Abstract
Background
Lyme disease (LD) is a common tick-borne disease in Europe. Diverse factors at various scales determine the spatial distribution of Borrelia burgdorferi infection risk and a better understanding of those factors in a spatially explicit framework is needed for disease management and prevention. While the ecology of ticks and the landscape favoring their abundance have been extensively studied, the environmental conditions favoring an intense contact with susceptible humans, including groups at risk, are sparse. The aim of this study is to assess which individual and environmental factors can favor B. burgdorferi infection in a Belgian group professionally at risk.
Methods
Serological results of 127 veterinarians and farmers enrolled in this study were analyzed, taking into account their municipality of residence. Using binary logistic regression and considering interaction terms, the joint effects of landscape composition and configuration, and forest and wildlife management were examined.
Results
Seven of the 127 workers were seropositive for LD, leading to a seroprevalence of 5.51%. Seropositivity was higher in older persons. The proportion of forest and semi-natural habitats and wetland had a positive impact on LD seroprevalence while arable land–grassland ecotones had a negative one. Our results confirmed the need to consider complex interactions between landscape variables in order to model risk.
Conclusions
Our data show that LD has to be considered as a risk for farmers and veterinarians. Rather than focusing either on ecological aspects of tick and pathogen distribution or on purely epidemiological aspects such as individual risk factors, our model highlights the role of human–environment interactions in LD risk assessment.
Considered the most common tick-borne disease in Europe, Lyme disease (LD) is a multisystemic disease caused by the spirochete Borrelia burgdorferi (Citation1, Citation2) transmitted to humans during an infected tick's blood meal.
Land users at high risk of infection are people exposed to areas potentially infested with infected ticks through professional or recreational activities or through their residential environment (Citation3). LD and exposure to B. burgdorferi has been documented in many occupational groups, including forestry workers, farmers, veterinarians, military recruits, outdoor workers, and also orienteers and scouts (Citation3–Citation8). Vegetation hospitable to ticks is likely the main factor contributing to the high seropositivity in these groups (Citation8).
LD transmission relates to environmental conditions: landscape composition (land cover proportion) and configuration (arrangement) have an impact on tick and host distributions, and therefore, on the infected tick distribution and abundance. The landscape also affects the spatial distribution of human activities and thus contacts between susceptible humans and infected ticks (Citation9). Complex and fragmented landscapes provide more ecotones (transition area between two adjacent biomes) and can increase contacts between species associated with diverse habitats, including reservoir hosts and ticks, and increase accessibility to forests (Citation10, Citation11). Furthermore, landscape fragmentation can influence biodiversity (Citation12). Ticks require blood meal hosts, whose role in tick reproduction and pathogen transmission vary (Citation13, Citation14). Wildlife management for species such as roe deer thus likely affects tick abundance too (Citation15, Citation16). However, most recent studies focused on tick habitat and distribution without considering human-environment interactions, including exposure of humans, or pathogen distribution in ticks or in humans.
LD has been endemic for several decades in many temperate countries, including Belgium, where LD has been reported since 1977 (Citation1). Most cases are reported along a North–South axis, in the provinces Antwerp, Walloon Brabant, Flemish Brabant, Namur, and Luxemburg (Citation15, Citation17). The incidence of LD is high in Belgian municipalities, having large forest-settlement interfaces in wealthy peri-urban areas (Citation15). Concerns have thus arisen regarding LD but few studies investigated seroprevalence (Citation1, Citation17).
In this context, there is a need for a better understanding of B. burgdorferi transmission in a spatially explicit framework and of factors that influence infection presence. Studying the link between the seroprevalence of B. burgdorferi and the environment frequented by people is of public health interest, also because ticks are capable of transmitting several pathogens to humans and livestock.
The aim of this study was to use an integrated landscape approach to determine individual and environmental factors associated with the seroprevalence of B. burgdorferi in a group professionally at risk, Belgian veterinarians and farmers.
Methods
Survey design and laboratory procedures
The survey protocol was detailed by De Keukeleire et al. (Citation18). Briefly, a survey was conducted in November 2011 in five sampling sites in Belgium (Mons, Liege, Libramont, Ciney, and Gembloux). Volunteers comprised veterinarians, farmers, hunters, and gamekeepers. All participants completed a questionnaire covering socio-demographic and economic characteristics, as well as items related to their potential clinical history of zoonotic diseases. Blood samples were then taken by a physician in order to test for the presence of anti-Borrelia IgG antibodies.
