Abstract
Background
The increasing prevalence of penicillin non-susceptible pneumococci (PNSP) throughout the world threatens successful treatment of infections caused by this important bacterial pathogen. The rate at which PNSP clones spread in the community is thought to mainly be determined by two key determinants; the volume of penicillin use and the magnitude of the fitness cost in the absence of treatment. The aim of the study was to determine the impacts of penicillin consumption and fitness cost on pneumococcal transmission dynamics in a developed country setting.
Methods
An individual-based network model based on real-life demographic data was constructed and applied in a developed country setting (Sweden). A population structure with transmission of carriage taking place within relevant mixing groups, i.e. families, day care groups, school classes, and other close contacts, was considered to properly assess the transmission dynamics for susceptible and PNSP clones. Several scenarios were simulated and model outcomes were statistically analysed.
Results
Model simulations predicted that with an outpatient penicillin use corresponding to the sales in Sweden 2010 (118 recipes per 1,000 inhabitants per year), the magnitude of a fitness cost for resistance must be at least 5% to offset the advantage of penicillin resistance. Moreover, even if there is a fitness cost associated with penicillin resistance, a considerable reduction of penicillin usage appears to be required to significantly decrease the incidence of PNSP in a community.
Conclusion
The frequency of PNSP clones is hard to reverse by simply reducing the penicillin consumption even if there is a biological cost associated with resistance. However, because penicillin usage does promote further spread of PNSP clones, it is important to keep down penicillin consumption considering future resistance problems.
Infections caused by Streptococcus pneumoniae, pneumococcus, remain a major cause of serious illness in children and adults worldwide, despite the availability of vaccines. One reason is that current vaccines only protect against a limited number of the more than 90 pneumococcal serotypes identified so far (Citation1). Also, vaccine-induced immunity often leads to replacement of targeted clones because other serotypes fill the newly opened ecological niche. Infections caused by S. pneumoniae have for many years been treated successfully with penicillin. However, the increasing prevalence of resistant clones has now complicated the treatment of these infections. Epidemiological studies have previously reported a correlation between the prevalence of penicillin non-susceptible pneumococci (PNSP; pneumococci with minimum inhibitory concentration of penicillin G≥0.5 µg/ml) and the community utilisation of penicillin (Citation2–Citation4). In comparison with other countries, Sweden has had a situation with a relatively low frequency of PNSP and a low rate of penicillin sale (Citation5, Citation6).
Several studies have reported that reduction in antibiotic consumption results in a decrease of the frequency of antibiotic-resistant pneumococci in the community (Citation7–Citation10), whereas other studies could not observe such an effect (Citation11–Citation13). An important parameter to consider when evaluating the effects of such interventions is the extent to which non-susceptible clones have any biological cost in competition with fully susceptible clones. For most pathogenic microorganisms, the acquisition of resistance usually carries a cost on the fitness and/or virulence of the microbe (Citation14). An important factor of fitness for pathogenic bacteria is the ability of a bacterial clone to be transmitted from host to host. The biological fitness also includes the ability of a bacterial clone to reproduce itself and the ability of the clone to avoid being cleared from the infected host. However, resistant bacteria can ameliorate the costs of resistance by acquiring additional fitness-compensatory mutations, which counteract the deleterious effects of resistance (Citation14). For example, the successful global spreading of multi-resistant pneumococcal clones indicates that if such fitness costs exist, they may be offset in certain clones by association with other genetic features that enhance the fitness (Citation15). If there is no fitness cost, there is neither any disadvantage of being resistant nor selective pressure to drive down the rate of resistant clones even in the absence of an antibiotic (Citation16, Citation17). The frequency at which resistant clones spread in a population is thought to depend mainly on a balance between the two parameters: the volume of antibiotic use in the population and the magnitude of the fitness cost of antibiotic resistance in the absence of treatment.
Several mathematical and computational models have been developed to study the different aspects of pneumococcal transmission dynamics (Citation17–Citation25). However, none of the previous models have addressed the impacts of penicillin resistance and fitness cost. For example, McCormick et al. (Citation21) used a mathematical transmission model to study the observed variation in proportions of strains resistant to beta-lactams and macrolides between geographic sites in the United States. Analyses of the model showed that the observed variation is best explained by geographic variation in selection pressure for resistance, rather than by clonal dynamics. In a recent study by Mitchell et al. (Citation23), a dynamic stochastic model of paediatric pneumococcal carriage was developed to assess impacts on the emergence of new resistant lineages following the introduction of a vaccine targeting more common resistant serotypes. They reported that as long as vaccination targets only a subset of pneumococcal serotypes, the antibiotic pressure will likely lead to the emergence of resistant lineages. Furthermore, we have previously published results based on simulations of probabilistic network models for pneumococcal transmission to address the impacts of penicillin consumption and between-strain competition on the spreading of PNSP; analyses of model simulations indicated that the age distribution of carriage prevalence of PNSP, in contrast to penicillin-susceptible pneumococci (PSP), is affected by penicillin consumption (Citation17). Besides, under the assumption that reduced penicillin susceptibility does not confer any fitness cost for the pneumococci, it appears extremely difficult to decrease the incidence of PNSP by simply controlling the penicillin consumption.
