Abstract
Background
Ebola virus disease (EVD) is a public health emergency of international concern. There is limited laboratory and clinical data available on patients with EVD. This is a meta-analysis to assess the utility of clinical signs, symptoms, and laboratory data in predicting mortality in EVD.
Aim
To assess the utility of clinical signs, symptoms, and laboratory data in predicting mortality in EVD.
Method
Study selection criterion: EVD articles with more than 35 EVD cases that described the clinical features were included. Data collection and extraction: Articles were searched in Medline, PubMed, Ovid journals, and CDC and WHO official websites. Statistical methods: Pooled proportions were calculated using DerSimonian Laird method (random effects model).
Results
Initial search identified 634 reference articles, of which 67 were selected and reviewed. Data were extracted from 10 articles (N=5,792) of EVD which met the inclusion criteria. Bleeding events (64.5% vs. 25.1%), abdominal pain (58.3% vs. 37.5%), vomiting (60.8% vs. 31.7%), diarrhea (69.9% vs. 37.8%), cough (31.6% vs. 22.3%), sore throat (47.7% vs. 19.8%), and conjunctivitis (39.3% vs. 20.3%) were more often present in pooled proportion of fatal cases as compared to EVD survivors.
Conclusions
Clinical features of EVD that may be associated with higher mortality include bleeding events, vomiting, diarrhea, abdominal pain, cough, sore throat, and conjunctivitis. These patients should be identified promptly, and appropriate management should be instituted immediately.
In March 2014, the World Health Organization (WHO) was first apprised of the Ebola virus disease (EVD) outbreak in Guinea, West Africa (Citation1). Little was known at that time that this local outbreak of a biosafety level 4 pathogen would transform into a public health emergency of international concern (Citation2).
EVD, formerly Ebola hemorrhagic fever, was first identified in 1976 from two simultaneous outbreaks of hemorrhagic fever in the Democratic Republic of Congo and Sudan (Citation2–(Citation4)). The virus name was derived from the Ebola River where the first case was identified. Ebola virus is an enveloped, single-stranded, negative sense RNA virus. It belongs to the family of Filoviridae viruses, which are known causal agents of viral hemorrhagic fevers. The genus Ebola virus has five species: Zaire ebola, Tai Forest ebola, Sudan ebola, Bundibugyo ebola, and Reston ebola virus. Of these, the Z. ebola species is the most notorious for causing sporadic EVD outbreaks in the sub-Saharan African region (Citation5, Citation6). The recent EVD epidemic in West Africa has been the largest and most severe to date with a conservatively estimated case fatality rate of 71% (range 46–72%) (Citation1, Citation7).
Patients with EVD typically develop signs and symptoms within 9–11 days of exposure to the Ebola virus (Citation7). The initial prodrome is most often characterized by fever, chills, myalgia, and malaise. Gastrointestinal symptoms such as watery diarrhea, nausea, vomiting, or abdominal pain typically follow. Other symptoms and signs including chest pain, dyspnea, headache, confusion, conjunctival injection, and bleeding may occur (Citation1, Citation7). The virus enters the host through breaks of the skin or mucosal surfaces; invades the monocytes, macrophages, and dendritic cells; replicates; and then disseminates via lymphatic and hematogenous routes. The characteristic hemorrhagic nature of EVD is believed to occur due to the release of cytokines that cause vascular instability, endothelial leakage, and coagulation abnormalities. The end result of the disease process mimics septic shock with impaired host immune response, hypotension, and multi-organ failure (Citation6).
Given that the non-specific viral prodromal presentation and the limited clinical and laboratory resources in Ebola virus endemic regions (Citation8, Citation9), the identification of prognostic features in EVD is a significant challenge. Although such prediction of risk factors for fatality has been attempted with individual outbreaks, there has not been a comprehensive assessment of factors across multiple outbreaks. Here, we present the findings of a meta-analysis done to assess the utility of clinical signs, symptoms, and laboratory markers in predicting EVD case mortality. The major focus of our study is to help in effective triage of EVD patients based on the clinical features and laboratory values. Such strategies could potentially improve the management and prognosis of Ebola-infected patients.
Methods
Study selection criteria
Studies and reports describing clinical EVD from 1976 to November 2014, with a minimum of 35 patient cases, were included. The number 35 was chosen to improve the reliability of results. Only studies that described patients’ clinical signs and symptoms along with case fatality were included.