A total of 148 workers took part in the survey, representing a participation rate of about 80%. Individuals who did not communicate their profession and those who could not be accurately geolocated during their professional activities, such as hunters and gamekeepers, were excluded from this analysis. Therefore, this study reports on 127 workers.
The detection of Borrelia-specific antibodies was performed with the LIAISON® Borrelia IgG assay on the Liaison XL instrument (DiaSorin S.p.A., Saluggia, Italy) according to the manufacturer's recommendations. In brief, DiaSorin-based chemiluminescence immunoassay uses VlsE recombinant antigen, which plays an important role in the immune response to Borrelia infection, to detect specific IgG. Following manufacturer recommendations, IgG ≤ 16 UI were considered negative, IgG>22 UI were positive, and the remaining values were borderline. The ELISA test showed a high sensitivity (100%) with a good specificity (90%) for the detection of Borrelia infection.
The study protocol was approved by the Ethics Committee of Université catholique de Louvain Medical School, Belgian Registry N° B40320096360. All participants signed an informed consent.
Data set
Veterinarians and famers are exposed to tick bites during their professional activities (Citation6). Farmers were assumed to be exposed close to their farms and in their pastures. Veterinarians were assumed to visit farms and pastures located in various municipalities around their resident municipalities. The municipality level was therefore the most representative of the two sub-groups: all independent variables were calculated at residence municipality level.
The Wallonia land cover map (Carte d'occupation du sol de Wallonie (2007) from the ‘Service public de Wallonie’ (Copyright-SPW-n°140225-1407)) was used to measure landscape composition and configuration in each municipality. The proportions of artificial land, forest and semi-natural habitats, arable land, grassland, and permanent crops and wetland were calculated for each municipality.
Landscape configuration focused on an aggregated forest class (forest and semi-natural habitats) as a key tick habitat: edge density (sum of the lengths of all edges around forests patches divided by the total landscape area), patch density (number of forest patches divided by the total landscape area), and area-weighted mean shape index of patches and area-weighted mean patch fractal dimension (which measures shape complexity by comparing patches to a square) were examined. All indices were calculated using Patch Analyst in ArcGIS.
Farmers and veterinarians frequent grassland, so we investigated the role of pasture ecotones using the proportion of specific edge type (i.e. arable land, artificial land, and agricultural building) in 2-m ecotones around grassland in the municipality (sum of area of the adjacent land cover type in a 2-m buffer around grassland divided by the total area of this buffer).
Estimates of red deer, roe deer, and wild boar by forest management unit (cantonnement) were retrieved from the Department of Hunting and Fishing of Wallonia. Because we do not know the date of infection, we considered the mean game density over the 2002–2011 period. As in previous work (Citation4, Citation16) and using dasymetric mapping (Citation19) and equation 1, an estimation of the density of red deer, roe deer, and wild boar at the municipality level was obtained. The density of these animals shot by municipality was also calculated.
All environmental variables are listed in . As we focused on professions linked to rural areas, we added categorical variable coding for densely populated areas, intermediate zones, and sparsely populated areas, according to the concept of Eurostat (Citation20).
Table 1 Summary statistics of environmental covariables
Data analysis
Because farmers and veterinarians are nested within municipalities, they share some characteristics linked to their environments. Therefore, to determine the relevance of using a multilevel model, we first checked the Intraclass Correlation Coefficient (ICC). According to the within-group variance, which was higher than the between-group variance (ICC = 0), observations within groups were not more similar than observations from different groups, and it was unnecessary to account for the nested structure of the data.
Since our dependent variable was binary (infected, yes/no), we applied binary logistic regression (Citation4). Briefly, variables were standardized to avoid scale dependence. Then, all variables with a univariate p<0.20 were selected together with their first-level interactions. When two terms were strongly correlated (r>0.90 or VIF>10), one was eliminated to avoid multicollinearity.
Afterwards, interaction terms with a p>0.10 were considered candidates for elimination. Using the Schwartz criterion and the chi-square likelihood ratio test (LRT) with a p>0.10, they were removed if there was no loss in likelihood. We worked with groups of terms to avoid false-positive results. The same procedure was used with the main terms, if not involved in a remaining interaction term, in order to keep hierarchical models. The procedure was stopped when all remaining covariates significantly contributed to likelihood, either because of an LRT with a p<0.10 or because of an increase in Schwartz criterion.