The present study investigates the relation between penicillin consumption and the magnitude of fitness cost on transmission dynamics of resistant and susceptible pneumococcal clones in a developed setting. More specifically, the questions addressed in this study are what magnitude the fitness cost must have to offset the advantage of being resistant in a community using penicillin and to what extent the penicillin consumption should be reduced so as to significantly reduce the prevalence of resistant S. pneumoniae in the community.
Methods
Model definitions
An in silico population consisting of 50,000 individuals was generated (). Each individual was assigned age according to demographic data of Sweden (Citation26). All simulations were initiated with equivalent sets of carriers, that is, same initial numbers and age distribution, to reflect observed age distribution for pneumococcal prevalence (Citation17, Citation20) (Citation27) and to permit the simulations to start with established pneumococcal prevalence as described elsewhere (Citation17, Citation20). The model considered the simultaneous spread of two clones, one susceptible clone and one PNSP clone. Each clone was assigned an initial number of 250 carriers. This corresponds proportionally to the prevalence of a major pneumococcal clone (i.e. genetically closely related isolates) in Sweden (Citation17, Citation20) (Citation28–Citation31). Discrete, fixed time-steps were used (1 week) and the simulations were run for 520 weeks (i.e. 10 years). The update of the model was implemented to occur synchronously at each time-step. The age structure and the contact network were held static over time and were constant in all simulations. Because this model is non-deterministic, each run produced different results because of chance. Each scenario was therefore simulated 100 times, and the results were averaged to find the most likely outcome. Thus, the number of simulations was sufficiently large to assume an approximately normal distribution for the output data according to the central limit theory. The distributions of the outcome variables were also examined using the Shapiro–Wilk normality test, and no significant departure from normality was found (p>0.05). The output data were statistically analysed using independent two-sample t-test or one-way analysis of variance (ANOVA) with post hoc Tukey test for comparisons of sample means. The significance level was set at 5% (p<0.05). Simple linear regression analysis was performed to assess the relationship between the average number of transmissions and the fitness cost for the PNSP clone. The simulation procedures were programmed in MATLAB R2013b (The Mathworks Inc., United States), and IBM SPSS Statistics version 22.0 (IBM Corp., United States) was used for the statistical analyses.
Fig. 1 Simplified graphic illustration of the network model concept. The blue boxes represent households, the green box represents day care centre, whereas the yellow box represents school. Individuals within the same household have contact with each other, whereas individuals within the same day care centre or school have contact with each other only if they belong to the same group or class, respectively. Disease transmission can only occur via edges, i.e., contacts between vertices, i.e., individuals. The edges are bidirectional, that is, disease may be transmitted in both directions. Adapted from Karlsson et al. (Citation20).
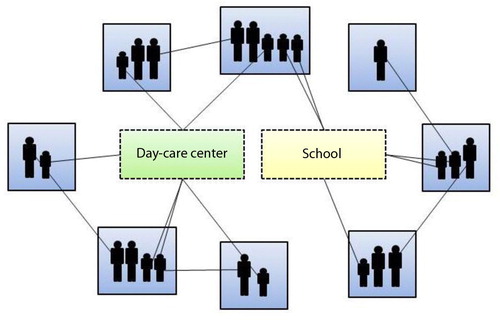
Contact network structure
In the model, individuals who were colonised with pneumococci could potentially transmit the bacteria to susceptible individuals with whom they had close contacts with. Only those contact sites that are considered to be important for the community spread of pneumococci, i.e. families, day care groups, school classes, and other close contacts, were included in the model as previously described (Citation17, Citation20) (). The contact structure was constructed based on available data of Sweden from the mid-2000s (Citation32–Citation38). Each generated contact comprised a possible route for transmission of pneumococci as there was a certain probability in each time-step for transmission to occur via an existing contact between a colonised individual and a susceptible one. The transmission probability varied depending on the type of contact and the age of the exposed individual (). The defined baseline values for the transmission probabilities assumed no penicillin consumption. Even if large variations in duration of carriage have been observed, there seems to be an inverse relationship between carriage duration and age (Citation39, Citation40). During the simulations, an individual was assigned a carriage duration at the time point for colonisation. The carriage duration was drawn from an exponential distribution with a mean dependent on the age of the colonised individual ().
Fig. 2 Model flowchart. The age-structured population and the contact structure were held constant between and during simulations. All simulations were initiated with equivalent sets of pneumococcal carriers, and all parameters, except for volume of outpatient penicillin consumption and transmission probabilities reflecting fitness costs, were held constant for each scenario. PSP=penicillin-susceptible pneumococci.
