Abstract
Background
The annual incidence of a small indeterminate pulmonary nodule (IPN) on computed tomography (CT) scan remains high. While traditional paradigms exist, the integration of new technologies into these diagnostic and treatment algorithms can result in alternative, potentially more efficient methods of managing these findings.
Methods
We report on an alternative diagnostic and therapeutic strategy for the management of an IPN. This approach combines electromagnetic navigational bronchoscopy (ENB) with an updated approach to placement of a pleural dye marker. This technique lends itself to a minimally invasive wedge resection via either video-assisted thoracoscopic surgery (VATS) or a robotic approach.
Results
Subsequent to alterations in the procedure, a cohort of 22 patients with an IPN was reviewed. Navigation was possible in 21 out of 22 patients with one patient excluded based on airway anatomy. The remaining 21 patients underwent ENB with pleural dye marking followed by minimally invasive wedge resection. The median size of the nodules was 13.4 mm (range: 7–29). There were no complications from the ENB procedure. Indigo carmine dye was used in ten patients. Methylene blue was used in the remaining 11 patients. In 81% of cases, the visceral pleural marker was visible at the time of surgery. In one patient, there was diffuse staining of the parietal pleura. In three additional patients, no dye was identified within the hemithorax. In all cases where dye marker was present on the visceral pleural surface, it was in proximity to the IPN and part of the excised specimen.
Conclusions
ENB with pleural dye marking can provide a safe and effective method to localize an IPN and can allow for subsequent minimally invasive resection. Depending on the characteristics and location of the nodule, this method may allow more rapid identification intraoperatively.
As a consequence of increased utilization of computed tomography (CT) scans of the chest, every year thousands of patients are discovered to have an indeterminate pulmonary nodule (IPN) (Citation1). Recently, the National Lung Cancer Screening trial demonstrated a relative risk reduction in lung cancer death of 20% in patients at high risk of lung cancer who were randomized to three serial low-dose CT scans compared to single posterior–anterior chest radiography. It is important to note that of those patients with positive screening results, 96.4% were ultimately found to be false positives (Citation2). CT scanning of the chest is used to interrogate a variety of pulmonary related complaints including shortness of breath and chest pain. As a result, numerous asymptomatic IPNs are discovered annually. In addition, CT scanning is often used as a tool for surveillance imaging in patients with a history of prior malignancies. While there are effective paradigms for the management of these nodules, the advent of new technologies may alter these approaches and result in new treatment algorithms to obtain an accurate diagnosis in a minimally invasive fashion.
The current approaches in the evaluation of an IPN include a variety of different methods from continued surveillance imaging, as well as procedural approaches to obtain tissue for a diagnosis. The options for biopsy include CT-guided transthoracic needle aspiration (TTNA), standard flexible bronchoscopy (FOB) with transbronchial biopsies (TBBx), and surgical resection (Citation3). A variety of factors are accounted for when determining the optimal investigational approach for each patient. The modality selected to diagnose a suspected lung cancer is often based on the size and location of the primary lesion in the lung, the presence of potential metastatic spread, and the anticipated treatment plan (Citation4). While each approach has its benefits as well as its limitations, a combined approach that utilizes the advantages of different modalities might result in a more efficient method in the management of IPNs. Such an approach might ensure a higher likelihood of accurately diagnosing both benign and malignant disease processes and improve the selection of appropriate patients for resection. To date, several techniques have been described to localize IPNs for operative excision (Citation5–Citation8). The vast majority of these approaches use a percutaneous technique, wherein pleural markers, wires, coils or radioactive tracers are placed using CT guidance. These approaches are typically followed by a video-assisted thoracoscopic surgery (VATS) exploration to identify the IPN that is then followed by resection. An additional option utilizes CT-fluoroscopy-guided bronchoscopy in conjunction with Teflon coated catheter followed by dye marking of the pleural surface. The IPNs were not biopsied during these approaches for several reasons and VATS was still performed (Citation8).