For model validation, risk scores were calculated for each person based on the estimated β coefficients. Distinguishing the negative, positive, and borderline results, the distributions of risk scores were visualized using boxplots. The discriminant power of our model was assessed using the area under the receiver operating characteristic (ROC) curve (AUC).
Results
Sample description
Most included volunteers were men (14% women) (). Age distribution was 48±11 years (range: 25–72 years). There were 96 veterinarians (76% of the sample) and 31 farmers but 7 veterinarians reported farming as well. Focusing on the landscape with which people have had contact during their professional activities, these seven veterinarians were considered as veterinarians in subsequent analyses. More than 61% of veterinarians (n=59) were specialized in livestock, 27% (n=26) were veterinarians for livestock/pets, and five were administrators.
Table 2 Stratified seroprevalence of Borrelia burgdorferi antibodies in veterinarians and farmers
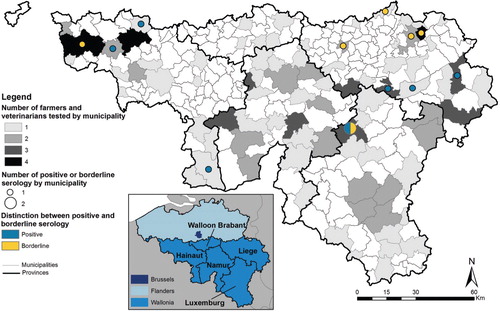
Participants were from 84 municipalities: 34.5% of municipalities counted over one worker. Almost three-quarters originated from the provinces of Liege, Luxemburg, and Namur, including, respectively, 29.1% (n=37), 20.5% (n=26), and 26.0% (n=33) of volunteers. 20.5% (n=26) were from Hainaut and 3.9% (n=5) from Walloon Brabant, which reflects the spatial distribution of farmers, veterinarians, and farms in this province (Citation21, Citation22).
Clinical history
Eleven workers reported a history of LD (8.7%) and 6 reported diagnosis with erythema migrans (EM). Two of those 11 declared a recent fever (preceding week).
Seroprevalence
Seven out of 127 were seropositive for Borrelia (seroprevalence 5.5%) (). Considering borderline serological results (n=6) as positive, seroprevalence amounted to 10.2%.
No women who participated in the survey (n=18) were seropositive. Seroprevalence increased with age: seropositivity reached 8.9% for 50- to 59-year-old participants and 10% for ≥ 60 years of age.
Seropositive individuals were mainly from Hainaut or Liege (). With three seropositive individuals, Hainaut had a seroprevalence of 11.5%, whereas Liege and Luxemburg were, respectively, estimated at 8.1% (3 seropositive individuals) and 3.8% (one seropositive). One borderline result was detected in Hainaut and Luxemburg and four in Liege. No seropositive or borderline tests were located in Namur or Walloon Brabant.
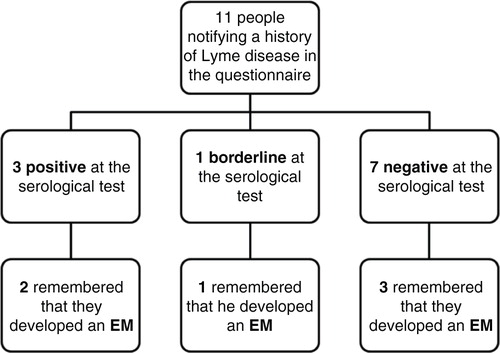
Among the 11 people who were reported previously with Lyme infection, 3 were positive at the serological test and 1 was borderline.
Factors influencing the seroprevalence of B. burgdorferi
The results of univariate and multivariate regression are presented in . Age, proportion of forest and semi-natural habitats (PFS), and proportion of wetland (PW) were associated with increased risk and edge proportion grassland–arable land (EPGA) was associated with decreased B. burgdorferi seroprevalence.