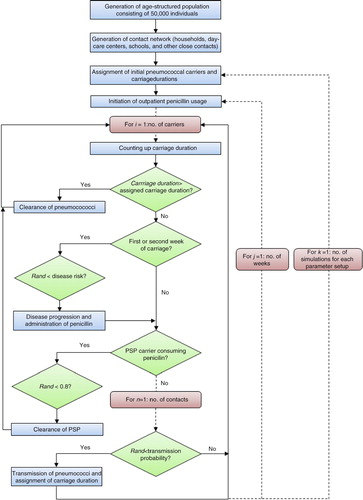
Table 1 Baselines values for the model parameters
Disease progression
Upon colonisation, individuals may become either asymptomatic carriers of the bacteria or develop clinical disease, herein defined as a condition requiring outpatient penicillin prescription. Because most cases of pneumococcal diseases appear to occur within a few days of pneumococcal acquisition (Citation41), disease progression in the model was assumed to occur during the first or second week of colonisation. The risk for disease progression per colonisation event was implemented to be age dependent as previously estimated based on incidence rates in Sweden (Citation17, Citation42) (Citation43). The model did not consider any individual risk factors other than age for disease progression. Also, the model did not implicate the progression of invasive pneumococcal infections; those patients are hospitalised for medical care and, therefore, not included in this outpatient category.
Outpatient penicillin consumption
The administration of outpatient penicillin was implemented according to available data of penicillin sales in Sweden during 2008 for different age groups (Citation44) and unrelated to pneumococcal carriage (). Depending on the indication, outpatient penicillin courses generally proceed between 5 and 10 days. In the model, the therapy period was approximated to 1 week. If an individual receiving penicillin was carrying pneumococci, the consequences depended on the resistance properties of the colonising clone. Carriages of the susceptible clone were considered to be cleared in 80% of the cases as previously reported (Citation45) because a successful antibiotic treatment does not always clear the nasopharynx from pneumococci (Citation46). In contrast, carriages of the PNSP clone were assumed not to be eradicated by penicillin treatment (Citation46). During an ongoing penicillin course, a treated individual could be colonised by the PNSP clone, but not by the susceptible one. Moreover, individuals who developed clinical diseases were administered penicillin and then handled in the same way as the asymptomatic carriers receiving penicillin. However, these penicillin courses were not included in the initial total prescription rate. As very few of the colonisation events progressed to clinical disease (), the number of penicillin courses administered because of clinical disease was considered low enough to be within the margin of random variability for the outpatient penicillin consumption.
Table 2 Outpatient penicillin (PcV) sales in Sweden 2010 (Citation44)
Fitness cost for penicillin resistance and simultaneous carriage of clones
There are inconsistent reports on whether penicillin resistance implies a fitness cost. If such a fitness cost exists, it probably varies between clones (Citation47). Besides, some clones might have compensated for the fitness cost (Citation15, Citation48). For example, Melnyk et al. (Citation49) reported mean relative fitness values ranging from 1.02 to 0.8 depending on bacterial species and antibiotics. For S. pneumoniae, it has been shown that the cost of penicillin resistance increases with the number of resistant pbp alleles acquired (Citation50). Therefore, varying fitness costs were investigated in this study. The fitness costs in the model were implemented by decreased transmission probabilities for the PNSP clone relative to the susceptible clone. The model allowed simultaneous carriage of the PNSP clone and the susceptible clone in the same individual (Citation47, Citation51). However, the acquisition rate of an additional serotype was implemented to be ten times slower in an individual already carrying another serotype (Citation51) ().
Model scenarios
Several model scenarios were implemented using different parameter value combinations. First, two reference scenarios were simulated; one reflecting the transmission dynamics of two pneumococcal clones in the absence of penicillin consumption and fitness cost, whereas in the second, penicillin consumption was implemented but the PNSP clone did not have any fitness cost. Next, a number of scenarios where simulated to examine the transmission dynamics using varying fitness costs for the PNSP clone in a penicillin-consuming setting. Finally, additional scenarios were set up to investigate how much the volume of penicillin use must be reduced to observe a significant decrease in the frequency of PNSP in the community.
Results
Impact of penicillin consumption on pneumococcal transmission dynamics
The first scenarios implemented, the so-called reference scenarios, were simulated to examine the impact of penicillin consumption on the transmission dynamics for a PNSP clone bearing no fitness cost for resistance in competition with a susceptible clone in a community. In the first reference scenario, the spread of two susceptible clones with equal fitness was simulated. No statistically significant difference (p=0.16) in the average number of transmissions for the two clones could be observed. In the next reference scenario, the transmission of one susceptible clone and one PNSP clone in a community setting consuming penicillin was simulated. The number of transmissions for the susceptible clone was then almost halved (decreased by 46%) compared with the first reference scenario; from 16,333 transmissions in the first reference scenario to 8,859 transmissions in the second reference scenario (p<0.001). For the PNSP clone, the number of transmission was increased by 25%; from 15,422 in the first reference scenario to 19,229 in the second one (p<0.001). The difference in the number of transmissions for the PNSP clone and the susceptible clone was statistically significant (p<0.001) in the second reference scenario.