Data collection and extraction
Articles were searched in Medline, PubMed, Ovid journals, EMABSE, Cumulative Index for Nursing and Allied Health Literature, ACP journal club, DARE, International Pharmaceutical Abstracts, old Medline, Medline non-indexed citations, Ovid Healthstar, Cochrane Central Register of Controlled Trials (CENTRAL), and CDC and WHO official websites. The search was performed for the years 1976 to November 2014. Abstracts were manually searched in the major infectious disease journals for the past 3 years. Study authors for the abstracts included in this analysis were contacted when the required data for the outcome measures could not be determined from the publications. The search terms used were Ebola, Ebola hemorrhagic fever, clinical illness, clinical signs, clinical symptoms, case fatality, mortality, and blood chemistry measurements. Four authors (HM, VM, SD, and RB) independently searched and extracted the data into an abstraction form. Any differences were resolved by mutual agreement. The agreement between reviewers for the collected data was quantified using the Cohen's κ (Citation10).
Quality of studies
Clinical trials designed with control and treatment arms can be assessed for quality of the study. A number of criteria could be used to assess the quality of a study (e.g., randomization, selection bias of the arms in the study, concealment of allocation, and blinding of outcome) (Citation11, Citation12). There is no consensus on how to assess studies designed without a control arm. Hence, these criteria do not apply to studies without a control arm (Citation12). All the articles included in this meta-analysis were observational studies. There were no randomized controlled trials where interventions were compared.
Statistical methods
Proportion meta-analysis was performed on pooled data. Freeman–Tukey variant of arcsine square root transformed proportion was used to transform individual study proportion into a quantity. Random effects model was performed using DerSimonian Laird weights (Citation13, Citation14). Pooled results and individual study results were displayed using forest plots. The weight of each study was indicated by the width of point estimates on forest plots. P-value of more than 0.10 rejects the null hypothesis that the studies are heterogeneous. Cochran's Q-test was used to test the heterogeneity among studies (Citation15). Harbord Egger and Begg Mazumdar bias indicators (Citation16, Citation17) were used to estimate selection and publication bias. Publication bias was also evaluated by the construction of funnel plots (Citation18, Citation19).
Results
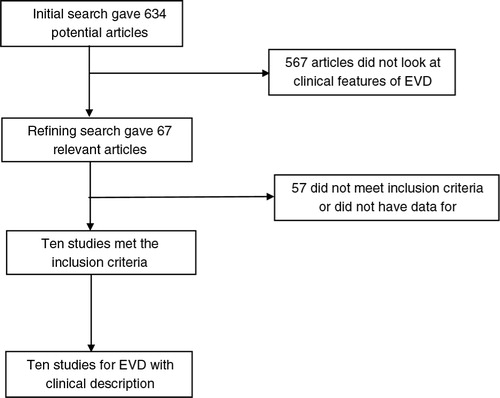
Initial search identified 634 articles from which 67 were selected for review. Data were extracted from 10 articles or studies (N=5,792) of Ebola viral disease (Citation2–(Citation4, Citation8, Citation9) (Citation20–Citation24)), which met the inclusion criteria. shows the flow chart with study selection criteria. Four of 10 articles are WHO Ebola reports collected from WHO and CDC official websites. The 10 studies included in this meta-analysis are published as full text articles. All the pooled proportions given are the estimates calculated by the random effects model. Random effects model was preferred due to the heterogeneity of the results. shows the basic study characteristics.
