Abstract
The pronounced prevalence of delirium in geriatric patients admitted to the intensive care unit (ICU) and its increased morbidity and mortality is a well-established phenomenon. The purpose of this review is to explore the potential use of dexmedetomidine in preventing or managing ICU delirium in older patients. Articles used were identified and selected through multiple search engines, including Google Scholar, PubMed, and MEDLINE. Keywords such as dexmedetomidine, delirium, geriatric, ICU delirium, delirium in elderly, and palliative were used to obtain the specific articles used for this paper and restricted to articles published in 1990 or later. Articles specifically looking at the use of dexmedetomidine as compared to a study drug and its potential for use in ICU patients, as opposed to overall reviews of dexmedetomidine, were compared. When compared to benzodiazepines for the prevention or treatment of ICU delirium in the elderly, dexmedetomidine was associated with a reduction in delirium, as well as decreased morbidity and mortality. Dexmedetomidine has also been shown to be effective in limiting risk factors associated with ICU delirium such as length and depth of sedation. As opposed to benzodiazepines or opiates, dexmedetomidine provides effective analgesia, sympatholysis, and anxiolysis without causing respiratory depression and allows a patient to more effectively interact with practitioners. The review of these nine articles indicates that these favorable attributes and overall decreased duration and incidence of delirium make dexmedetomidine a viable option in preventing or reducing ICU delirium in high-risk geriatric patients and as a palliative adjunct to help control symptoms and stressors.
Delirium is an acute change in mental status characterized by temporal fluctuations with disturbances in attention and cognition that develops rapidly over a short period of time. It is not related to an underlying neurocognitive disorder, such as dementia, and is usually precipitated by an underlying medical condition (Citation1). The stressors of the inpatient hospital setting often confound or precipitate this acute medical condition. The geriatric patient, who usually has more comorbidities, a higher degree of frailty, more functional impairment, and less physiologic reserve, is particularly susceptible to delirium and its associated increases in morbidity and mortality (both short and long term), length of stay, costs, and likelihood of institutionalization (Citation2, Citation3).
Dexmedetomidine (Precedex®) is a selective alpha-2 adrenoreceptor agonist that was initially approved by the FDA in 1999 for ‘sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting … sedation of non-intubated patients prior to and/or during surgical and other procedures’ (Citation4). Several studies have documented safe usage of dexmedetomidine for a week or longer in mechanically ventilated and critically ill patients (Citation5). Although most guidelines focus on the prevention of delirium by non-pharmacologic modifications by an interdisciplinary team, we must also consider pharmacologic interventions when non-pharmacologic factors fail.
This begs the question: Are we helping or hindering the delirium by introducing traditional medications that may potentiate the symptoms we are already seeing? Given that the cornerstone of delirium management is prevention, we hypothesize that there may be a role in giving prophylactic dexmedetomidine in high-risk geriatric intensive care unit (ICU) patients, either at the beginning of their acute episode or prior to extubation. This paper will focus on reviewing articles that have broached this topic or derivations of this idea as well as its potential use in palliative patients.
Methods
A search for articles was conducted regarding the use of dexmedetomidine in ICU patients, especially with elderly patients, through multiple search engines, including Google Scholar, PubMed, and MEDLINE. Keywords such as dexmedetomidine, delirium, geriatric, ICU delirium, delirium in elderly, and palliative were used to obtain articles used for this paper and restricted to articles published in 1990 or later.
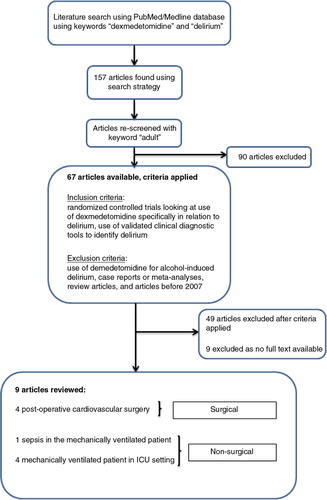
To specifically look at articles comparing dexmedetomidine to another study drug (), a search was conducted using PubMed/MEDLINE using the keywords ‘dexmedetomidine’ and ‘delirium’, which brought back a result of 157 articles. After adding the keyword ‘adult’, 67 of these 157 articles were found to be potential for inclusion. The inclusion criterion was randomized controlled trials looking at use of dexmedetomidine specifically in relation to delirium. Exclusion criteria included use of dexmedetomidine for alcohol-induced delirium, case reports or meta-analyses, review articles, and articles before 2007. After applying the exclusion criteria, our search returned 19 articles. Of the 19, nine were available as full-text English articles and were included ().