Effects of fitness costs on pneumococcal transmission dynamics
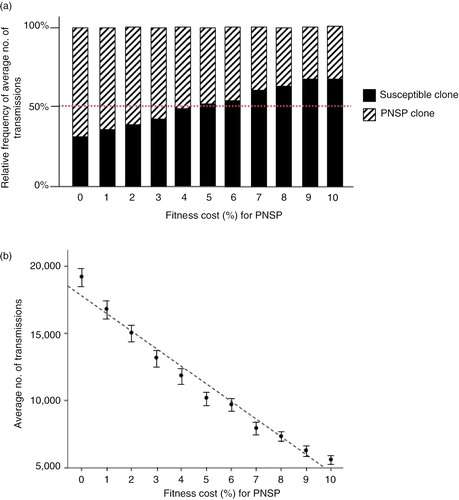
The next step was to investigate how the balance between the volume of penicillin use in the community and the magnitude of the fitness cost affects the pneumococcal transmission dynamics. Several scenarios with varying fitness costs for the PNSP clone were therefore simulated (). The lowest fitness cost was set to 1% and was then gradually increased by one percentage point up to 10%. For the scenarios simulated with a fitness cost ≤4%, the frequency of the PNSP clone was higher than that for the susceptible clone (). A turning point for the balance between fitness cost and penicillin consumption could be observed for a fitness cost between 4 and 5% (). The difference in the mean number of transmissions for the susceptible clone and the PNSP clone was not statistically significant using the 4% fitness cost (p=0.017) or the 5% fitness cost (p=0.046). With a fitness cost higher than 5%, the susceptible clone was more successful in spreading than the PNSP clone (, p<0.001).The relationship between the average number of transmissions and the magnitude of fitness cost for the PNSP clone appeared to be linear (). Linear regression analysis showed that the number of transmissions of PNSP decreased significantly with increased fitness cost (p<0.001; average number of transmissions=17,843 – 1,326×fitness cost (%), r 2=0.972).
Fig. 3 Outcomes for scenarios simulated using varying fitness cost for the PNSP clone. For each scenario, 100 simulations were performed and the outcomes were averaged to find the most probable outcome. The implemented fitness costs for the PNSP clone ranged between 0 and 10%. (a) Relative frequency of average number of transmissions for the penicillin-susceptible pneumococci clone and the PNSP clone. (b) Plot of average number of transmissions for the PNSP clone against fitness cost with fitted regression line (F 1,9=311, p<0.001; average number of transmissions=17,843 – 1,326×fitness cost (%), r 2=0.97). Error bars indicate 95% confidence interval. PNSP, penicillin non-susceptible pneumococci.
By how much must the volume of penicillin consumption be reduced to affect the frequency of PNSP in the community?
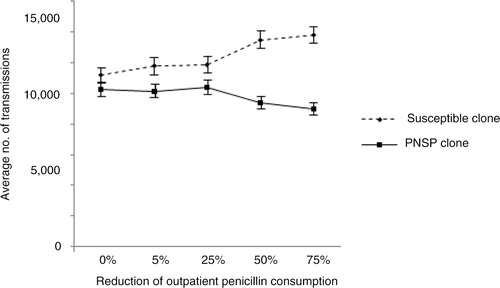
One of the most interesting questions concerning the resistance problems is to what extent penicillin use should be reduced so as to affect resistance among S. pneumoniae in the community. This was examined by assuming a fitness cost of 5% for the PNSP clone, whereas the penicillin consumption was reduced in varying extents by 5, 25, 50 and 75% (). The fitness cost for the PNSP clone was determined based on previous model simulations indicating that the magnitude of the fitness cost must be at least 5% to offset the advantage of penicillin resistance. With decreasing penicillin consumption, the trend moved toward an increased incidence of the susceptible clone and decreased incidence of the PNSP clone (). Besides, the total number of transmissions (PNSP and susceptible) was increased. Reducing the penicillin consumption by 5, 25 and 50% did not result in any significant change in the incidence of the PNSP clone in comparison with the scenario where default penicillin consumption was observed. (p=0.79, p=0.76, and p=0.063, respectively). When reducing the penicillin consumption by 75%, a significant change (p=0.0016) in the incidence of the PNSP clone in comparison with the scenario with default penicillin consumption was observed.
Fig. 4 Average number of transmissions for susceptible clone and PNSP clone by implementing reduced outpatient penicillin consumption. The fitness cost for the PNSP clone was fixed at 5% and the default penicillin consumption was set according Sweden year 2010 (Citation44). Error bars indicate 95% confidence interval. PNSP, penicillin non-susceptible pneumococci.
