Abstract
In Sweden, human cases of Puumala hantavirus (PUUV) infections are reported from the northern endemic regions. We found hantavirus-specific antibodies in yellow-necked mice (Apodemus flavicollis) trapped in human dwellings in the surroundings of the cities of Uppsala and Stockholm, which are situated far south from the traditional endemic areas of PUUV. Because the yellow-necked mouse is the most common rodent in human dwellings, hantaviruses in this rodent species may be important for the public health.
The objective of the present study was to investigate the variety of pathogens in wild rodents over-wintering in human dwellings in the surrounding areas of the city of Uppsala () in eastern Sweden. In total, 185 rodents were trapped between October 2009 and February 2010 in houses, garages, and storage areas by the houseowners, using commercial snap-traps. The animals were sampled at the National Veterinary Institute (SVA) in Uppsala, Sweden. The most commonly occurring rodent in anthropogenic buildings was the yellow-necked mouse (Apodemus flavicollis), which constituted 70% of the trapped animals. Only 8.1% of the trapped rodents were bank voles (Myodes glareolus), the natural host for Puumala hantavirus (PUUV), and only 1,6% were field voles (Microtus agrestis), a common host of Tula hantavirus (TULV) in Central Europe (Citation1). Hantavirus-reactive antibodies were detected in 10 of the yellow-necked mice (out of 129, ) by using an in-house ELISA and confirmed as hantavirus-specific by the focus reduction neutralization test (FRNT), the gold standard for hantavirus serology (Citation2). No hantavirus-reactive antibodies were found in the bank voles (n=15) or field voles (n=3). This is the first study showing the occurrence of hantaviruses so far south from its endemic areas in northern Sweden, and also the first observation of hantavirus-specific antibodies in Swedish yellow-necked mice. Furthermore, the study may implicate the existence also of TULV, and/or a new, possibly unknown hantavirus in this area.
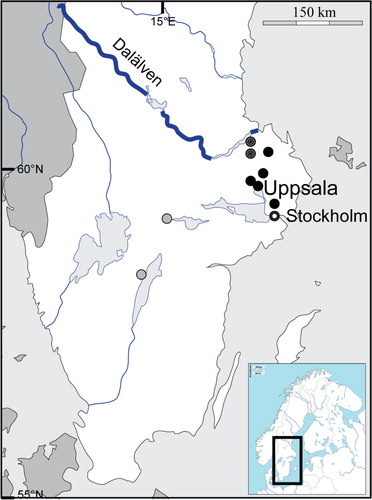
Fig. 1 Map over central/southern Sweden. Black dots indicate the geographic locations for the hantavirus-infected yellow-necked mice; the two striped dots indicate the previously reported southernmost cases of PUUV-infected bank voles in Sweden; the gray dots, Hjo and Örebro, indicate where human PUUV infections have been suspected (Hjo) and confirmed (Örebro).
Background
Much knowledge is missing concerning agents, prevalence, geographic distributions, etc. for the many different zoonotic infections that are spread by small rodents. Hantaviruses are globally distributed enveloped RNA viruses (family Bunyaviridae), carried by rodent or insectivore hosts (Citation3). Hantaviruses are transmitted to humans via aerosols of virus-contaminated excreta (urine, feces, saliva) from chronically infected wild rodents (Citation4). Hantavirus infections in rodents are typically asymptomatic and lifelong (Citation4). As earlier shown for PUUV, hantavirus-infected bank voles can secrete the virus for several months after the infection, and the virus is infective for weeks outside the rodent (Citation3, Citation5) (Citation6). Recently, we showed that the secretion of PUUV from wild bank voles in the wild is sometimes extremely long, probably lifelong (Citation7). Hantaviruses are usually rodent host species-specific, most often infecting only a single rodent or insectivore (Soricomorpha) species, although spillover infections have been described on several occasions (Citation8–Citation14).