Table 1 General characteristics of studies in this meta-analysis
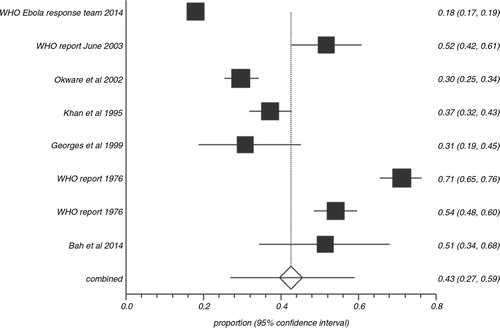
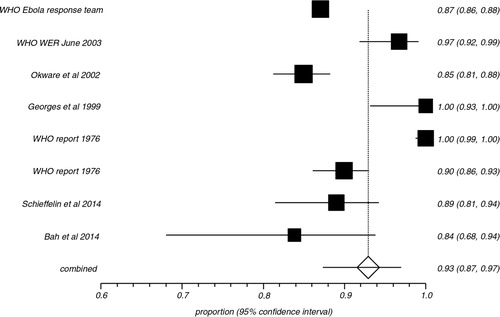
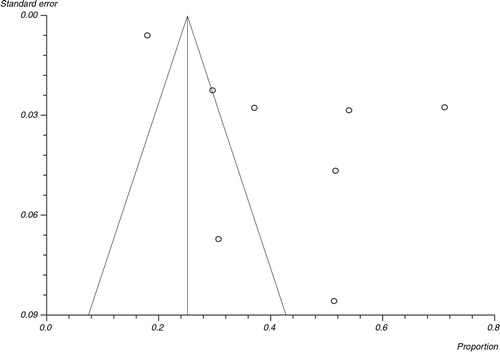
In the pooled proportion of EVD patients, bleeding events occurred in 42.5% (95% CI=26.9–58.9), fevers were present in 92.9% (95% CI=87.3–96.9), headaches in 73.4% (95% CI=52.9–89.7), and diarrhea in 73.3% (95% CI=66.4–79.7). shows the overall association of clinical symptoms and signs with EVD. compares the association of clinical signs and symptoms of EVD in patients with fatal outcomes versus survivors. Bleeding events (64.5% vs. 25.1%), abdominal pain (58.3% vs. 37.5%), vomiting (60.8% vs. 31.7%), diarrhea (69.9% vs. 37.8%), cough (31.6% vs. 22.3%), sore throat (47.7% vs. 19.8%), and conjunctivitis (39.3% vs. 20.3%) were more often present in the pooled proportion of fatal cases compared to the pooled proportion of EVD survivors. Meaningful association of fevers, fatigue, headache, dyspnea, arthralgia, myalgia, elevated aspartate transaminase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), and creatinine with case fatality could not be made either due to similar prevalence of these symptoms in both groups or due to the limited sporadic data available. shows the heterogeneity data and risk of bias associated with individual symptoms in pooled patient population. and are Forest plots for bleeding events and fever respectively. is a Funnel plot to assess publication bias.
Table 2 Overall association of clinical symptoms and signs with EVD
Table 3 Association of clinical signs and symptoms of EVD patients with fatal outcome versus survivors
Table 4 Heterogeneity data and risk of bias associated with individual symptoms in pooled patient population
Discussion
Fever, fatigue, and headache were the commonly described symptoms in most outbreaks of EVD (Citation25, Citation26). A maculopapular rash that appeared between days 5 and 7 of the disease was also common. The current outbreak though has predominantly gastrointestinal symptoms that include abdominal pain, nausea, vomiting, and diarrhea (Citation5). Patients who succumb to the illness usually die of shock and multi-organ failure as evident on the hematological and chemistry labs (Citation5). Hematological changes include lymphopenia and neutropenia at the onset of the disease. As the disease progresses, some patients develop thrombocytopenia and increased prothrombin and partial thromboplastin time suggesting consumptive coagulopathy (Citation5). In patients who survive, clinical improvement is seen with the development of antibodies, and it has been observed that sometimes antibody response may be absent in lethal cases suggesting the possible role of antibody response as a prognostic factor (Citation27, Citation28).
The WHO response team found that, in the recent West African epidemic, a higher rate of fatal outcomes was associated with the clinical features of diarrhea, conjunctivitis, difficulty breathing or swallowing, confusion or disorientation, coma, hemorrhagic symptoms (unexplained bleeding, bleeding gums, bloody nose, bleeding at the injection site, and bleeding from the vagina), and patient's age>45 years (Citation2).
Schieffelin et al. (Citation8) analyzed that the risk factors associated with fatal outcomes in EVD patients included fever higher than 38°C (100.4°F), weakness, dizziness, and diarrhea on presentation, evidence of hepatocellular damage, and impaired kidney function. Similarly, Rollin et al. (Citation9) showed that the increased levels of blood AST, D-dimer, BUN, creatinine, and amylase were associated with fatal outcomes, hence of prognostic importance. In a recent study by Bah et al. (Citation20), dehydration associated with vomiting and diarrhea was the primary finding contributing to the case fatality.
In the current meta-analysis, bleeding events, diarrhea, vomiting, abdominal pain, cough, sore throat, and conjunctivitis were more often associated with the pooled proportion of fatal EVD cases when compared to the pooled proportions of survivors (). Bleeding events (64.5% with the 95% CI=14.8–99.0) and gastrointestinal symptoms [abdominal pain 58.3% (95% CI=30.8–83.3), vomiting 60.8% (95% CI=49.9–71.2), and diarrhea 69.9% (95% CI=60.7–78.4)] were seen in more than half of the pooled proportion of dead patients indicating the importance of aggressive management of these symptoms by fluid and electrolyte replacement. Fever, headache, myalgia/arthralgia, and dyspnea were commonly seen in both groups (survivors and non-survivors) and no association with mortality could be made for these signs and symptoms. Unfortunately, the laboratory data (AST, ALT, BUN, amylase, and D-dimer) reported are limited and sporadic (Citation8, Citation9) and, hence, a meaningful association of these factors with case fatality could not be made in this meta-analysis. Predictive score based on the combination of symptoms should be explored to identify the risk profile of patients. This exploration can be done only with patient-level data.
