Abstract
Background
Apixaban, a novel oral anticoagulant, is also used for deep vein thrombosis (DVT) prophylaxis. In this study, we sought to critically evaluate the differences in the rates of symptomatic DVT and bleeding, and analyze the rates of pulmonary embolism (PE) in subgroups of patients from ADVANCE I and II trials given their similar indication and design.
Methods
Studies were identified through electronic literature searches of MEDLINE, clinicaltrial.gov, SCOPUS, and EMBASE up to January 2014. Phase III RCTs involving use of apixaban and enoxaparin for thromboprophylaxis in patients undergoing total knee or hip replacement were included. Study-specific odds ratios were calculated and between-study heterogeneity was assessed using the I 2 statistics.
Results
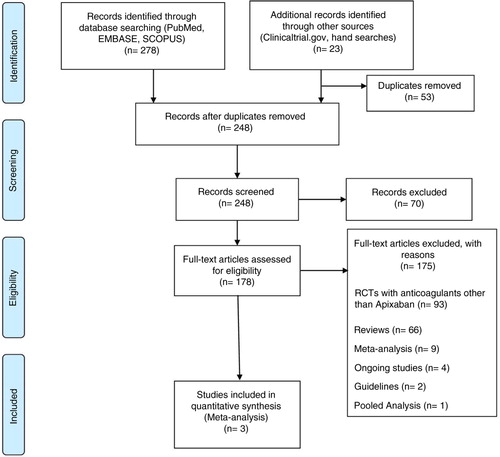
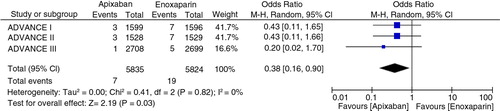
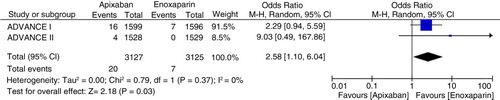
In three studies involving 11,659 patients, the risk of symptomatic DVT (pooled OR 0.38, 95% CI 0.16–0.90, I 2=0%, p=0.03) and bleeding (pooled OR 0.87, 95% CI 0.77–0.99, I 2=0%, p=0.03) was less in apixaban group compared to the enoxaparin group. However, it was interesting to note that on subgroup analysis, the risk of PE was higher with apixaban when used for thromboprophylaxis in knee replacement surgery (pooled OR 2.58, 95% CI 1.10–6.04, I 2=0%, p=0.03).
Conclusion
Apixaban was found to be associated with lower risk of symptomatic DVT and bleeding compared to enoxaparin when used for thromboprophylaxis in patients undergoing knee and hip replacement surgeries. However, it was associated with higher risk of PE in patients undergoing knee replacement.
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
Low molecular weight heparin (LMWH) and vitamin K antagonist (VKA) have established role in the prevention of venous thromboembolism (VTE) (which includes deep vein thrombosis and pulmonary embolism) during hip and knee replacement surgery (Citation1–(Citation3)). However, their efficacy and safety may be limited by the route of administration for heparins and significant drug–drug and drug–food interactions, requiring careful dose adjustment for VKA (Citation4, Citation5). Apixaban (factor Xa inhibitor) offers advantages of having fixed dosing schedules and oral route of administration and does not require any laboratory monitoring (Citation6, Citation7). Studies have shown that apixaban is as clinically effective as LMWH and appear to be safer in terms of major bleeding risks, composite outcome of symptomatic and asymptomatic deep vein thrombosis (DVT), non-fatal pulmonary embolism (PE), and death from any cause (Citation8–(Citation10)).
Several studies (Citation8–(Citation10)) comparing the efficacy and safety of apixaban to enoxaparin for VTE prevention have been carried out with results showing superior efficacy in terms of composite outcomes and similar bleeding rates. Although ADVANCE I trial reported higher rates of PE in the apixaban group, this was attributed to chance. In this study, we sought to critically analyze the rates of PE with apixaban incorporating data from additional trials and further study PE rates in subgroup of patients undergoing knee replacement surgery (ADVANCE I and II) trials given their similar indication and design.
Methods
A protocol containing background, objectives, and inclusion and exclusion criteria of the studies along with outcomes and statistical methods can be obtained for review upon request to investigators.