PUUV causes a milder form of hemorrhagic fever with renal syndrome (HFRS) named nephropathia epidemica (NE) and is the major causative agent of hantavirus-related clinical cases in Europe (Citation15). Despite its wide distribution in Europe, approximately 90% of all NE cases in Sweden are found in the PUUV-endemic northernmost regions (Citation16). Human cases outside of this region are most often found during the holiday season when southern residents visit more northern latitudes (Citation16). Although the bank vole has been clearly identified as the main reservoir of PUUV, other species have occasionally been found positive for PUUV-specific antibodies in Europe, for example, Apodemus sylvaticus in Belgium and moose in northern Sweden (Citation8, Citation17). TULV, shown to occasionally infect humans but not yet clearly associated to human disease, has never been described in Sweden.
Virus antibody and RNA detection
Analyses were conducted at the Zoonosis Science Centre (ZSC) at the Department of Medical Biochemistry and Microbiology, Uppsala University, and The Public Health Agency of Sweden, Stockholm, Sweden. Rodents were trapped from October 2009 to February 2010 in houses, garages, and storage areas by the house-owners (all were employees at SVA), using commercial snap-traps. The trapped animals were transported whole to SVA in Uppsala, on the morning following the trapping. Internal organs were sampled immediately and separately frozen at −18°C until used. Rodent hearts were vortexed together with 1 ml of PBS and centrifuged. The samples were assumed to have a dilution equivalent to serum dilution of approximately 1:25.
We used an in-house PUUV ELISA, essentially as described earlier (Citation8, Citation18) (Citation19). Briefly, ELISA plates were coated with native PUUV or bac-PUUV N antigens, followed by post-coating (3% BSA in PBS). Samples were diluted 1:16 (equivalent to an approximate final serum dilution of 1:400, see above) and control sera were incubated at a dilution of 1:400, followed by alkaline-phosphatase-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch, Suffolk, UK), and p-nitrophenyl phosphate substrate. The reactions were measured after 30 min at 405 nm. All incubations were for 1 h at 37°C, and the plates were washed four times between each step. Ten out of 185 rodent samples were found clearly positive by the ELISA ().
Table 1 Summary of ELISA and FRNT results
To finally confirm the hantavirus reactivity and specificity, the 10 ELISA-positive yellow-necked mice samples were examined by FRNT against PUUV, Dobrava virus (DOBV), Saaremaa virus (SAAV), Seoul virus (SEOV), and TULV as previously described (Citation2). Briefly, the samples were serially diluted and mixed with diluted viruses containing 30–70 focus forming units/100 µl. Confluent Vero E6 cell monolayers in 6-well tissue culture plates were used and incubated at 37°C for 1 h after addition of the virus/antibody solution. Agarose medium was subsequently added to the wells, and the plates were incubated for 7–10 days, depending on the hantavirus. To indicate virus-infected cells, monkey anti-PUUV polyclonal serum (Citation20), followed by peroxidase-labeled goat antibodies to human IgG (BioRad Laboratories, Hercules, CA), were added. 3, 3’, 5, 5’-Tetramethylbenzidine substrate (Sigma) was used as substrate and foci were enumerated.
Eight of the 25 ELISA-reactive samples were initially found FRNT PUUV-positive at dilution 1:50, whereas two samples (#87 and #183) only neutralized TULV. These 10 FRNT-positive samples, all from Apodemus flavicollis, were subsequently titrated and end-point titers determined ( and ). They were further tested at 1:50 dilution with DOBV, SAAV, and SEOV – the three other hantaviruses known to circulate in Europe. In some cases, a weak cross-reactivity was observed also against SEOV, DOBV, and SAAV. In conclusion, all 10 yellow-necked mice samples showed a clear hantavirus specificity, several with an at least four-fold higher titer to TULV (#87, #89, and #183), or PUUV (#85), whereas the remaining six showed similar titers, or just over the cutoff level (1:50) ( and ).
A hantavirus genus-specific-nested RT-PCR was applied as described earlier (Citation21). All samples showed negative results.
Discussion
This is the first finding of hantavirus-infected rodents so far south of its known endemic area in northern Sweden. To our best knowledge, until now, the southernmost reported occurrence of PUUV in Sweden has been from two bank voles trapped in Munga (outside Tierp, 65 km north of Uppsala) and in Mångelbo (35 km northwest of Uppsala) (Citation22), respectively. In the present study, we detected hanta-infected yellow-necked mice within central Uppsala (5 km south of the Castle in central Uppsala) and in Vallentuna (40 km southeast of Uppsala, 30 km from central Stockholm). In 2003, Olsson and coworkers did a careful study on human hantavirus infections in Sweden (Citation23). Because approximately 90% of the human cases were reported from the four northernmost counties, they focused their study on Norbotten, Västerbotten, Jämtland, and Västernorrland counties. Our present work indicates that a similar study is now justified also in more southern counties: Gävleborg, Uppsala, and Stockholm.
