Abstract
Central Poststroke Pain syndrome (CPSP) can occur due to disruption of the somatosensory pathways of the brain at any level such as the thalamus, medulla, or cerebral cortex. It is characterized by sensory abnormalities and hyperesthesia in the part of the body correlating to the central lesion. The treatment of this pain syndrome is often difficult, and it does not usually respond to traditional analgesics. The first line of treatment is drugs aimed at lowering neuronal hyperexcitability, for example, amitriptyline or lamotrigine, with gabapentin considered a second line.
Dejerine Roussy syndrome or thalamic pain syndrome is a rare form of central pain syndrome occurring as a result of a vascular lesion in the sensory nucleus of the thalamus. It is characterized by sensory abnormalities and hyperesthesia in the region of the body correlating to the cerebrovascular lesion. The treatment of this pain syndrome is often difficult. Therapies that have been found to be of benefit include medications that reduce neuronal hyperexcitability of the neurons, for example, amitriptyline and lamotrigine.
Case
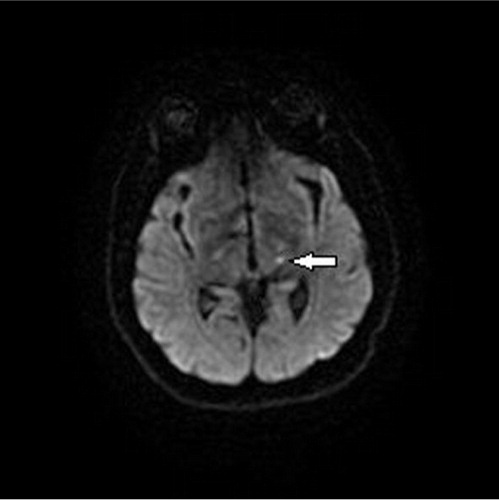
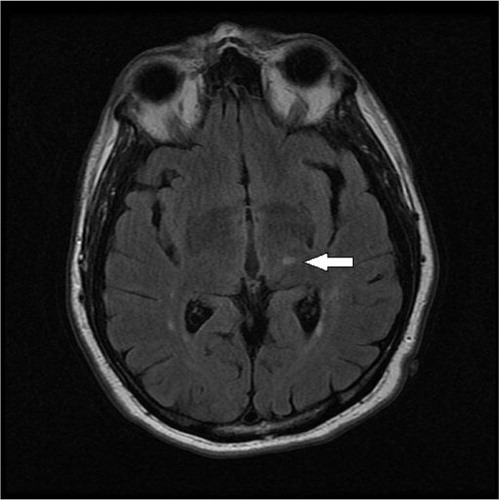
A 63-year-old right handed male with a history of hypertension, diabetes mellitus, and active tobacco abuse presented with a 5-day history of right-sided numbness and sharp pain in his left lower extremity for 1 day. He denied symptoms of fever, chills, seizure-like activity, nausea, vomiting, weakness, bowel or bladder incontinence, slurring of speech, loss of consciousness, chest pain, shortness of breath, or palpitations. Additional medical history included anxiety, depression, and chronic back pain. On examination, he was alert and oriented to person, place, and time. His vital signs revealed marked elevation of blood pressure to 190/102 mmHg, pulse rate of 88 beats per minute (regular), and temperature 37.8°C. On neurological examination, he had hyperesthesia on the right side of the body demonstrated by exaggerated pain on light touch. No facial asymmetry, cranial nerve involvement, or any motor deficits were noted. Babinski reflexes were bilaterally down going and deep tendon reflexes were bilaterally equal. CT scan of the head showed right-sided old infarct in the corona radiata. Diffusion-weighted magnetic resonance imaging (MRI) of the brain revealed subacute infarcts in left corona radiata and punctate subacute lacunar infarct in left thalamus ( and ). Echocardiogram showed no evidence of thrombus in the heart chambers or atrial fibrillation/flutter, and Doppler imaging of the carotid artery was within normal limits. His right-sided hyperesthesia was attributed to thalamic stroke. Gabapentin was started at 300 mg at bedtime and gradually advanced to 300 mg three times daily. His pain responded well to morphine initially which was later transitioned to tramadol. Over the next 4 days, he reported a marked decrease in his right-sided discomfort. At 6 months’ follow-up, his pain has been fairly well controlled with gabapentin and tramadol.