Herein, we describe a novel modality using an endobronchial approach that combines electromagnetic navigational bronchoscopy (ENB) with dye marker placement, and in this application done in conjunction with minimally invasive wedge resection as an alternative approach to managing IPNs. This provides a means to accurately diagnose and potentially treat IPNs while minimizing risk and invasiveness. Being able to visualize the dye marker during operation may allow the surgeon to localize the nodule with greater ease and thus potentially allow procedures to be completed in a minimally invasive fashion. We initially reported on this technique in abstract form several years ago (Citation9). While this approach demonstrated potential, there were several difficulties. These included soiling of the pleural space with the dye as well as communication issues with respect to the physician placing the marker and the physician performing the resection in the setting where these were not the same people. These technical issues have been addressed with the new technique and subsequent results described. The goal was to facilitate the diagnosis of the IPN as well as to improve the efficiency of the procedure by decreasing the time associated with the resection and minimize the morbidity by decreasing the conversion rate to open thoracotomy.
Patients and methods
This is a retrospective, multi-institutional study to review the safety and results of ENB dye marker placement followed by minimally invasive wedge resection given the alterations in the technique. Specifically, these alterations included a tenting technique whereby the subpleural space was ‘tented’ with the locatable guide (LG) and extended working channel (EWC) under fluoroscopy to enhance localization, described below, and, in the instances where the physicians placing the marker and doing the resection were not the same, communication issues and directed review of the site of placement utilizing the navigational software was conducted. The procedures were completed at four different institutions. Each institution's IRB approved this study. Individual patient informed consent was obtained where appropriate. The technique of dye marker placement is described in the following paragraph.
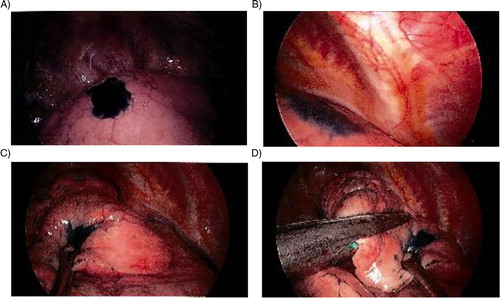
ENB (SuperDimension, Plymouth, MN) was performed under general anesthesia or monitored anesthesia care with sedation. A therapeutic bronchoscope (Olympus BF 1T180) in conjunction with the navigational system was used in accordance with the manufacturer's instructions and as described in the literature (Citation10). Occasionally, proximity to the target IPN was confirmed by passing an ultrasound miniprobe (Olympus UM-20-S20) through the EWC. If biopsies were performed, these were done prior to dye marking and submitted for pathologic examination. Once the target lesion was reached, the LG and EWC were advanced using fluoroscopy to approximate the visceral pleural surface. This allowed for the most direct route from the lesion to the visceral pleural surface The LG has a blunt tip and is able to ‘tent’ the pleural surface when advanced under fluoroscopic visualization. The EWC was then locked at this position and a 25-gauge sclerotherapy needle replaced the LG. Approximately 0.25 ml of dye was then injected at this site. Again, either indigo carmine dye or methylene blue was utilized for dye marker placement. Both have been found to be safe and effective in humans and have utility in many organ systems including the lung (Citation11, Citation12). Once the dye was injected, if there was no soiling of the airways, then an additional 0.25 ml was injected for a total of 0.5 ml. Again, in those instances where the physicians injecting the dye and performing the resections where not one and the same – a directed review of the navigational software was then conducted to access the position of the mark.
Following dye marker placement, patients were taken to the operating room for minimally invasive wedge resection either by a VATS or robotic approach. Depending on the patient characteristics and frozen section analysis, a formal anatomic resection with lymph node dissection was completed at the same time.