Table 3 Multivariate logistic regression
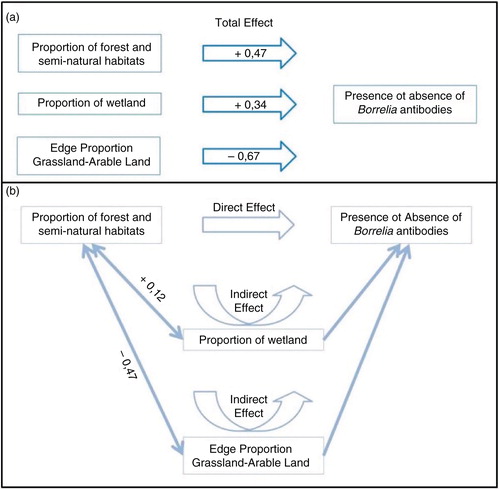
As PFS is in interaction with both PW and EPGA, the effect of PFS on the risk of being seropositive is composed by a direct effect and two indirect effects (). Using this approach, we can attest that an increase of PFS relates to ():
a direct increase of the risk of being seropositive (according to the coefficient in univariate regression)
an increase of PW and so an indirect increase of the risk of being seropositive (PFS and PW are positively correlated and PW has a positive direct effect on the risk);
a decrease of EPGA and so an indirect increase of the risk of being seropositive (PFS and EPGA are negatively correlated and EPGA has a negative direct effect on the risk).
Therefore, the global impact of PFS on the risk is positive.
Fig. 2 Total effects of the proportion of forest and semi-natural habitats, the proportion of wetland, and the edge proportion grassland–arable land on the presence or absence of Borrelia infection (without mediation) (a) and the impact of the proportion of forest and semi-natural habitats on the presence or absence of Borrelia infection (b).
Using multivariate logit regression, where all variables are expressed as number of standard deviation from the mean, the estimated equation for logit risk score was:2
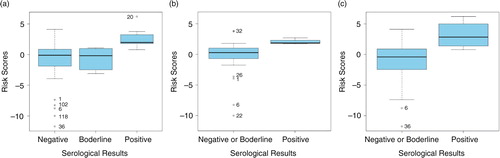
The logit risk score (equation 2) of all 127 participants is illustrated using boxplots (). a shows that negative and borderline sera had lower scores than positive sera. Therefore, our model based on personal and environmental conditions had good predictive power. The distribution of risk scores for borderline sera was close to the distribution of negative ones. Therefore, a borderline serology may be considered as a negative result. Our model was able to discriminate between positive and negative and/or borderline sera for farmers (b) as well as for veterinarians (c).
Fig. 3 Boxplots of logit risk scores derived from the estimated equation in all participants, regardless of professions (a), in farmers (b), and in veterinarians (c).
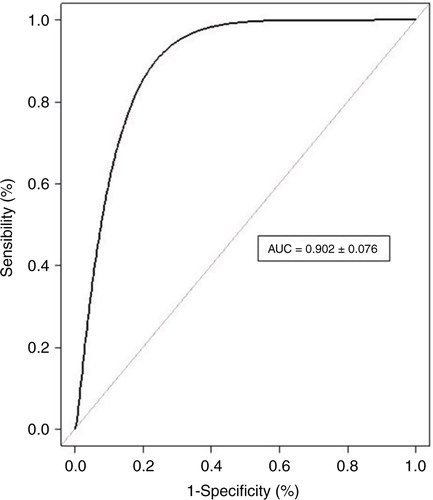
Performance of the score is also represented by the ROC curve in for all 127 volunteers. AUC indicates an accuracy of 90.2% (82.6–97.8%; 95% CI) of the score, predicting positive, negative, or borderline sera.
Discussion
Sample
In this study, we enrolled a group of land users at high risk of exposure to tick bites and in close contact with livestock: veterinarians and farmers (Citation3, Citation8). Consequently, this study is one of the first to analyze sera from subjects exposed to LD risk in Belgium and permits a contemporary insight into Borrelia seroprevalence in individuals professionally at risk.
Our sample is representative because there was no link between seroprevalence and recruitment. Participants were recruited during annual meetings of professional associations of veterinarians and farmers achieving a participation rate of about 80%. No spatial bias between responders and non-responders was observed. The sample of 127 volunteers is fairly limited. However, reaching out to at-risk professionals to encourage them to participate in studies is challenging, and this number should therefore be considered a success in recruiting participants.
Clinical history
Among those 11 people notifying a history of LD in the questionnaire, 3 were positive at the serological test and 1 was borderline (). Three of those four people recalled an EM (two seropositive and one borderline) but EM was also reported by three seronegative workers. This low serological performance is well known in early stages of LD. Four other volunteers out of the 11 people notifying Lyme infections were also reported as seronegative. We neither have clinical data nor the date of LD occurrence. Knowing that anti-Borrelia antibodies can persist but decline over months and years in the body, we can hypothesize that such antibodies would have already disappeared. Moreover, if EM is the only manifestation of LD, some patients treated at this early disease stage may never seroconvert (Citation2, Citation23).