Discussion
Thalamic pain syndrome is a rare condition, first described by Dejerine and Roussy in 1906, which develops after a thalamic stroke. It is one of the causes of Central Poststoke Pain syndrome (CPSP) which occurs as a result of a vascular lesion of the ventroposterolateral nucleus of the thalamus. A lesion at any level of the spinothalamic tract is thought to be essential for the development of thalamic CPSP (Citation1). Though common after ischemic or hemorrhagic strokes involving the thalamus, CPSP can occur secondary to disruption of the somatosensory pathways of the brain at any level, that is, thalamus, medulla, or cerebral cortex (Citation2). Diagnosis relies on a detailed history, accurate general and neurological examination, and imaging of the brain with the exclusion of other possible causes of pain (Citation2, Citation3). It is important to distinguish CPSP from other types of pain that can occur after a stroke, including persistent headache, painful contractures, hemiplegic shoulder pain, and other painful musculoskeletal conditions.
The syndrome commonly causes numbness and tingling in the part of the body corresponding to the lesion in the brain. Patients can present with sensory deficits on one side of the body, mild motor deficits, abnormal movements, and other minor stroke symptoms. Weeks to months later, patients can present with severe and chronic pain disproportional to an environmental stimulus, called dysesthesia or allodynia. In one prospective study, 63% of patients developed these symptoms within 1 month, 19% within 6 months, and the rest 19% after 6 months of having the stroke (Citation4). Patients often suffer chronic pain which can be disabling, often described as sharp, stabbing, or burning. The area of pain corresponds to the stroke-associated sensory deficit with pinprick or thermal sensation (Citation3).
Neuropathic pain, in contrast to the regular pain occurring from the triggering of nociceptors, arises from abnormal pain signaling within the nervous system itself. Diagnosis of the syndrome in itself can be reassuring to the patient. To date, only a few randomized controlled studies on the treatment of CPSP have been published, showing, at best, modest treatment response to some agents. None of the agents, however, have been found to have any preventive benefit following an acute thalamic stroke (Citation2). Currently, agents directed at lowering neuronal hyperexcitability, such as amitriptyline and lamotrigine, are considered first line as they have been shown to have moderate benefit in some randomized controlled trials (RCTs) (Citation3, Citation5) (Citation6). There have been no clinical trials using gabapentin specifically for CPSP. It has proven efficacy for other neuropathic pain syndromes such as diabetic neuropathy. It is considered a second-line agent in clinical practice. A recent RCT, including 219 patients, failed to show any improvement in pain scales in the pregabalin treatment arm (Citation3). Opioids and lidocaine have been used with variable efficacy. Although not proven in clinical trials, combination therapy, as frequently used in clinical practice, may be effective. Noninvasive stimulation therapy can be considered in refractory cases (Citation3, Citation7) (Citation8). In conclusion, CPSP remains a significant impediment in the lives of certain poststroke patients, with little in the way of effective treatment in the standard armamentarium, and further research is needed into novel treatments.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Krause T, Brunecker P, Pittl S, Taskin B, Laubisch D, Winter B, etal. Thalamic sensory strokes with and without pain: Differences in lesion patterns in the ventral posterior thalamus. J Neurol Neurosurg Psychiatry. 2012; 83(8): 776–84.
- Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009; 8(9): 857–68.
- Flaster M, Meresh E, Rao M, Biller J. Central poststroke pain: Current diagnosis and treatment. Top Stroke Rehabil. 2013; 20(2): 116–23.
- Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995; 61(2): 187–93.
- Leijon G, Boivie J. Central post-stroke pain – a controlled trial of amitriptyline and carbamazepine. Pain. 1989; 36(1): 27–36.
- Vestergaard K, Andersen G, Gottrup H, Kristensen BT, Jensen TS. Lamotrigine for central poststroke pain: A randomized controlled trial. Neurol. 2001; 56(2): 184–90.
- Holtom N. Gabapentin for treatment of thalamic pain syndrome. Palliat Med. 2000; 14(2): 167.
- Bharadwaj P, Danilychev M. Central post-stroke syndrome treated with parenteral lidocaine. J Pain Symptom Manage. 2006; 32(5): 400–1.