Results
Patient demographics are listed in . Twenty-two patients with a pulmonary lesion suspicious for malignancy were recruited, with one exclusion secondary to airway anatomy resulting in the inability to advance the catheter. This was subsequently located thoracoscopically. Twenty-one patients underwent ENB with dye marker placement followed by minimally invasive wedge resection. Indigo carmine dye was used in ten patients and methylene was used in the remaining 11 patients. VATS performed 17 operations and four were completed by a completely portal robotic technique. At the time of operation, the dye marker was visible in 81% patients and outlined the area for excisional biopsy (). One patient had diffuse dye marker staining of the parietal pleura and in three patients no dye marker was visualized within the hemithorax. No cases required conversion to an open procedure to identify the nodule. Characteristics of the nodules are listed in . The mean nodule size was 13.4 mm with size range from 7 to 30 mm. Pathologic examination showed that eight patients (38%) had benign disease. Eight patients (38%) had metastatic lesions. The remaining five patients (24%) had primary non-small cell lung cancer and underwent subsequent lobectomy and thoracic lymphadenectomy.
Table 1 Baseline characteristics of patients undergoing ENB prior to VATS
Table 2 Characteristics of nodules
Comment
The renewed interest in screening CTs for the detection of lung cancer in at-risk patients, the increased utilization of CT scanning to evaluate respiratory symptoms as well as to survey those patients with prior malignancies will result in the detection of many pulmonary nodules, the majority of which will be benign (Citation2). Historically, thoracoscopies done in these settings, i.e., an indeterminate chest lesion or nodule, typically results in an approximately 50% conversion rate to an open, and consequently more invasive procedure (Citation13). Thus, it would appear that the combination of navigational bronchoscopy with pleural dye marking represents a distinct, stepwise approach in the management of the IPN with the potential to decrease the rate to an open thoracotomy.
In this cohort of patients, with the exception of the pleural dye mark, the visceral pleura was otherwise unremarkable and there were no other visual cues to suggest the location of the IPNs. Further, the presence of the dye marker allowed efficient localization of the nodule/area of interest and completion of the operation. In addition to the potential to decrease the conversion rate to an open thoracotomy, and thus a more invasive procedure, the advantages of this technique include the potential for shorter operative time due to more rapid localization of the nodule, as well as providing a visual cue in difficult to palpate or non-palpable aberrancies such as ground glass opacities, etc. This approach, as a consequence of the enhanced localization, also has the potential to decrease the amount of resected tissue necessary during the excisional phase of the procedure.
The options for blue dye marker include indigo carmine dye or methylene blue. The technique for injection through a 25-gauge sclerotherapy needle as described above is the same for both dye markers. The duration of time between dye marker placement and operation does influence the choice of marker. Both dyes have been used to help localize pulmonary nodules for resection. Indigo carmine dye has an advantage in that the dye can be visible to the surgeon at least 3 days after marking (Citation11). In contrast, methylene blue will diffuse more rapidly through the tissues in several hours, which may inhibit precise localization of the nodule to allow for a more limited resection (Citation14). If the resection is to occur immediately following dye marker placement, either indigo carmine or methylene blue can be used. However, if the operation is going to occur on a different day, indigo carmine dye should be used. Either dye can be used successfully with the technique described as the resection occurs immediately following dye marking.
Other techniques have been described to assist in intraoperative localization of a pulmonary nodule. These include needle localization by interventional radiology, percutaneous or CT guided bronchoscopic injection of dye marker, and placement of a fiducial marker for digital palpation (Citation15–Citation17). An additional option includes injection of a radionuclide followed by intraoperative gamma probe localization of the nodule (Citation18). When properly performed, all of these techniques can be useful. Disadvantages of needle localization include concerns of needle movement, creation of a parenchyma hematoma, or symptomatic pneumothorax prior to resection. Percutaneous placement of dye or a fiducial can also be complicated by pneumothorax. Additionally, the relative inability to provide any diagnostic tissue no matter the situation would seem to further limit these approaches. Based upon our personal experience, the CT-guided approach to dye placement is cumbersome. Although the actual procedure time has been reported as approximately 30 minutes, there is additional time to obtain CT scanner availability as well as patient positioning in the CT scanner and equipment positioning around the CT scanner (Citation11). Additionally, the CT-guided bronchoscopic method is without the steering capability associated with the navigational approach.
