and markedly higher than the 13% estimated for countries such as the USA (Citation31) and China (Citation32). Although emphasis is often given to infection risk in rural populations, in this study, urban slum populations had a high seroprevalence as well, indicating suitable conditions for wide exposure.
The three communities were analyzed separately based on the rationale that risk factors would be different, reflecting the distinct living conditions and practices. Income, for example, was one variable that showed varying effects depending on the community type. The association between seropositivity and low income in the farm community analysis has previously been reported in the literature (Citation33), where an increasing income could result in improved overall living conditions and less contact with a contaminated environment. Interestingly, this effect was significant only for women in farms and was even reversed in urban slum communities, where income was positively associated with being seropositive. Considering that slum communities overall represent very low socioeconomic status, people from households with relatively higher income may have had distinctly higher exposure risk that correlates with the observed higher seroprevalence. We can speculate that it could be due to more meat consumption (a benefit of higher income) or simply a correlation with other factors specific to the study communities (location of houses, access to water, etc.) that put them at higher risk but were not captured by the covariates analyzed or were obscured by low statistical power. The cross-sectional nature of this study and the fact that antibodies can only be interpreted as evidence of exposure at some point in the past are limiting factors in the interpretation of the observed associations.
Practices associated with increased risk of exposure included consumption of sheep, consumption of locally produced vegetables, and cleaning barns. Sheep meat in this region is produced locally and, particularly in rural communities, processed by the families for self-consumption. The prevalence of T. gondii infection in sheep from the study area is high (33.3%) (Citation34), and the finding is consistent with other studies documenting consumption of undercooked lamb as a risk factor (Citation35). Similar to this study, eating unwashed raw vegetables or fruits has been previously associated with increased risk of infection (Citation36). This risk factor is the result of a local environment (e.g. soil and water sources) that is extensively contaminated with oocysts as suggested by previous studies (Citation37–Citation39). Furthermore, always boiling water for household use was found to be a protective factor, which may reflect the direct benefit of consuming decontaminated water (Citation40) as well as the benefit of an overall higher level of awareness about hygiene and food safety.
Barns or sheds were common in the study households but were also often in poor condition and provided shelter to animals, including rodents and cats. Cleaning barns or sheds was associated with higher seroprevalence in rural villages and farms. This risk factor could indicate exposure to oocysts present in the barns/sheds that are ingested when performing cleaning activities. Infection after manipulation of an oocyst-contaminated environment has been document in the context of children playing in a sandy yard also visited by cats (Citation41) and an outbreak linked to possible inhalation or hand-to-mouth transmission of oocysts in a horse barn (Citation42). People, specifically men, who reported occupations linked to spending time outdoors or in domestic environments, as opposed to indoor or office type of jobs, had greater odds of being seropositive, which is also consistent with the previously mentioned risk conditions. Although shown as a risk factor in farms, the adjusted model in this study did not support a statistically significant association between cat ownership and increased seroprevalence, which is consistent with other reports, including a multicenter study in Europe (Citation43). This alludes to more complex mechanisms of infection beyond the commonly mentioned ‘cat litter box’. All of these findings underscore the household environment as a target area for more investigation to understand patterns of oocyst contamination as well as properly measured risk behaviors.
Research on spatial factors associated with Toxoplasma infection risk has been limited probably because of the lack of awareness about the environment as a contributing factor to infection. We found that living in a sloped terrain was a protective factor in the analysis of the urban communities and living in an area with higher water flow accumulation index was a risk factor in the rural villages (). Water is a well-documented source of occyst-associated infections and of outbreaks (Citation44); consequently, landscape features that regulate flow and accumulation of contaminated water could result in specific areas with relative higher or lower infection risk. Houses in sloped terrain may benefit from better water drainage that cleans away oocysts. Conversely, in an environment contaminated with oocysts, houses in low areas to which water tends to flow and accumulate are likely to represent sources of infection. This seemingly important role of a contaminated environment may explain the high observed level of exposure at a very early age in this study population. Description of the genetic diversity of T. gondii in Chile has been limited to genotyping of strains detected in free roaming chickens from central Chile, which revealed predominance of the Type II lineage (Citation45). Further research needs to be carried out to fill the gaps in our understanding of population structure T. gondii, pathogenesis, immunogenetics, and the entire spectrum of health outcomes associated with this highly prevalent infection.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Supplementary Material
Download PDF (294.4 KB)Acknowledgements
We thank the study participants and acknowledge Marcelo Gonzalez and Gunther Heyl for their contribution to data collection. This work was funded partially by grants from the Global Spotlight Program, University of Minnesota, and the National Science Foundation, Ecology of Infectious Diseases Program (Project No. 0913570).
Notes
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
References
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004; 363: 1965–76. [PubMed Abstract].