Literature search, data extraction, and study selection
A systematic search of MEDLINE (via PubMed), EMBASE, SCOPUS, and clinical trials.gov (from inception to January 2014) was carried out to identify eligible phase III randomized controlled trials (RCTs). MEDLINE, EMBASE, SCOPUS, and clinicaltrials.gov databases were searched using the search terms under two search themes and combined using the Boolean operator ‘AND’. For the theme ‘apixaban’, we used a combination of MeSH, entry terms and text words: (apixaban OR Factor Xa/antagonists and inhibitors OR factor Xa inhibitor). For the theme ‘hip or knee surgery’, we used a combination of MeSH, entry terms and text words: (hip surgery OR hip arthroplasty OR hip replacement OR joint replacement OR knee surgery OR knee replacement OR knee arthroplasty OR joint surgery). No language restriction was used. Multiple papers with same titles and same authors were excluded to minimize duplications.
Two authors (MRA and SG) screened and retrieved reports excluded irrelevant studies and finalized the studies to be included. An additional author (AP) participated in the resolution process when uncertainty was encountered. From the included studies, we extracted and tabulated mean age, female gender, mean days of hospitalization, history of VTE, creatinine clearance, duration of prophylaxis, dose of apixaban and enoxaparin, primary efficacy outcome, safety outcome, type of surgery, and follow-up period ().
Table 1 Baseline characteristics of the included randomized clinical trials
In order to conduct this meta-analysis, the PRISMA statement for reporting systematic reviews as recommended by the Cochrane Collaboration was followed () (Citation11). The inclusion criteria for this study were: 1) phase III RCTs; 2) participants randomized to apixaban as intervention and LMWH as control group; 3) thromboprophylaxis used for knee or hip replacement/surgery; and 4) studies that assessed the occurrence of VTE as an outcome. The RCTs were excluded if they did not primarily assess prevention of VTE or if any other anticoagulants (including factor Xa inhibitor other than apixaban) were used. The primary outcomes of interest in our study were symptomatic DVT, PE, and bleeding events. Other outcomes, including asymptomatic DVT, major VTE, all DVT, VTE-related deaths, all-cause deaths, major bleeding, were not analyzed to prevent redundancy in publication as these have already been done in previous studies (Citation12).
Statistical analysis and assessment of risk of bias
Data from each study were compared and interpreted using RevMan version 5.2 (Cochrane Collaboration, Oxford, United Kingdom). The summary odds ratio (OR) and 95% confidence intervals (CI) were estimated using a Mantel–Haenszel random effect method to account for heterogeneity as their assumption account for presence of variability among the studies. Using I 2 statistics the percentage of heterogeneity was calculated. When interpreting heterogeneity, I 2 values less than 30% were considered as low heterogeneity, less than 60% as moderate, and greater than 60% as high (Citation13). A p value of <0.05 was used as the level of significance. Assessment of risk of bias was performed using Jadad score (Citation14).
Results
Characteristics of included studies
Out of 248 studies screened only 3 studies met the inclusion criteria. The steps of literature review and selection are summarized in . Three studies included a total of 11,659 patients randomized to apixaban (n=5,835) and enoxaparin (n=5,824).
The three included studies are slightly different in terms of the design and characteristics (). Specifically, in ADVANCE III trial apixaban was used for hip replacement and the duration of apixaban therapy was for 4 weeks while ADVANCE I and II trials were done for knee replacement and the duration of apixaban therapy was for 2 weeks. The mean follow-up period was 60 days in all three studies. Each study looked at the same outcomes, which included composite outcome of asymptomatic and symptomatic DVT, non-fatal PE, and all-cause death during treatment. For our analysis, we included the incidence of symptomatic DVT and bleeding events in three studies. Further subgroup analyses for the rates of PE, major VTE, asymptomatic DVT, all DVTs, VTE-related deaths, all-cause deaths, and major bleeding were done based on the type of surgery. Each study got Jadad score of 5, which suggests high-quality studies.
Outcomes
Symptomatic DVT
In the three clinical trials involving 11,659 patients, DVT occurred in 7 of 5,835 (0.12%) treated with apixaban and 19 of 5,824 (0.32%) treated with enoxaparin. The combined effect size was significant (OR=0.38, 95% CI 0.16–0.90, Z=2.19, p=0.03, I 2=0%). The absolute risk reduction was 0.2%. The number needed to treat to prevent one DVT was 500 ().
Overall bleeding
Overall bleeding occurred in 502 of 5,770 (8.70%) in apixaban group compared to 568 of 5,755 (9.86%) in enoxaparin group (pooled OR 0.87, 95% CI 0.77–0.99, p=0.03, I 2=0%) as shown in . The absolute risk reduction was 1.16%. The number needed to treat to prevent one overall bleeding was 82 ().