Seroprevalence
The seroprevalence for Borrelia IgG was 5.5% for farmers and veterinarians. Comparison with other study results must be done with care due to possible differences in the specificity and positivity thresholds. For agricultural and forestry workers, the seroprevalence varies from 5.4 to 19.3% in neighboring countries (France, Italy, Germany, and the Netherlands) (Citation7, Citation24) (Citation25).
We previously compared the seroprevalence obtained for veterinarians and farmers to two control groups of blood donors (one of rural and one of urban blood donors). De Keukeleire et al. (Citation18) can attest that veterinarians and farmers were more exposed to B. burgdorferi antigens than the general population because antibodies against B. burgdorferi were less often recorded in the overall population: 3.1 and 4.3% for urban and rural blood donors, respectively. In Germany and Spain, prevalence of antibodies to B. burgdorferi in the general population was similar, 4.8 and 3.4%, respectively (Citation26, Citation27). Seroprevalence in farmers (9.68%) is higher than in veterinarians (4.17%). An explanation may be that farmers are more frequently out in natural environments, where they can be at risk of tick bites. In other European countries (UK and Italy), the data reported on farmers are similar to our results: 10.1 to 14.4% (Citation6).
Our data showed that seropositive individuals were found in the provinces of Hainaut, Liege, and Luxemburg. This distribution is in line with reported tick collections in Belgium. In 2009 and 2010, Ixodes ricinus ticks were found in all 10 provinces of Belgium and, in Wallonia, the sampled tick abundance was higher in Namur (7.3–22.1 nymphs/100 m2), Hainaut (2.8–25.0 nymphs/100 m2), and Luxemburg (12.1–17.2 nymphs/100 m2) (Citation16). However, our distribution differs from the spatial distribution of Borrelia-positive results of laboratory tests reported by a sentinel network of laboratories in 2011 and 2012, coordinated by the Scientific Institute of Public Health (ISP) (Citation28): those data indicate high incidence of B. burgdorferi–positive results in the east part of the provinces of Antwerp and Flemish Brabant (not covered in this study) and in the province of Walloon Brabant and Luxemburg. But those data differ from ours because they are spatially linked to the place of residence which does not always reflect the place of infection. If we compare to a sero-epidemiological study of patients diagnosed at different stages of LD, most patients were infected in the central and south-eastern part of Belgium, as suggested by our results (Citation1).
In Belgium, LD is due to at least five species: Borrelia afzelii, B. garinii, B. burgdorderi sensu stricto (ss), B. spielmanii, and B. valaisiana (Citation29, Citation30). A prior study indicated the most prevalent genospecies in Belgian ticks was B. garinii (53% of infected ticks), followed by B. burgdoreferi ss (38%) and B. afzelii (9%) (Citation31). A subsequent study indicated the dominance of B. garinii (54% of infected ticks), followed by B. valaisiana (27%), B. burgdoreferi ss and B. afzelii (9%) (Citation32). Another study showed that some genospecies of B. burgdorferi s.l. are structured ecologically into clusters that are host specific: B. afzelii is more adapted to mammalian hosts, whereas B. burgdorferi ss appear to be less specialized (Citation33). These host associations are likely to have an impact on the geographical population structure of the genospecies. This may lead to diverse clinical manifestations in different regions but could not be assessed through our data.
Presence of antibodies is not necessarily associated with symptoms, and it is not known how long antibodies persist (Citation1, Citation34). It is therefore impossible to distinguish past and newly acquired infections based solely on serology (Citation2). Nevertheless, given that the survey was conducted in November and that ticks are generally active from March to October in Belgium (Citation16), the chances of encountering newly acquired infections are limited. Moreover, results cannot be extended to the general population as veterinarians and farmers usually have a higher level of knowledge about ticks and tick-borne diseases (TBDs) and are likely to protect themselves or limit contacts with ticks.
Consequently, our study shows that veterinarians and farmers constitute a population at risk. Even if human infection by B. burgdorferi does not necessarily lead to symptoms, LD has to be considered as a risk for farmers and veterinarians.
Factors influencing the seroprevalence of B. burgdorferi
Our results highlight three major elements associated with the seroprevalence of B. burgdorferi in individuals professionally at risk.
First, landscape composition is associated with the seroprevalence of B. burgdorferi. The PFS has a positive impact on the risk of being seropositive, as shown by univariate regression. This positive link has previously been published (Citation8, Citation15) (Citation16, Citation35). A positive impact of wetland on the risk was also found. Because of the low desiccation resistance of ticks (Citation36), the suitability of an area for ticks depends on environmental variables such as humidity and land cover type. Wetlands do not strictly constitute a suitable land cover type for ticks, but they can provide humidity to surrounding environments and make them more favorable for ticks.