- Jones JL, Dubey JP. Foodborne toxoplasmosis. Clin Infect Dis. 2012; 55: 845–51. [PubMed Abstract].
- McAuley J, Boyer KM, Patel D, Mets M, Swisher C, Roizen N, etal. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994; 18: 38–72. [PubMed Abstract].
- Novotna M, Havlicek J, Smith AP, Kolbekova P, Skallova A, Klose J, etal. Toxoplasma and reaction time: role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism. Parasitology. 2008; 135: 1253–61. [PubMed Abstract].
- Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007; 33: 757–60. [PubMed Abstract] [PubMed CentralFull Text].
- Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010; 82: 464–5. [PubMed Abstract] [PubMed CentralFull Text].
- De-la-Torre A, Sauer A, Pfaff AW, Bourcier T, Brunet J, Speeg-Schatz C, etal. Severe South American ocular toxoplasmosis is associated with decreased Ifn-gamma/Il-17a and increased Il-6/Il-13 intraocular levels. PLoS Negl Trop Dis. 2013; 7: e2541. [PubMed Abstract] [PubMed CentralFull Text].
- Cohen BA. Neurologic manifestations of toxoplasmosis in AIDS. Semin Neurol. 1999; 19: 201–11. [PubMed Abstract].
- Coutinho SG, Lobo R, Dutra G. Isolation of Toxoplasma from the soil during an outbreak of toxoplasmosis in a rural area in Brazil. J Parasitol. 1982; 68: 866–8. [PubMed Abstract].
- Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med. 1982; 307: 666–9. [PubMed Abstract].
- Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012; 25: 264–96. [PubMed Abstract] [PubMed CentralFull Text].
- Isaac-Renton J, Bowie WR, King A, Irwin GS, Ong CS, Fung CP, etal. Detection of Toxoplasma gondii oocysts in drinking water. Appl Environ Microbiol. 1998; 64: 2278–80. [PubMed Abstract] [PubMed CentralFull Text].
- Tenter AM. Toxoplasma gondii in animals used for human consumption. Mem Inst Oswaldo Cruz. 2009; 104: 364–9. [PubMed Abstract].
- Dubey JP, Ferreira LR, Martins J, Jones JL. Sporulation and survival of Toxoplasma gondii oocysts in different types of commercial cat litter. J Parasitol. 2011; 97: 751–4. [PubMed Abstract].
- Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001; 154: 357–65. [PubMed Abstract].
- Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000; 64: 607–23. [PubMed Abstract] [PubMed CentralFull Text].
- Bobic B, Klun I, Nikolic A, Vujanic M, Zivkovic T, Ivovic V, etal. Seasonal variations in human Toxoplasma infection in Serbia. Vector Borne Zoonotic Dis. 2010; 10: 465–9. [PubMed Abstract].
- Contopoulos-Ioannidis D, Talucod J, Maldonado Y, Montoya JG. Seasonal variation of acute toxoplasmic lymphadenopathy in the United States. Epidemiol Infect. 2015; 143: 1893–7. [PubMed Abstract].
- Gomez-Marin JE, de-la-Torre A, Angel-Muller E, Rubio J, Arenas J, Osorio E, etal. First Colombian multi-centric newborn screening for congenital toxoplasmosis. PLoS Negl Trop Dis. 2011; 5: e1195. [PubMed Abstract] [PubMed CentralFull Text].
- Dumetre A, Darde ML. How to detect Toxoplasma gondii oocysts in environmental samples?. FEMS Microbiol Rev. 2003; 27: 651–61. [PubMed Abstract].
- Munoz-Zanzi C, Williams-Nguyen J, Belongia EA. A sero-survey of toxoplasmosis in farm and non-farm children from Wisconsin, United States, 1997–1999. BMC Public Health. 2013; 13: 837. [PubMed Abstract] [PubMed CentralFull Text].
- Taylor MR, Lennon B, Holland CV, Cafferkey M. Community study of Toxoplasma antibodies in urban and rural schoolchildren aged 4 to 18 years. Arch Dis Child. 1997; 77: 406–9. [PubMed Abstract] [PubMed CentralFull Text].
- Mohan B, Dubey ML, Malla N, Kumar R. Seroepidemiological study of toxoplasmosis in different sections of population of Union Territory of Chandigarh. J Commun Dis. 2002; 34: 15–22. [PubMed Abstract].
- Contreras M, Schenone H, Salinas P, Sandoval L, Rojas A, Villarroel F, etal. Seroepidemiology of human toxoplasmosis in Chile. Rev Inst Med Trop Sao Paulo. 1996; 38: 431–5. [PubMed Abstract].
- Munoz-Zanzi C, Saavedra F, Otth C, Domancich L, Hott M, Padula P. Serological evidence of hantavirus infection in apparently healthy people from rural and slum communities in southern Chile. Viruses. 2015; 7: 2006–13. [PubMed Abstract] [PubMed CentralFull Text].