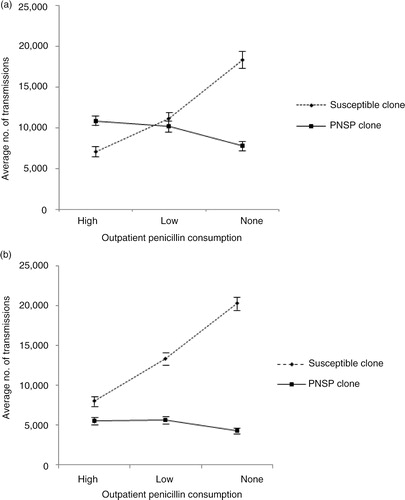
Further simulations were then performed by implementing 5 and 10% fitness costs, respectively, for the PNSP clone (). The impact of penicillin consumption was examined by implementing outpatient penicillin consumption at three levels; none (no penicillin consumption), low (penicillin consumption according to default volume, Sweden, year 2010; ), and high (doubled penicillin consumption compared with the default volume). A one-way ANOVA showed a significant difference in the number of PNSP transmissions among the simulations performed using 5% fitness cost for the PNSP clone (F 2,297=27.9, p<0.001). An a posteriori Tukey test showed no significant decrease in the number of transmissions for PNSP when the penicillin use was reduced from high to low level (, p=0.32), but showed a statistically significant decrease when reduced from high to none (p<0.001). A significant decrease could also be observed when the penicillin consumption was reduced from low to none (p<0.001). For the simulations performed using 10% fitness cost for the PNSP clone, a one-way ANOVA showed a significant difference in the number of PNSP transmissions (F 2,297=14.4, p<0.001). An a posteriori Tukey test showed no significant difference in the number of transmissions for PNSP when the penicillin use was reduced from high to low level (, p=0.96). However, a statistically significant decrease could be observed when penicillin consumption was reduced from high to none (p<0.001) and from low to none (p<0.001). Moreover, the mean number of PNSP transmissions was significantly less for simulations performed with 10% fitness cost compared with simulations performed with 5% fitness cost (p<0.0001).
Fig. 5 Average number of transmissions for susceptible clone and PNSP clone by implementing varying degrees of penicillin consumption. (a) 5% fitness cost for the PNSP relative to the susceptible clone. One-way ANOVA ( F 2,297=27.9, p<0.001) followed by post hoc Tukey test revealed significant differences in the number of transmissions for the PNSP clone between low and no penicillin consumption (p<0.001) as well as between high and no penicillin consumption (p<0.001). (b) 10% fitness cost for the PNSP relative to the susceptible clone. One-way ANOVA (F 2,297=14.4, p<0.001) followed by post hoc Tukey test revealed significant differences in the number of transmissions for the PNSP clone between low and no penicillin consumption (p<0.001), as well as between high and no penicillin consumption (p<0.001). Error bars indicate 95% confidence interval. PNSP, penicillin non-susceptible pneumococci.
Discussion
The present study was designed to evaluate the impacts of penicillin resistance and fitness cost on pneumococcal transmission dynamics in a developed setting. For that purpose, a network model based on real-life demographic data (Table and 2 ) was constructed, and several model scenarios were simulated. The model simulations suggested that cost-driven reversibility of penicillin resistance at the community level appeared to be of modest importance ( and ). The difficulties to reduce the rates of these resistant bacteria imply that it is even more important to avoid the overuse of antibiotics. Despite whether a significant decrease in the prevalence of PNSP can be observed after reduced penicillin consumption, the reduction may at least contribute to slowing down the spread of resistant pneumococci in the community. The pneumococcal population biology is affected by antibiotic therapy in several ways. A direct effect is mediated by the antibiotic-mediated killing or inhibition of susceptible clones in a treated individual, thereby promoting the growth of resistant clones given that pneumococcal clones compete to colonise nasopharynx (Citation53). Even if no resistant clone is present in the treated individual, inhibition of susceptible clones in an antibiotic-treated person could promote the spread of resistant clones in the community by reducing transmission of the susceptible clones with which the resistant ones compete (Citation54). Any used antibiotic that inhibits susceptible clones more than resistant ones will exert natural selection in favour of the clones against which it is less active (). The relative efficacies against the susceptible and the resistant clones determine the degree of selection. Thus, it is highly important to keep all antibiotic consumption as low as possible. The increasing globalisation implies increased transfer of bacteria including resistant ones between countries (Citation55) making the resistance problems a global issue. Not only the human antibiotic use is of concern as a significant proportion of the antibiotic usage occurs within the agriculture area.