Table 1 Comparison of the nine articles
Pharmacology
Dexmedetomidine works centrally in the locus coeruleus with a high affinity for the α2-adrenoreceptor predominantly as a sedative but also with analgesic, sympatholytic, and anxiolytic properties. It has a half-life of 2 h, does not accumulate with prolonged infusion, is metabolized in the liver, and has no active metabolites (Citation6). Peak onset of action is 1 h from the onset of infusion. In intubated and mechanically ventilated patients, it is recommended to start with an initial 1 µg/kg loading dose, then an infusion of 0.2–1.5 µg/kg/h for a maximum of 24 h. Because of an increased risk of hypotension in the bolus phase, the initial loading dose is used less commonly. Doses greater than 1.5 µg/kg/h do not increase clinical efficacy (Citation7).
Devabhakthuni et al. compared the use of doses less than 0.7 µg/kg/h versus doses greater than 0.7 µg/kg/h in a retrospective review of 127 mechanically ventilated trauma ICU patients and found a higher incidence of hypotension, longer length of stay, and increased use of adjunctive (sedative, analgesic, and antipsychotics) medications in the high-dose group with no significant clinical benefit (Citation8). There are currently no specific guidelines for modifying the dose for elderly patients or patients with kidney or liver dysfunction but, as is the case in all geriatric (both chronologic and physiologic) patients, it is prudent to start at the lowest dose possible for the least amount of time to achieve the anticipated clinical endpoint.
Potential side effects of prolonged use
The indication for use via continuous infusion for no longer than 24 h was based on initial trials showing up to a 30% incidence of transient hypotension occurring shortly after initial dosing and was thought to be related to activation of post-synaptic alpha-2 adrenoreceptors in vascular smooth muscle (Citation4, Citation9). This duration limit was also meant to prevent rebound hypertension that could theoretically occur if used for longer periods, which was similar to that seen with clonidine (Citation4).
Shehabi et al. showed this not to be the case by maintaining individuals in the ICU for a mean of 81 h on dexmedetomidine infusion (without initial bolus). A 16% reduction in mean systolic blood pressure and 21% reduction in heart rate occurred over the first 4 h followed by minimal (±10%) changes throughout the infusion (Citation5). These blood pressure and heart rate changes were anticipated and less severe than those seen during initiation boluses with the recommended dosing. No significant cardiovascular rebound phenomenon was seen with abrupt cessation of dexmedetomidine, and systolic blood pressure and heart rate monitored for 24 h rose by only 7 and 11%, respectively (Citation5).
Additional reported adverse events with dexmedetomidine, in addition to hypotension, include hypertension (in setting of bolus or rapid intravenous administration when alpha-2b adrenoreceptors are activated in the periphery resulting in vasoconstriction), nausea, bradycardia, heart block, and sinus arrest (Citation10). The latter occurred in the setting of the loading bolus dose, providing even more evidence to not use an initial bolus dose and also explaining why dose titrations should occur slowly and not exceed an interval less than 30 min. Given these reported adverse events, dexmedetomidine should theoretically be used with caution or avoided in those older individuals with significant cardiac disease, especially conduction abnormalities. Due to changes in pharmacokinetics and pharmacodynamics often seen in the elderly, small dose or frequency changes can have exaggerated medication effects, especially with respect to hypotension and bradycardia. Most elderly patients are on more than 4–5 medications to begin with, and when combined with a medication such as dexmedetomidine, this can produce rapidly worsening side effects and consequences. Hence, the ‘starting low, going slow’ approach must be used.