Furthermore, hantavirus-specific antibodies were found in yellow-necked mice, a rodent species not previously reported for PUUV or TULV infections in nature. The yellow-necked mouse is the well-studied natural reservoir for DOBV, causing severe HFRS in southeastern Europe (Citation2, Citation3). However, none of our hantavirus-positive yellow-necked mice showed DOBV-specificity. TULV is carried by Microtus arvalis, M. rossiameridionalis, and M. agrestis in nature (Citation1, Citation3) (Citation24). M. agrestis is the only Microtus vole present in Sweden but never shown to carry TULV. At present, we have no knowledge concerning a potential amplification and transmission of the hantaviruses infecting Apodemus flavicollis in central Sweden, or whether this rodent species is only a dead-end host, that is, does not allow any further virus transmission.
Because aerosols, contaminated by virus-infected bank-vole excreta, are the main source for human infection of hantaviruses, no physical contact between rodents and humans is required for disease transmission (Citation4). Consequently, it may be difficult for both the infected humans and for medical specialists to suspect transmission of hantaviruses, especially if the transmission has occurred outside the known endemic area. Therefore, disseminating information about the occurrence of hantavirus-infected rodents outside its traditional endemic areas has great importance for both medical workers and the general public. This information helps with disease transmission prevention and leads to faster determination of diagnoses by medical specialists.
Our new observations, together with a laboratory-confirmed NE case believed to have been infected in central Uppsala, several suspected NE cases from Hjo (58°18′N 14°17′Ö), and a laboratory-confirmed NE case from Örebro (59°16′26.2′′N 15°12′48.1′′Ö) (our unpublished observations) may indicate that there are still unknown hantaviruses circulating in Sweden, and/or that the PUUV endemic area in Sweden is by far larger than earlier believed, which warrants further extended and detailed analyses. Additional studies have now been initiated, for example, to trace the source of PUUV- and TULV-like infections in Apodemus flavicollis, that is, by investigating the circulation of PUUV in the bank vole population, and search for TULV in field voles, in the Uppsala/Stockholm area, and by further viral genetic studies on hantavirus-infected yellow-necked mice.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Schmidt-Chanasit J, Essbauer S, Petraityte R, Yoshimatsu K, Tackmann K, Conraths FJ, etal. Extensive host sharing of central European Tula virus. J Virol. 2010; 84: 459–74.
- Lundkvist Å, Hukic M, Hörling J, Gilljam M, Nichol S, Niklasson B. Puumala and Dobrava viruses cause hemorrhagic fever with renal syndrome in Bosnia-Herzegovina: evidence of highly cross-neutralizing antibody responses in early patient sera. J Med Virol. 1997; 53: 51–9.
- Vaheri A, Henttonen H, Voutilainen L, Mustonen J, Sironen T, Vapalahti O. Hantavirus infections in Europe and their impact on public health. Rev Med Virol. 2013; 23: 35–49.
- Vapalahti O, Mustonen J, Lundkvist Å, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect Dis. 2003; 3: 653–61.
- Kallio ER, Klingström J, Gustafsson E, Manni T, Vaheri A, Henttonen H, etal. Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. J Gen Virol. 2006; 87: 2127–34.
- Hardestam J, Karlsson M, Falk KI, Olsson G, Klingström J, Lundkvist Å. Puumala hantavirus excretion kinetics in bank voles (Myodes glareolus). Emerg Infect Dis. 2008; 14: 1209.
- Voutilainen L, Sironen T, Tonteri E, Bäck AT, Razzauti M, Karlsson M, etal. Life-long shedding of Puumala hantavirus in wild bank voles (Myodes glareolus). J Gen Virol. 2015; 96(Pt 6): 1238–47. doi: http://dx.doi.org/10.1099/vir.0.000076 [PubMed Abstract].