In comparison, the advantages of this approach include: the lower pneumothorax rate associated with bronchoscopy, the ability to perform marker placement and resection in one procedure, the steerability and enhanced endoscopic localization of navigation as well as the ability to obtain tissue biopsies at the same time if needed. Additionally, if at the time of the bronchoscopic biopsy, a lesional, confirmatory non-malignant diagnosis is found, this might obviate the need for additional procedures unlike the other methods described above. This technique can also be performed in the same operating theater just prior to the VATS procedure while the patient is anesthetized, thus potentially allowing for a more efficient and cost-effective approach.
There are many techniques and treatment algorithms available to evaluate the IPN. The methods and techniques described herein are not meant to replace other options. Rather, ENB with dye marker placement followed by operative resection provides another approach in the evaluation and management of the IPN with an efficient and stepwise approach. In addition, it appears to be safe and reproducible in a variety of different practice settings.
Conflict of interest and funding
The authors have no financial disclosures or conflicts of interest to report.
The views expressed in this presentation are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Army, Department of Defense, nor the U.S. Government.
I certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document; and that each author takes public responsibility for it.
References
- Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS, etal. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006; 355(17): 1763–71.
- The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365(5): 395–409.
- Gambhir SS, Shepherd JE, Shah BD, Hart E, Hoh CK, Valik PE, etal. Analytical decision model for the cost-effective management of solitary pulmonary nodules. J Clin Oncol. 1998; 16(6): 2113–25.
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer. Summary of published evidence. Chest. 2003; 123: 115S–28S.
- Sortini D, Feo C, Maravegias K, Carcoforo P, Pozza E, Liboni A, etal. intrathoracoscopic localization techniques: review of the literature. Surg Endosc. 2006; 20: 1341–7.
- De Kerviler E, Gossot D, Celerier M, Frija J. Limitations of intraoperative sonography for the localization of pulmonary nodules during thoracoscopy. Am J Roentgenol. 1998; 170: 214–5.
- Mack MJ, Shennib H, Landreneau RJ, Hazelrigg SR. Techniques for localization of pulmonary nodules for thoracoscopic resection. J Thorac Cardiovasc Surg. 1993; 106: 550–3.
- Chella A, Lucchi M, Ambrogi MC, Menconi G, Melfi FM, Gonfiotti A, etal. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg. 2000; 18: 17–21.
- Krimsky W, Sethi S, Cicenia JC. Tattooing of pulmonary nodules for localization prior to VATS. Chest. 2007; 132: 425a.
- Glidea T, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med. 2006; 12(1): 9–13.
- Sakamoto T, Takada Y, Endoh M, Matsuoka H, Tsubota N. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg. 2001; 72: 296–7.
- Endo M, Kotani Y, Satouchi M, Takada Y, Sakamoto T, Tsubota N, etal. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest. 2004; 125: 1747–52.
- Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, etal. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999; 115(2): 563–8.
- Vandoni RE, Cuttat JF, Wicky S, Suter M. CT-guided methylene-blue labeling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998; 14(3): 265–70.
- Shepard JA, Mathisen DJ, Muse VV, Bhalla M, McLoud TC. Needle localization of peripheral lung nodules for video-assisted thoracoscopic surgery. Chest. 1994; 105(5): 1559–63.
- Wicky S, Mayor B, Cuttat JF, Schnyder P. CT-guided localizations of pulmonary nodules with methylene blue injections for thoracoscopic resections. Chest. 1994; 106(5): 1326–8.
- Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, etal. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009; 250: 576–85.
- Sugi K, Kaneda Y, Kirasawa K, Kunitani N. Radioisotope marking under CT guidance and localization using handheld gamma probe for small or indistinct pulmonary lesions. Chest. 2003; 124(1): 155–8.