The difficulties to reverse the frequency of PNSP in the community by a reduction in penicillin consumption as indicated by model simulations ( and ) are partly supported by previous observational studies. In a study performed on Iceland, Arason et al. (Citation56) reported that a reduction of penicillin use by 18% among children ≤7 years did not have any significant effect on the proportion of PNSP carriers among 1–7-year-old children (8.5% in 1993; 8.1% in 1998; p=0.8). Furthermore, the prevalence of PNSP increased in two out of four study areas despite reduced consumption during 5 years. Similar findings were reported in a study from southern Israel (Citation11). During a 6-year period, the association between prescribing antibiotics and resistance in S. pneumoniae among children <5 years of age with acute otitis media was investigated. No decrease in PNSP could be observed, although there was reduction by approximately 30% in the prescription rates of penicillin. An observational study of invasive S. pneumoniae conducted in Europe during 1999–2001 (Citation7) observed an overall rate of decrease in the proportion of single penicillin non-susceptibility, but the authors did not report the penicillin consumption for the countries included. Moreover, when they analysed by country, none of the countries showed a significant trend in the proportions of single penicillin non-susceptibility.
The decrease in the frequency of PNSP expected after a decline in penicillin use depends on both the fitness cost of resistance for pneumococci and the extent of the reduction in penicillin consumption. Model simulations predicted that with an outpatient penicillin use corresponding to the sales in Sweden in the year 2010 (118 recipes per 1,000 inhabitants and year), the magnitude of a fitness cost for resistance must be at least 5% to offset the advantage of penicillin resistance (). In countries with higher penicillin consumption than Sweden, the threshold at which the magnitude of fitness cost offsets the advantage of being resistance is most probably higher than 5% as indicated by . Thus, whether it is advantageous to be resistant is determined by the fitness cost as well as the volume of penicillin use, for example, for PNSP clones with 10% fitness cost, model simulations suggested that the outpatient penicillin consumption must be higher than the levels investigated herein for the resistance property to be beneficial for the clone. Irrespective of the magnitude of fitness cost, model simulations indicated that a dramatic reduction in penicillin consumption is required to have an impact on the frequency of PNSP ( and ). Moreover, the relationship between penicillin consumption and penicillin resistance in pneumococci is further complicated by herd immunity and clonal spread. Some pneumococcal clonal types are more prone to be spread globally (Citation57). For example, the rapid increase of PNSP observed in Iceland from 1989 to 1993 was largely caused by the spread of a single multiresistant serotype 6B clone (the Spanish-Icelandic clone Spain-26B) (Citation58).
Results of model simulations highlight the importance of fitness cost for resistance in pneumococcal transmission dynamics. For example, if there is no fitness cost for resistance, the PNSP clone has the ability to disseminate widely even at a relatively low selection pressure as mediated by a penicillin consumption corresponding to Sweden year 2010 (). Also the results from the simulations performed with 5 and 10% fitness costs, respectively, for the PNSP clone and varying penicillin consumption () indicate the impact of fitness cost for resistance; the mean number of PNSP was significantly less in the simulations conducted using 10% fitness cost in comparison with the simulations performed with 5% fitness cost (p<0.0001).
A limitation with the constructed model is that it only considers the spread of two clones in a community; however, the model is thought to capture the major dynamics in pneumococcal transmission. In addition, the model does not consider the effects of pneumococcal conjugate vaccine or herd immunity. It would be interesting in a future study to explore the effects of vaccination such as serotype replacement and herd immunity in an extended transmission model.
In conclusion, the most important finding of this study is that the reversibility of PNSP clones appears to be very hard to affect by only reducing the penicillin consumption even if there is a biological cost for resistance. The difficulties to reduce the rates of PNSP imply that it is even more important to avoid unnecessary use of penicillin because its usage does promote further spread of PNSP clones.
Competing interests and funding
The author has declared that no conflict of interest exists. No funding or benefits have been received to conduct this study.
Acknowledgements
I thank Andreas Tilevik, University of Skövde, for helpful comments on the manuscript.
References
- Browall S, Backhaus E, Naucler P, Galanis I, Sjostrom K, Karlsson D, etal. Clinical manifestations of invasive pneumococcal disease by vaccine and non-vaccine types. Eur Respir J. 2014; 44: 1646–57.
- Arason VA, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996; 313: 387–91.
- Kristinsson KG. Epidemiology of penicillin resistant pneumococci in Iceland. Microb Drug Resist. 1995; 1: 121–5.
- Melander E, Ekdahl K, Jonsson G, Molstad S. Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr Infect Dis J. 2000; 19: 1172–7.
- Backhaus E, Berg S, Trollfors B, Andersson R, Persson E, Claesson BE, etal. Antimicrobial susceptibility of invasive pneumococcal isolates from a region in south-west Sweden 1998–2001. Scand J Infect Dis. 2007; 39: 19–27.
- van de Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H, etal. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008; 14: 1722–30.
- Bruinsma N, Kristinsson KG, Bronzwaer S, Schrijnemakers P, Degener J, Tiemersma E, etal. Trends of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae in Europe. J Antimicrob Chemother. 2004; 54: 1045–50.