In the MIDEX trial comparing dexmedetomidine with midazolam, although hypotension and bradycardia were observed with increased frequency in the study group [midazolam: 11.6% hypotension, 5.2% bradycardia versus dexmedetomidine: 20.6% hypotension (p=0.007), bradycardia 14.2% (p<0.001)], these adverse effects rarely necessitated stopping the study in all study groups despite noting cardiovascular dysfunction in 61% of the patients in the dexmedetomidine arm and 60.2% of the midazolam arm of patients at baseline (Citation10). Menon et al. showed that the sympatholysis caused by the centrally acting alpha-2 receptor blockade of dexmedetomidine may mitigate the deleterious cardiovascular effects of acute cocaine overdose (Citation11).
Advantages of use of dexmedetomidine
The primary advantage of dexmedetomidine over agents such as benzodiazepines or opiates is that it is able to adequately sedate the patient but with less respiratory depression and allows patients to remain more awake and interactive (Citation6, Citation10). Its use is advantageous during extubation, as it exerts sedative and analgesic properties to counterbalance stress while maintaining hemodynamic stability and preserving cognitive function (Citation12). In situations where patients must be able to interact with clinicians, this combination of properties is useful. For example, in neurosurgical patients, post-operative ventilated patients must be woken up to obtain serial neurological exams. The reliability of the clinical neurologic exam hampered by the sedation caused by other agents is preserved with dexmedetomidine.
It has also been used as a palliative adjunct to help control symptoms and stressors, while maintaining a level of alertness not seen with high-dose benzodiazepines and/or opiates. Although the cost of the medication itself may be more than older agents, the potential cost savings that could result from less mechanical ventilation time, decreased time in the ICU, and decreased incidence of anesthetic or ICU-related delirium are enormous.
Preventing and treating delirium
A large proportion of studies have focused on treating delirium once its onset has been identified. Strategies to identify delirium include the Confusion Assessment Method (CAM) (Citation3), the Delirium Rating Scale–Revised-98 (Citation13), and the Delirium Symptom Interview (Citation14). Among the slew of causes of delirium, including infection, cardiogenic, metabolic, physical injury, and endocrine, almost all classes of medications can also precipitate delirium, but some, such as haloperidol, can treat and even prevent the onset of such symptoms (Citation15). summarizes the findings of the nine specific studies included in our analysis of dexmedetomidine and other study drugs such as lorazepam, propofol, and midazolam. Overall, it was noted that dexmedetomidine had much better outcomes with regard to delirium than any of the other study drugs used.
A few studies in particular are important to note. A double-blind, randomized controlled trial was done by Pandharipande et al. on 106 adult mechanically ventilated medical and surgical ICU patients at two tertiary care centers (Citation16). This study compared lorazepam to dexmedetomidine to determine whether the duration of delirium and coma in mechanically ventilated ICU patients could be reduced while providing adequate sedation.
They were able to show more delirium-free days as compared to the study drug lorazepam. Patients were also more apt to be able to complete post-ICU neuropsychological testing evaluating global, cognitive, motor speed, and attention functions (Citation16). The 28-day mortality in the dexmedetomidine group was 17% vs. 27% in the lorazepam group (p=0.18) with the 12-month time to death was 363 days in the dexmedetomidine group vs. 188 days in the lorazepam group (p=0.48). This reduction in delirium, coupled with the overall decreased morbidity and mortality, makes the use of dexmedetomidine a very appealing agent in ICU care.
Riker et al. (Citation17) conducted a prospective, double-blind, randomized trial in 68 centers in five countries looking at the use of dexmedetomidine versus midazolam in 375 medical/surgical ICU patients with expected mechanical ventilation for more than 24 h. The CAM-ICU negative and positive patients were assessed at baseline and after administration of dexmedetomidine versus midazolam with encouraging results.
For the CAM-ICU–negative at study enrollment, the effect of dexmedetomidine treatment measured by generalized estimating equation (GEE) was a 15.4% decrease (95% CI, 2 to 29%; p=0.02), with a delirium prevalence of 32.9% (25/76) in dexmedetomidine-treated patients versus 55.0% (22/40, p=0.03) in the midazolam arm of treatment. For those CAM-ICU–positive at baseline, the dexmedetomidine treatment effect measured by GEE was a 32.2% reduction (95% CI, 21 to 43%; p=0.001), with a prevalence of 68.7% (90/131) for dexmedetomidine-treated patients versus 95.5% (63/66) for midazolam-treated patients (p=0.001). The overall prevalence of delirium during treatment was 54% in dexmedetomidine group and 76.6% in the midazolam group.