Second, the seroprevalence of B. burgdorferi is influenced by the landscape configuration. Our model showed the negative effect of ecotone between arable land and grasslands on the risk of seropositivity. Arable land is considered the most unfavorable agricultural habitat for ticks because of drastic changes in plant cover and soil management throughout a year (Citation4, Citation37). But we know that the occurrence of ticks is influenced by the neighboring habitat (Citation38): a contact between grassland and woodland is more favorable than a contact between grassland and arable lands. Therefore, the presence of unfavorable environments near pastures decreases the risk of infection by tick bites for veterinarians and farmers.
Beyond their direct relation to tick habitat suitability and bite exposure, these variables also reflected, as indicated by their interaction with PFS, that they are associated to favorable or unfavorable landscapes; wetlands are more present in natural landscapes (more suitable for ticks) and arable land is dominant in cultivated landscapes (less favorable to ticks). Our results showed that the impact of the proportion of forest and semi-natural habitats is modulated by other variables. This suggests that landscape suitability, for ticks and for tick-borne pathogen circulation, is determined by combinations of landscape factors, rather than the presence of tick suitable habitat. The suitability of a landscape for disease transmission also depends on human–vector contact, host presence, and interactions, all of which are affected by landscape composition configuration and combination. We ascertain that, when assessing environment risk factors, landscape has to be analyzed using a more complex approach instead of using single-class perspective (Citation39).
Third, our results indicated that B. burgdorferi antibodies are more frequent among men (Citation27, Citation40) and as they age (Citation25, Citation40) (Citation41). As we do not know how persistent anti-Borrelia antibodies are in the body, the age distribution for seropositivity may reflect the population's cumulative exposure to B. burgdorferi.
Due to the unknown persistence of antibodies (Citation1, Citation34), linking landscape factors to seroprevalence asks the question of the temporality of tick bites as we have to situate the infection in the right landscape. Errors could occur in our study in relation to landscape change or occupation change for the participants. However, the Belgian landscape is stable, in relation to a strict framework defining land use. Veterinarians and farmers do not change their employment regularly. It is conceivable that volunteers contracted tick bites during leisure time, but this seems unlikely.
Conclusions
Our study was one of the first that linked B. burgdorferi seroprevalence in a Belgian group professionally at risk with environmental factors. The objective was to determine individual and environmental factors associated with the seroprevalence of B. burgdorferi in Belgian veterinarians and farmers, and we showed that both individual and environmental factors were associated with the seroprevalence of B. burgdorferi. As reported in other studies, our study also showed that the risk of being seropositive to B. burgdorferi was higher in older persons. Our results also highlighted the impact of landscape and the positive impact of the proportion of forest and semi-natural habitats. Moreover, we showed that interactions existing between landscape variables need to be considered in modeling. Our results may be extrapolated to other tick-borne diseases and other professionals at risk of contact with ticks.
Our study showed that veterinarians and farmers constitute a population at risk. Therefore, we want to underline the importance of studying B. burgdorferi serporevalence and relate individuals to their environment in other exposed populations to have a comprehensive picture of factors affecting the risk of LD. This picture will help decision-makers to target high-risk areas or populations for public health policies.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Université catholique de Louvain Medical School, Belgian Registry N° B40320096360. All participants signed informed consent.
Authors’ contributions
MDK derived land-cover variables, performed the statistical analyses, interpreted the results, and wrote the first draft of the manuscript. AR conceptualized and designed the study, oversaw the data analysis and interpretation, and corrected the article. BK carried out the laboratory analysis of blood samples and helped in interpretation of results. ED helped organizing the collection of data. VL organized, supervised, and participated in the collection of blood samples. SOV conceptualized and designed the study, supported the statistical analysis, read back, and critically reviewed and revised the manuscript drafts. All authors read and approved the final manuscript.
Conflict of interest and funding
No competing financial interests exist. This study was kindly supported by Special Research Fund (FSR) grants of the Université catholique de Louvain.
Acknowledgements
We also thank UCL/Rily Emporiatrie ASBL for scientific and technical support. We are very grateful to all participating veterinarians and farmers and to the ‘Association Régionale de Santé et d'Identifications Animale’ (ARSIA) through which we had contacts with those professionals at risk. Moreover, the authors thank the ‘Service public de Wallonie’ for providing the land cover map (Carte d'occupation du sol de Wallonie (COSW)).