Pulmonary embolism
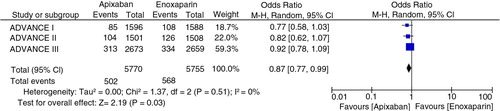
In the three trials, PE occurred in 23 out of 5,835 (0.39%) in apixaban group and in 12 out of 5,824 (0.2%) in enoxaparin group. The pooled odds ratio was OR=1.71 (95% CI 0.52–5.64, Z=0.89, p=0.38, I 2=47%). However, in patients undergoing knee replacement surgery, the rates of PE was 20 of 3,127 (0.63%) in apixaban group compared to 7 of 3,125 (0.22%) in enoxaparin group (pooled OR 2.58, 95% CI 1.1–6.04, Z=2.18, I 2=0%, p=0.03) as shown (). There was significantly increased rate of PE in apixaban group of patients undergoing knee replacement surgery. The number needed to harm was 240.
Further subgroup analyses in knee replacement surgery patients showed similar rates of all DVT (10.93% vs. 15.81%, OR 0.70, 95% CI 0.40–1.24, p=0.22, I 2=89%) (Supplementary file 1), VTE-related deaths (0.07% vs. 0.00%, OR = 3.00, 95% CI 0.12–73.80, p=0.50, I 2=not applicable) (Supplementary file 2), and all-cause deaths (0.16% vs. 0.10%, OR 1.42, 95% CI 0.34–5.85, p=0.63, I 2=0%) (Supplementary file 3), whereas lower rates of major VTE (1.09% vs. 2.17%, OR 0.50, 95% CI 0.25–0.97, p=0.04, I 2=not applicable) (Supplementary file 4), asymptomatic DVT (0.67% vs. 2.09%, OR 0.32, 95% CI 0.14–0.71, p=0.005, I 2=not applicable) (Supplementary file 5), and major bleeding (0.65% vs. 1.16%, OR 0.56, 95% CI 0.32–0.96, p=0.03, I 2=0%) (Supplementary file 6).
Discussion
Findings
The main aim of this study was to critically analyze the efficacy of apixaban over enoxaparin in thromboprophylaxis of patients undergoing knee or hip replacement as well as to assess the safety outcome in terms of overall bleeding events. Our analysis showed that while the risk of symptomatic DVT and overall bleeding was significantly less with the use of apixaban than with enoxaparin, risk of PE was significantly higher in patients undergoing knee replacement surgery. Earlier, it was hypothesized that increased rates of PE in ADVANCE I trial was due to chance (Citation8). However, after combining the data from two studies, ADVANCE I and II (Citation8, Citation10), our analysis shows that there is definitely an increased risk of PE with apixaban when used for thromboprophylaxis in patients undergoing knee replacement surgery. As both DVT and PE are considered to be a part of the same disease process, it is surprising that higher rates of PE were seen in these patients although the rates of DVT were actually lower. Although biological plausibility of this finding is difficult to explain, it might be due to 1) complete embolization of thrombi from peripheral veins, leaving no detectable DVT upon imaging; 2) clots originating in the upper extremities, which were not studied in these trials; and 3) de novo clot formation in the pulmonary circulation. The increased risk of PE with patients undergoing knee surgery in comparison to those undergoing hip replacement might be due to the shorter duration of therapy (2 weeks vs. 4 weeks). However, it should be noted that there was a non-significant increased risk of PE in ADVANCE III, which had longer duration of apixaban therapy (up to 4 weeks). Another explanation could be different dosing regimen of enoxaparin. Both studies ADVANCE I and II compared same apixaban dosing with different dosing and timing of enoxaparin. In ADVANCE I, 30 mg enoxaparin, two times daily, starting 20 hours after surgery was used compared to 40 mg enoxaparin per day that was started before surgery and resumed 19 hours after surgery in ADVANCE II trial. The rates of PE in enoxaparin group in ADVANCE I trial was slightly higher than in ADVANCE II. Whether this difference was due to different dosing and timing of enoxaparin in 2 studies remains uncertain, as there has been no direct comparison between them.
Comparison with similar studies
Two previous meta-analyses published on thromboprophylaxis with apixaban in orthopedic population have not investigated the role of apixaban in risk of PE in orthopedic patients undergoing knee replacement. The first meta-analysis published in 2012 performed pooled analysis of ADVANCE II and III (Citation12). The pooled analysis showed beneficial effect of apixaban and concluded that apixaban 2.5 mg was more effective than enoxaparin 40 mg once daily in preventing major VTE. While this meta-analysis showed no difference in non-fatal PE, we believe the data were biased because ADVANCE II was designed to see efficacy in patients with knee replacement and ADVANCE III was designed for hip replacement. The duration of apixaban was significantly different from 2 weeks in ADVANCE II and 4 weeks in ADVANCE III. Another meta-analysis published later in 2012 that included all three trials ADVANCE I, II, and III did not perform sub-group analysis based on the type of surgery (i.e., knee replacement vs. hip replacement) (Citation15).