Patients also showed improved communication and interaction while on dexmedetomidine in the study done by Jakob et al. (Citation10), with a visual analog scale (VAS) estimated score difference of 19.7 (95% CI, 15.2–24.2), p=0.001, as compared to midazolam and 11.2 (95% CI, 6.4–15.9), p=0.001, as compared to propofol in the MIDEX and PRODEX trials, respectively. The intent of this study was to compare these agents in maintaining sedation, reducing duration of mechanical ventilation, and improving patients’ interaction with nursing care. This was carried out as a two-phase three multicenter, randomized, double-blind trial.
As per the studies evaluated in this paper, one may extrapolate that improved communication and appropriate sedation level could lead to more appropriate use of medications known to precipitate delirium, including analgesics (opiates), anxiolytics (benzodiazepines), and antipsychotics. Dexmedetomidine was effective in reducing many of the major triggers of delirium in the elderly ICU patient, namely time on mechanical ventilation, time in the ICU, and stress related to inability to make needs known.
Dexmedetomidine in palliative care
There is a growing body of literature in the palliative care arena advocating the use of a low-dose infusion of dexmedetomidine as an adjunct to treating opiate resistant pain. There has been success in the use of this drug as both a fast-acting analgesic and light sedative that manages symptoms and, by allowing for continued patient interaction, avoids palliative sedation in situations of intractable pain (Citation24). Coyne et al., who looked at the potential role of dexmedetomidine as a bridge to obtaining effective analgesia, proposed a protocol for a low-dose infusion of dexmedetomidine in patients on a palliative care service to be used as, ‘an adjunctive analgesic for refractory pain, may be opioid sparing, and provides a mild sedative effect (Citation24)’. Case reports in the palliative care field report on the ability to provide analgesia, anxiolysis, and ‘conscious sedation’ to allow for control of symptoms while preserving precious time with patients and loved ones (Citation25).
Future studies may look at this type of protocol in patients at high risk of delirium, admitted to the ICU, but not in a terminal state requiring palliative care, to see if symptoms can be controlled adequately with decreased incidence of delirium. This is an exciting hypothesis that could have vast potentials in managing the critically ill geriatric patients.
Conclusion
Delirium triggers a cascade of iatrogenic reflexes often leading to polypharmacy and adverse drug effects; unnecessary and uncomfortable tests, procedures, and interventions; cognitive decline; deconditioning; hospital-acquired infections; skin breakdown; and family/caregiver stress. The ICU environment, as well as the severity of diagnostic and therapeutic interventions used there, is incredibly distressing to any patient, and even more so to the elderly individual.
Patients with delirium have a 62% increase in mortality at 1 year compared to those without delirium (Citation26). Compared to those who avoid delirium, individuals who become delirious have significantly higher rates of institutionalization and cognitive decline in the year following an episode (Citation2, Citation26). Delirium in the ICU portends adverse clinical outcomes at 6 months, including a threefold risk of death, longer hospitalizations, longer times on mechanical ventilation, and higher rates of cognitive impairment (Citation27). Therefore, prevention and, if needed, treatment are the keys to improving outcomes.
A natural conclusion is that delirium should decrease with both less exposure to delirium-precipitating medications, as well as the patient being more awake, alert, and participatory in their care. Non-pharmacologic treatments should always be explored first, especially concerning the geriatric population of patients; however, when pharmacologic agents are warranted, those that can achieve stated goals with the least amount of side effects and least risk of precipitating delirium should be utilized. Habit and comfort level should no longer be acceptable reasons for choosing a medication, which is not substantiated by evidence.