References
- Bigaignon G, Tomasi JP, Goubau P, Martin P, Pierard D, Sindic CJ, etal. A clinical and sero-epidemiological study of 190 Belgian patients suffering from Lyme borreliosis. Acta Clin Belg. 1989; 44: 174–81.
- Borchers AT, Keen CL, Huntley AC, Gershwin ME. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015; 57: 82–115.
- Lindgren E, Jaenson TGT. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. 2006; Copenhagen, Denmark: World Health Organization Regional Office for Europe. EUR/04/5046250.
- De Keukeleire M, Vanwambeke SO, Somassè E, Kabamba B, Luyasu V, Robert A. Scouts, forests, and ticks: impact of landscapes on human-tick contacts. Ticks Tick Borne Dis. 2015; 6: 636–44.
- Lakos A, Igari Z, Solymosi N. Recent lesson from a clinical and seroepidemiological survey: low positive predictive value of Borrelia burgdorferi antibody testing in a high risk population. Adv Med Sci. 2012; 57: 356–63.
- Piacentino JD, Schwartz BS. Occupational risk of Lyme disease: an epidemiological review. Occup Environ Med. 2002; 59: 75–84.
- Tomao P, Ciceroni L, D'Ovidio MC, De Rosa M, Vonesch N, Iavicoli S, etal. Prevalence and incidence of antibodies to Borrelia burgdorferi and to tick-borne encephalitis virus in agricultural and forestry workers from Tuscany, Italy. Eur J Clin Microbiol Infect Dis. 2005; 24: 457–63.
- Pfäffle M, Littwin N, Muders SV, Petney TN. The ecology of tick-borne diseases. Int J Parasitol. 2013; 43: 1059–77.
- Méha C. Influence of forest landscape structures on human populations’ exposure to ticks. The case of Lyme Borreliosis in senart forest. Bull Assoc Geogr Fr. 2012; 89: 255–66.
- Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr. 2010; 9: 54.
- Zeimes CB, Olsson GE, Hjertqvist M, Vanwambeke SO. Shaping zoonosis risk: landscape ecology vs. landscape attractiveness for people, the case of tick-borne encephalitis in Sweden. Parasit Vectors. 2014; 7: 370.
- Fahrig L. Effects of habitat fragmentation on biodiversity. Annu Review Ecol Evol Syst. 2003; 34: 487–515.
- Killilea ME, Swei A, Lane RS, Briggs CJ, Ostfeld RS. Spatial dynamics of Lyme disease: a review. EcoHealth. 2008; 5: 167–95.
- Vu Hai V, Almeras L, Socolovschi C, Raoult D, Parola P, Pagès F. Monitoring human tick-borne disease risk and tick bite exposure in Europe: available tools and promising future methods. Ticks Tick Borne Dis. 2014; 5: 607–19.
- Linard C, Lamarque P, Heyman P, Ducoffre G, Luyasu V, Tersago K, etal. Determinants of the geographic distribution of Puumala virus and Lyme borreliosis infections in Belgium. Int J Health Geogr. 2007; 6: 15.
- Li S, Heyman P, Cochez C, Simons L, Vanwambeke SO. A multi-level analysis of the relationship between environmental factors and questing Ixodes ricinus dynamics in Belgium. Parasit Vectors. 2012; 5: 149.
- Bleyenheuft C, Lernout T, Berger N, Rebolledo J, Leroy M, Robert A, etal. Epidemiological situation of Lyme borreliosis in Belgium, 2003 to 2012. Arch Public Health. 2015; 73: 33.
- De Keukeleire M, Vanwambeke SO, Cochez C, Heyman P, Fretin D, Deneys V, etal. Vector-Borne and Zoonotic Diseases. 2016, doi: http://dx.doi.org/10.1089/vbz.2016.1954 [Epub ahead of print].
- Mennis J. Generating surface models of population using dasymetric mapping. Prof Geogr. 2003; 55: 31–42.
- SPF Economie. DG Statistique et Information économique: Typologie des communes selon 2 concepts différents. OCDE et EUROSTAT. Available from: http://statbel.fgov.be/fr/statistiques/chiffres/environnement/geo/typologie_communes/ [cited 15 January 2016].