- Guillemot D, Varon E, Bernede C, Weber P, Henriet L, Simon S, etal. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin G-nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005; 41: 930–8.
- Karcic E, Aljicevic M, Bektas S, Karcic B. Antimicrobial susceptibility/resistance of Streptococcus pneumoniae. Mater Sociomed. 2015; 27(3): 180–4.
- Low DE. Changing trends in antimicrobial-resistant pneumococci: it's not all bad news. Clin Infect Dis. 2005; 41(Suppl 4): S228–33.
- Barkai G, Greenberg D, Givon-Lavi N, Dreifuss E, Vardy D, Dagan R. Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005; 11: 829–37.
- Belongia EA, Sullivan BJ, Chyou PH, Madagame E, Reed KD, Schwartz B. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001; 108: 575–83.
- Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, etal. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004; 190: 2031–8.
- Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance?. Nat Rev Microbiol. 2010; 8: 260–71. [PubMed Abstract].
- Sjostrom K, Blomberg C, Fernebro J, Dagerhamn J, Morfeldt E, Barocchi MA, etal. Clonal success of piliated penicillin nonsusceptible pneumococci. Proc Natl Acad Sci USA. 2007; 104: 12907–12.
- Lipsitch M. Measuring and interpreting associations between antibiotic use and penicillin resistance in Streptococcus pneumoniae. Clin Infect Dis. 2001; 32: 1044–54.
- Karlsson D. Probabilistic network modelling of the impact of penicillin consumption on spread of pneumococci. Epidemiol Infect. 2011; 139: 1351–60.
- Andersson M, Ekdahl K, Molstad S, Persson K, Hansson HB, Giesecke J. Modelling the spread of penicillin-resistant Streptococcus pneumoniae in day care and evaluation of intervention. Stat Med. 2005; 24: 3593–607.
- Huang SS, Finkelstein JA, Lipsitch M. Modeling community- and individual-level effects of child-care center attendance on pneumococcal carriage. Clin Infect Dis. 2005; 40: 1215–22.
- Karlsson D, Jansson A, Normark BH, Nilsson P. An individual-based network model to evaluate interventions for controlling pneumococcal transmission. BMC Infect Dis. 2008; 8: 83.
- McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, etal. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med. 2003; 9: 424–30.
- Melegaro A, Gay NJ, Medley GF. Estimating the transmission parameters of pneumococcal carriage in households. Epidemiol Infect. 2004; 132: 433–41.
- Mitchell PK, Lipsitch M, Hanage WP. Carriage burden, multiple colonization and antibiotic pressure promote emergence of resistant vaccine escape pneumococci. Philos trans Rl Soc Lond B Biol Sci. 2015; 370: 20140342.
- Shiri T, Auranen K, Nunes MC, Adrian PV, van Niekerk N, de Gouveia L, etal. Dynamics of pneumococcal transmission in vaccine-naive children and their HIV-infected or HIV-uninfected mothers during the first 2 years of life. Am J Epidemiol. 2013; 178: 1629–37.
- Snedecor SJ, Strutton DR, Ciuryla V, Schwartz EJ, Botteman MF. Transmission-dynamic model to capture the indirect effects of infant vaccination with Prevnar (7-valent pneumococcal conjugate vaccine (PCV7)) in older populations. Vaccine. 2009; 27: 4694–703.
- Statistics Sweden. Sveriges befolkning efter ålder och kön 31/12/2006. 2007. Available from: http://www.scb.se/templates/tableOrChart_78315.asp [cited 15 March 2007].
- Hogberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J Clinl Microbiol. 2007; 45: 948–52.
- Borres MP, Alestig K, Krantz I, Larsson P, Norvenius G, Stenqvist K. Carriage of penicillin-susceptible and non-susceptible pneumococci in healthy young children in Goteborg, Sweden. J Infect. 2000; 40: 141–4.
- Kalin M. Bacteremic pneumococcal pneumonia: value of culture of nasopharyngeal specimens and examination of washed sputum specimens. Eur J Clin Microbiol. 1982; 1: 394–6.
- Sandgren A, Sjostrom K, Olsson-Liljequist B, Christensson B, Samuelsson A, Kronvall G, etal. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis. 2004; 189: 785–96.
- Söderström M . Respiratory tract infections in children. 1990. Aspects of etiology, incidence and recurrent infections. PhD thesis, Lund, Sweden.
- Statistics Sweden. Kapitel 4: Grundskole- och gymnasienivå 2003. Available from: http://www.scb.se/statistik/UF/UF0526/2003M00/UF100%C3%96P0201_04.pdf [cited 18 June 2007].
- Statistics Sweden. Children and their families 2005. 2006; Sweden Available from: http://www.scb.se/statistik/_publikationer/BE0701_2005A01_BR_BE51ST0603.pdf [cited 18 June 2007].
- Skolverket. Barn och personal i förskola 2006. 2007. Available from: http://www.skolverket.se/sb/d/1667/a/9255 [cited 18 June 2007].