The nine articles used in this study outlined the use of dexmedetomidine as a newer agent that has proven to be effective. The ages of the patients included show that patients, including the geriatric population, may benefit from such intervention as it may have applications in preventing delirium in high-risk geriatric ICU patients. It appears that dexmedetomidine might be a viable option for all, including those with more serious medical conditions such as underlying heart disease (as proven by the MIDEX trial), but more research is needed to safely conclude so. All variables still have to be individualized per each patient based on their medical history and overall functional state; however, these nine articles prove promising results, if dexmedetomidine is used correctly and with close monitoring.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2013; 5th ed, Arlington, VA: American Psychiatric Association.
- Inouye SK. Delirium in older persons. N Engl J Med. 2006; 354(11): 1157–65.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990; 113(12): 941–8.
- Precedex [package insert]. 2008. Lake Forest, IL: Hospira, Inc;.
- Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: Sedative and cardiovascular effects. Intensive Care Med. 2004; 30(12): 2188–96.
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014; 370(5): 444–54.
- Venn M, Newman J, Grounds M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003; 29(2): 201–7.
- Devabhakthuni S, Pajoumand M, Williams C, Kufera JA, Watson K, Stein DM. Evaluation of dexmedetomidine: Safety and clinical outcomes in critically ill trauma patients. J Trauma. 2011; 71(5): 1164–71.
- Jackson KC 3rd , Wohlt P, Fine PG. Dexmedetomidine: A novel analgesic with palliative medicine potential. J Pain Palliat Care Pharmacother. 2006; 20(2): 23–7.
- Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, etal. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012; 307(11): 1151–60.
- Menon DV, Wang Z, Fadel PJ, Arbique D, Leonard D, Li JL, etal. Central sympatholysis as a novel countermeasure for cocaine-induced sympathetic activation and vasoconstriction in humans. J Am Coll Cardiol. 2007; 50(7): 626–33.
- Venn RM, Karol MD, Grounds RM. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Br J Anaesth. 2002; 88(5): 669–75.
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale–Revised-98: Comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001; 13: 229–42.
- Albert MS, Levkoff SE, Reilly C, Liptzin B, Pilgrim D, Cleary PD, etal. The Delirium Symptom Interview: An interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992; 5: 14–21.
- Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry. 2004; 12(1): 7–21.
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, etal. Effect of sedation with dexmedetomidine vs. lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007; 298(22): 2644–53.
- Riker R, Shehabi Y, Bokesch P, Ceraso D, Wisemandle W, Koura F, etal. Dexmedetomidine vs. midazolam for sedation of critically ill patients a randomized trial. JAMA. 2009; 301(5): 489–99.
- Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, etal. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: A randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology. 2009; 111: 1075–84.
- Maldonado JR, Wysong A, van der Starre PJA, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009; 50(3): 206–17.
- Reade MC, O'Sullivan K, Bates S, Goldsmith D, Ainslie W, Bellomo R. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: A randomised open-label trial. Crit Care. 2009; 13: R75.
- Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, etal. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010; 14: R38.
- Park JB, Bang SH, Chee HK, Kim JS, Lee SA, Shin JK. Efficacy and safety of dexmedetomidine for postoperative delirium in adult cardiac surgery. Korean J Thorac Cardiovasc Surg. 2014; 47: 249–54. doi: 10.5090/kjtcs.2014.47.3.249.
- Wanat M, Fitousis K, Boston F, Masud F. Comparison of dexmedetomidine versus propofol for sedation in mechanically ventilated patients after cardiovascular surgery. Methodist DeBakey Cardiovasc J. 2014; 10(2): 111–17.
- Coyne PJ, Wozencraft CP, Roberts SB, Bobb B, Smith TJ. Dexmedetomidine: Exploring its potential role and dosing guideline for its use in intractable pain in the palliative care setting. J Pain Palliat Care Pharmacother. 2010; 24(4): 384–6.
- Soares LG, Naylor C, Martins MA, Peixoto G. Dexmedetomidine: A new option for intractable distress in the dying. J Pain Symptom Manage. 2002; 24(1): 6–8.
- Leslie DL, Zhang Y, Holford TR, Bogardus ST, Leo-Summers LS, Inouye SK. Premature death associated with delirium at 1-year follow-up. Arch Intern Med. 2005; 165(14): 1657–62.
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr , etal. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291(14): 1753–62.