- Delbrouck B, Tchinda C, Service d’études UCM. Statistiques Indépendants et Professions libérales 2011 – Focus sur la pénurie des médecins vétérinaires et des médecins Unplib. Brussels: UCM edition . 2011; 45.
- Agriculture - Chiffres agricoles de 2011. Direction Générale Statistique et Information Économique (DGSIE), Brussels. 2012
- Aguero-Rosenfeld ME, Nowakowski J, McKenna DF, Carbonaro CA, Wormser GP. Serodiagnosis in early Lyme disease. J Clin Microbiol. 1993; 31: 3090–5.
- Richard S, Oppliger A. Zoonotic occupational diseases in forestry workers – Lyme borreliosis, tularemia and leptospirosis in Europe. Ann Agric Environ Med. 2015; 22: 43–50.
- Thorin C, Rigaud E, Capek I, André-Fontaine G, Oster B, Gastinger G, etal. Seroprevalence of Lyme Borreliosis and tick-borne encephalitis in workers at risk, in Eastern France. Méd Mal Infect. 2008; 38: 533–42.
- Dehnert M, Fingerle V, Klier C, Talaska T, Schlaud M, Krause G, etal. Seropositivity of Lyme borreliosis and associated risk factors: a population-based study in children and adolescents in Germany (KiGGS). PLoS One. 2012; 7: e41321.
- Lledó L, Gegúndez MI, Saz JV, Beltrán M. Screening of the prevalence of antibodies to Borrelia burgdorferi in Madrid province, Spain. Eur J Epidemiol. 2004; 19: 471–2.
- Rebolledo J, Hammadi S, Van Beckhoven D. Zoonoses et maladies à transmission vectorielle – Surveillance épidémiologique en Flandre, en Wallonie, en Région de Bruxelles-Capitale et en Belgique, 2011 et 2012. Bruxelles: Institut scientifique de Santé publique (ISP) . 2015; 101.
- Obsomer V, Wirtgen M, Linden A, Claerebout E, Heyman P, Heylen D, etal. Spatial disaggregation of tick occurrence and ecology at a local scale as a preliminary step for spatial surveillance of tick-borne diseases: general framework and health implications in Belgium. Parasit Vectors. 2013; 6: 190.
- Kesteman T, Rossi C, Bastien P, Brouillard J, Avesani V, Olive N, etal. Prevalence and genetic heterogeneity of Borreliaburgdorferi sensu lato in Ixodes ricinus in Belgium. Acta Clin Belg. 2010; 65: 319–22.
- Misonne M-C, Van Impe G, Hoet PP. Genetic heterogeneity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in Belgium. J Clin Microbiol. 1998; 36: 3352–4.
- Badalamenti J. Prévalence et diversité génétique de Borrelia burgdorferi sensu lato chez Ixodes ricinus et étude des facteurs environnementaux influençant sa distribution en Belgique. 2015; Université catholique de Louvain: Unpublished thesis.
- Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell H-S, Brade V, etal. Host association of Borrelia burgdorferi sensu lato – the key role of host complement. Trends Microbiol. 2002; 10: 74–9.
- Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis. 2001; 33: 780–5.
- Lindström A, Jaenson TGT. Distribution of the common tick, Ixodes ricinus (Acari: Ixodidae), in different vegetation types in southern Sweden. J Medical Entomol. 2003; 40: 375–8.
- Gern L, Morán Cadenas F, Burri C. Influence of some climatic factors on Ixodes ricinus ticks studied along altitudinal gradients in two geographic regions in Switzerland. Int J Med Microbiol. 2008; 298: 55–9.
- Mierzejewska EJ, Alsarraf M, Behnke JM, Bajer A. The effect of changes in agricultural practices on the density of Dermacentor reticulatus ticks. Vet Parasitol. 2015; 211: 259–65.
- Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, etal. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013; 6: 1.
- Vanwambeke S, Van doninck J, Artois J, Davidson RK, Meyfroidt P, Jore S. Forest classes and tree cover gradient: tick habitat in encroached areas of southern Norway. Exp Appl Acarol. 2016; 68: 375–85.
- Wilking H, Fingerle V, Klier C, Thamm M, Stark K. Antibodies against Borrelia burgdorferi sensu lato among Adults, Germany, 2008–2011. Emerg Infect Dis. 2015; 21: 107–10.
- Hubálek Z, Lipsker D, Jaulhac B. Epidemiology of Lyme Borreliosis. Current problems in dermatology . 2009; 31–50. Basel: Karger; Vol. 37.