- Skolverket. Uppgifter på kommunnivå, tabell 1: Antal och andel inskrivna barn i olika åldersgrupper samt antal inskrivna barn på obekväma arbetstider 2006. 2007. Available from: http://www.skolverket.se/content/1/c4/92/23/BO - Barn och grupper - Kommunniv%E5 - Tabell1.xls [cited 18 June 2007].
- Skolverket. Efterfrågade mått – Förskolan 2007. 2007. Available from: http://www.skolverket.se/sb/d/1663/a/7697 [cited 18 June 2007].
- Skolverket. Tabell 3 A: Antal institutioner, inskrivna barn efter ålder samt kommuner med verksamhet 2009. 2009. Available from: http://www.skolverket.se/sb/d/1664 [cited 9 March 2011].
- Skolverket. Tabell 3B: Inskrivna barn efter ålder och kön 2003–2009. Andel av alla barn i befolkningen. 2009. Available from: http://www.skolverket.se/sb/d/1664 [cited 9 March 2011].
- Cauchemez S, Temime L, Valleron AJ, Varon E, Thomas G, Guillemot D, etal. S. pneumoniae transmission according to inclusion in conjugate vaccines: Bayesian analysis of a longitudinal follow-up in schools. BMC Infect Dis. 2006; 6: 14.
- Ekdahl K, Ahlinder I, Hansson HB, Melander E, Molstad S, Soderstrom M, etal. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: experiences from the south Swedish pneumococcal intervention project. Clin Infect Dis. 1997; 25: 1113–17.
- Gray BM, Converse GM3rd, Dillon HCJr.. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980; 142: 923–33.
- Enheten för öron- näsa- och hals, Institutionen för kliniska vetenskaper, Malmö. Infektioner i mellanörat 1998. Available from: http://www.oron.mas.lu.se/utbildning/infektioner.html [cited 9 March 2011].
- 2007; Sundbyberg: Säve Förlag. Iwarson-Norrby, editor. Infektionsmedicin. 4th ed.
- STRAMA. Antibiotikaförsäljning – Öppenvård. Tabeller med menyval: Alla antibiotikagrupper. 2009. Available from: http://www.strama.se/dyn/,227.html [cited 9 March 2011].
- Cohen R, Bingen E, Varon E, de La Rocque F, Brahimi N, Levy C, etal. Change in nasopharyngeal carriage of Streptococcus pneumoniae resulting from antibiotic therapy for acute otitis media in children. Pediatr Infect Dis J. 1997; 16: 555–60.
- Libson S, Dagan R, Greenberg D, Porat N, Trepler R, Leiberman A, etal. Nasopharyngeal carriage of Streptococcus pneumoniae at the completion of successful antibiotic treatment of acute otitis media predisposes to early clinical recurrence. J Infect Dis. 2005; 191: 1869–75.
- Dagan R , Lipsitch M. , Tuomanen EI , Mitchell TJ , Morrison DA , Spratt BG . Changing the ecology of pneumococci with antibiotics and vaccines. The pneumococcus. 2004; Washington, DC: ASM Press. 283–313.
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000; 31: 578–85.
- Melnyk AH, Wong A, Kassen R. The fitness costs of antibiotic resistance mutations. Evol Appl. 2015; 8: 273–83.
- Trzcinski K, Thompson CM, Gilbey AM, Dowson CG, Lipsitch M. Incremental increase in fitness cost with increased beta-lactam resistance in pneumococci evaluated by competition in an infant rat nasal colonization model. J Infect Dis. 2006; 193: 1296–303.
- Auranen K, Mehtala J, Tanskanen A, S Kaltoft M. Between-strain competition in acquisition and clearance of pneumococcal carriage–epidemiologic evidence from a longitudinal study of day-care children. Am J Epidemiol. 2010; 171: 169–76.
- Feikin DR, Klugman KP, Facklam RR, Zell ER, Schuchat A, Whitney CG. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin Infect Dis. 2005; 41: 481–7.
- Lipsitch M. The rise and fall of antimicrobial resistance. Trends Microbiol. 2001; 9: 438–44.
- Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis. 2002; 8: 347–54.
- Lindahl JF, Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol. 2015; 5: 30048,. doi: http://dx.doi.org/10.3402/iee.v5.30048.
- Arason VA, Gunnlaugsson A, Sigurdsson JA, Erlendsdottir H, Gudmundsson S, Kristinsson KG. Clonal spread of resistant pneumococci despite diminished antimicrobial use. Microb Drug Resist. 2002; 8: 187–92.
- Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013; 3(7): a010215.
- Soares S, Kristinsson KG, Musser JM, Tomasz A. Evidence for the introduction of a multiresistant clone of serotype 6B Streptococcus pneumoniae from Spain to Iceland in the late 1980s. J Infect Dis. 1993; 168: 158–63.