- Munoz-Zanzi C, Mason M, Encina C, Gonzalez M, Berg S. Household characteristics associated with rodent presence and Leptospira infection in rural and urban communities from Southern Chile. Am J Trop Med Hyg. 2014; 90: 497–506. [PubMed Abstract] [PubMed CentralFull Text].
- Munoz-Zanzi C, Mason MR, Encina C, Astroza A, Romero A. Leptospira contamination in household and environmental water in rural communities in southern Chile. Int J Environ Res Public Health. 2014; 11: 6666–80. [PubMed Abstract] [PubMed CentralFull Text].
- Bates D, Maechler M, Bolker B, Walker S. lme4: linear mixed-effects models using Eigen and S4. 2014. Available from: http://lme4.r-forge.r-project.org/ [cited 11 November 2015]..
- Schenone H, Sandoval L, Contreras MC, Salinas P, Rojas A. Epidemiology of toxoplasmosis in Chile. VII. Prevalence of human infection investigated by means of indirect hemagglutination reaction in the regions X, XI and XII. Bol Chil Parasitol. 1990; 45: 77–9. [PubMed Abstract].
- Zapata M, Reyes L, Holst I. Decreased prevalence of Toxoplasma gondii antibodies in adults form the Central Valley in Costa Rica. Parasitol Latinoam. 2005; 60: 32–7.
- Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg. 2007; 77: 405–10. [PubMed Abstract].
- Xiao Y, Yin J, Jiang N, Xiang M, Hao L, Lu H, etal. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis. 2010; 10: 4. [PubMed Abstract] [PubMed CentralFull Text].
- Prestes-Carneiro LE, Rubinsky-Elefant G, Ferreira AW, Araujo PR, Troiani C, Zago SC, etal. Seroprevalence of toxoplasmosis, toxocariasis and cysticercosis in a rural settlement, Sao Paulo State, Brazil. Pathog Glob Health. 2013; 107: 88–95. [PubMed Abstract] [PubMed CentralFull Text].
- Gorman T, Arancibia JP, Lorca M, Hird D, Alcaino H. Seroprevalence of Toxoplasma gondii infection in sheep and alpacas (Llama pacos) in Chile. Prev Vet Med. 1999; 40: 143–9. [PubMed Abstract].
- Baril L, Ancelle T, Goulet V, Thulliez P, Tirard-Fleury V, Carme B. Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand J Infect Dis. 1999; 31: 305–9. [PubMed Abstract].
- Kapperud G, Jenum PA, Stray-Pedersen B, Melby KK, Eskild A, Eng J. Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am J Epidemiol. 1996; 144: 405–12. [PubMed Abstract].
- Munoz-Zanzi CA, Fry P, Lesina B, Hill D. Toxoplasma gondii oocyst-specific antibodies and source of infection. Emerg Infect Dis. 2010; 16: 1591–3. [PubMed Abstract] [PubMed CentralFull Text].
- Sepulveda MA, Munoz-Zanzi C, Rosenfeld C, Jara R, Pelican KM, Hill D. Toxoplasma gondii in feral American minks at the Maullin river, Chile. Vet Parasitol. 2011; 175: 60–5. [PubMed Abstract].
- Munoz-Zanzi C, Tamayo R, Balboa J, Hill D. Detection of oocyst-associated toxoplasmosis in swine from southern Chile. Zoonoses Public Health. 2012; 59: 389–92. [PubMed Abstract].
- Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Orefice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. 2003; 9: 55–62. [PubMed Abstract] [PubMed CentralFull Text].
- Stagno S, Dykes AC, Amos CS, Head RA, Juranek DD, Walls K. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980; 65: 706–12. [PubMed Abstract].
- Teutsch SM, Juranek DD, Sulzer A, Dubey JP, Sikes RK. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979; 300: 695–9. [PubMed Abstract].
- Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, etal. Sources of toxoplasma infection in pregnant women: European multi-centre case-control study. European Research Network on Congenital Toxoplasmosis. BMJ. 2000; 321: 142–7. [PubMed Abstract] [PubMed CentralFull Text].
- Vieira FP, Alves MD, Martins LM, Rangel AL, Dubey JP, Hill D, etal. Waterborne toxoplasmosis investigated and analyzed under hydrogeological assessment: new data and perspectives for further research. Mem Inst Oswaldo Cruz. 2015; 110: 929–35. [PubMed Abstract] [PubMed CentralFull Text].
- Dubey JP, Patitucci AN, Su C, Sundar N, Kwok OCH, Shen SK. Characterization of Toxoplasma gondii isolates in free-range chickens from Chile, South America. Vet Parasitol. 2006; 140: 76–82. [PubMed Abstract].