- Klingström J, Heyman P, Escutenaire S, Sjölander KB, Jaegere FD, Henttonen H, etal. Rodent host specificity of European hantaviruses: evidence of Puumala virus interspecific spillover. J Med Virol. 2002; 68: 581–8.
- Weidmann M, Schmidt P, Vackova M, Krivanec K, Munclinger P, Hufert F. Identification of genetic evidence for Dobrava virus spillover in rodents by nested reverse transcription (RT)-PCR and TaqMan RT-PCR. J Clinl Microbiol. 2005; 43: 808–12.
- Delfraro A, Tomé L, D'Elía G, Clara M, Achával F, Russi JC, etal. Juquitiba-like hantavirus from 2 nonrelated rodent species, Uruguay. Emerg Infect Dis. 2008; 14: 1447.
- Allen LJ, Wesley CL, Owen RD, Goodin DG, Koch D, Jonsson CB, etal. A habitat-based model for the spread of hantavirus between reservoir and spillover species. J Theor Biol. 2009; 260: 510–22.
- Schlegel M, Radosa L, Rosenfeld UM, Schmidt S, Triebenbacher C, Löhr P-W, etal. Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Gene. 2012; 45: 48–55.
- Teixeira BR, Loureiro N, Strecht L, Gentile R, Oliveira RC, Guterres A, etal. Population ecology of hantavirus rodent hosts in Southern Brazil. Am J Trop Med Hyg. 2014; 91: 249–57.
- Stanojevic M, Nikolic V, Stajkovic N, Stamenkovic G, Bozovic B, Cekanac R, etal. Genetic detection of Dobrava-Belgrade hantavirus in the edible dormouse (Glis glis) in central Serbia. Epidemiol Infect. 2015; 143: 400–4.
- Brummer-Korvenkontio M, Vaheri A, Hovi T, Von Bonsdorff C-H, Vuorimies J, Manni T, etal. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980; 141: 131–4.
- Olsson GE, Hjertqvist M, Lundkvist Å, Hörnfeldt B. Predicting high risk for human hantavirus infections, Sweden. Emerg Infect Dis. 2009; 15: 104.
- Ahlm C, Wallin K, Lundkvist A, Elgh F, Juto P, Merza M, etal. Serologic evidence of Puumala virus infection in wild moose in northern Sweden. Am J Trop Med Hyg. 2000; 62: 106–11. [PubMed Abstract].
- Vapalahti O, Lundkvist A, Kallio-Kokko H, Paukku K, Julkunen I, Lankinen H, etal. Antigenic properties and diagnostic potential of Puumala virus nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1996; 34: 119–25. [PubMed Abstract] [PubMed CentralFull Text].
- Verner-Carlsson J, Lõhmus M, Sundström K, Strand TM, Verkerk M, Reusken C, etal. First evidence of Seoul hantavirus in the wild rat population in the Netherlands. Infect Ecol Epidemiol. 2015; 5 27215. doi: http://dx.doi.org/10.3402/iee.v5.27215. eCollection 2015..
- Klingström J, Plyusnin A, Vaheri A, Lundkvist Å. Wild-type Puumala hantavirus infection induces cytokines, C-reactive protein, creatinine, and nitric oxide in cynomolgus macaques. J Virol. 2002; 76: 444–9.
- Klempa B, Fichet-Calvet E, Lecompte E, Auste B, Aniskin V, Meisel H, etal. Hantavirus in African wood mouse, Guinea. Emerg Infect Dis. 2006; 12: 838.
- Nemirov K, Leirs H, Lundkvist Å, Olsson GE. Puumala hantavirus and Myodes glareolus in northern Europe: no evidence of co-divergence between genetic lineages of virus and host. J Gen Virol. 2010; 91: 1262–74.
- Olsson GE, Dalerum F, Hörnfeldt B, Elgh F, Palo TR, Juto P, etal. Human hantavirus infections, Sweden. Emerg Infect Dis. 2003; 9: 1395–401.
- Plyusnin A, Vapalahti O, Lankinen H, Lehväslaiho H, Apekina N, Myasnikov Y, etal. Tula virus: a newly detected hantavirus carried by European common voles. J Virol. 1994; 68: 7833–9. [PubMed Abstract] [PubMed CentralFull Text].