Clinical implications
US FDA has recently approved apixaban for the thromboprophylaxis during hip or knee surgery while this has been approved for use in Europe for a while. Our findings regarding apixaban and risk of PE are concerning and demand further investigation, as this is bound to have serious implications on the morbidity and mortality in patients undergoing knee replacement surgeries.
Strength and limitations
This is the first analysis that suggests that there could be increased risk of PE in patients undergoing knee replacement surgery with apixaban when used for thromboprophylaxis compared to enoxaparin. The potential limitation of this analysis can arise from the fact that the number of studies included was small. However, it should be noted that there were only two studies that have used the apixaban for the knee replacement and for the same duration of treatment. One study was not included since this was phase II trial (Citation16) and did not meet our inclusion criteria. We took rigorous steps in conduct of this meta-analysis using PRISMA statement; therefore, we believe that these findings are valid and robust. Since patient-level data were not available, time to event analysis could not be performed. One can speculate that PE could have occurred after completion of a 2-week course of apixaban in ADVANCE I and in ADVANCE II and that longer duration of apixaban in ADVANCE III could have prevented PE. The implications of having increased risk of PE in this subgroup of patients could not be assessed since the length of hospitalization, fatality rates, and cost could not be determined due to the lack of patient-level data.
Conclusion
In our study, apixaban compared to enoxaparin was found to be associated with lower risk of symptomatic DVT and bleeding when used for thromboprophylaxis in patients undergoing knee and hip replacement surgeries as was concluded by the individual studies. However, it was associated with higher risk of PE in patients undergoing knee replacement surgery. Furthermore, these findings need to be ascertained in larger RCTs looking at this subgroup.
Authors' contributions
AP conceived, designed, participated in data abstraction, analysis, interpretation, and drafting of manuscript. FS, MRA, SG, DRP, and RP analyzed, interpreted data and provided intellectual content, and approved the final manuscript.
Financial disclosures
Authors have no financial disclosures to disclose
Conflict of interest and funding
There is no role of any funding agencies in the conduct of this meta-analysis.
Notes
To access the supplementary material for this article, please see Supplementary files under ‘Article Tools’
References
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, etal. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008; 133(6 Suppl): 381S–453S.
- Levine MN, Hirsh J, Gent M, Turpie AG, Leclerc J, Powers PJ, etal. Prevention of deep vein thrombosis after elective hip surgery. A randomized trial comparing low molecular weight heparin with standard unfractionated heparin. Ann Intern Med. 1991; 114(7): 545–51.
- Hull R, Raskob G, Pineo G, Rosenbloom D, Evans W, Mallory T, etal. A comparison of subcutaneous low-molecular-weight heparin with warfarin sodium for prophylaxis against deep-vein thrombosis after hip or knee implantation. N Engl J Med. 1993; 329(19): 1370–6.
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126(3 Suppl): 204S–33S.
- Dahl OE, Pleil AM. Investment in prolonged thromboprophylaxis with dalteparin improves clinical outcomes after hip replacement. J Thromb Haemost. 2003; 1(5): 896–906.
- Bauer KA. Recent progress in anticoagulant therapy: Oral direct inhibitors of thrombin and factor Xa. J Thromb Haemost. 2011; 9(Suppl 1): 12–19.
- Wong PC, Crain EJ, Xin B, Wexler RR, Lam PYS, Pinto DJ, etal. Apixaban, an oral, direct and highly selective factor Xa inhibitor: In vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008; 6(5): 820–9.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009; 361(6): 594–604.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, etal. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010; 363(26): 2487–98.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, etal. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010; 375(9717): 807–15.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, etal. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009; 339: b2700.
- Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez L-M, Wright RT, etal. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: Pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J Bone Joint Surg Br. 2012; 94(2): 257–64.
- Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions, version 5.1.0, published 3/2011. Julian P.T. Higgins and Sally Green, Editors. Res Synth Methods. 2011; 2(2): 126–30.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, etal. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Control Clin Trials. 1996; 17(1): 1–12.
- Li X-M, Sun S-G, Zhang W-D. Apixaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: A meta-analysis of randomized controlled trials. Chin Med J (Engl). 2012; 125(13): 2339–45.
- Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost. 2007; 5(12): 2368–75.