There are some limitations to our study primarily due to the drawbacks of the individual studies in reporting the complete clinical and laboratory data. There has been some non-uniformity in the data collection because of inherent difficulties in collecting information from deceased persons (Citation24), and the challenges of clinical follow-up, limited laboratory services, and infection control precautions during the epidemic (Citation20). Although there are more than 10,000 documented EVD patients, detailed clinical data are available in only about 50–60% of the total patient population and thus may not be representing the entire patient population. There were many suspected Ebola deaths during the epidemics, and patients may have died before confirmation, skewing the case fatality rates. Also, case fatality is affected by a number of other factors including age and coexisting conditions, as well as biological characteristics of various strains of the virus (Citation2, Citation3). Signs and symptoms at presentation are derived from outbreaks in Africa where presentation can be close to five or more days on average after the day of symptom onset. The situation may be different elsewhere (e.g., in a Western facility), where individuals may present for care earlier. The pooled data in this analysis are from different viruses (Z. ebola and S. ebola), and because these viruses have different case fatality rates, a sign or symptom in an individual with one virus may not necessarily translate to the same implications in an individual infected with the other virus.
Studies with statistically significant positive results tend to be published and cited. Additionally, smaller studies may show larger treatment effects compared with larger studies. This publication and selection bias may affect the summary estimates. The bias can be estimated using Egger bias indicators and the construction of funnel plots whose shape can be affected by bias. In the present meta-analysis and systematic review, bias calculations using both Egger (Citation16) and Begg Mazumdar (Citation17) bias indicators showed no statistically significant bias. Furthermore, analysis using funnel plots showed no significant publication bias among the studies included in the present analysis.
There is a dire need to develop more reliable therapies for EVD. Should more reliable therapies become globally available for patients with active EVD, and if said therapies were in relatively short supply and needed to be rationed in an outbreak, then perhaps the signs and symptoms noted here could be used as a basis for determining who gets said therapy in addition to aggressive supportive care versus who does not.
Conclusions
In EVD, the presenting clinical features of vomiting, diarrhea, bleeding events, abdominal pain, cough, sore throat, and conjunctivitis may be associated with the increased risk of mortality. EVD with such features should promptly be identified, and rigorous management options should be pursued emergently. Although prior studies have shown that increased AST, BUN, and creatinine have been associated with high mortality (Citation2, Citation3), we could not synthesize meaningful outcomes in this regard due to the limited available data.
Conflict of interest and funding
None of the authors have a conflict of interest.
Acknowledgement
We would like to acknowledge the Research Open Access Article Publishing (ROAAP) Fund of the University of Illinois at Chicago for financial support towards the open access publishing fee for this article. Dr. Srinivas Puli's assistance with statistics and study design.
References
- Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, etal. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014 Oct; 371(15): 1418–25.
- World Health Organization. WHO Ebola Response Team. Ebola virus disease in West Africa – The first 9 months of the epidemic and forward projections. N Engl J Med. 2014 Oct; 371(16): 1481–95.
- World Health Organization. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ. 1978; 56(2): 247–70.
- World Health Organization. Ebola haemorrhagic fever in Zaire, 1976. Report of an International Convention. Bull World Health Organ. 1978; 56(2): 271–93.
- Goeijenbier M, van Kampen JJ, Reusken CB, Koopmans MP, van Gorp EC. Ebola virus disease: A review on epidemiology, symptoms, treatment and pathogenesis. Neth J Med. 2014; 72(9): 442–8.
- Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011; 377(9768): 849–62.
- Centers for Disease Control and Prevention. Ebola virus disease information for clinicians in U.S. healthcare settings. 2014. Available from: www.cdc.gov/vhf/ebola/hcp/clinician-information-us-healthcare-settings.html [cited 19 November 2014].
- Schieffelin SJ, Shaffer GJ, Goba A, Gbakie M, Gire SK, Colubri A, etal. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 Nov; 371(22): 2092–100.
- Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007 Nov; 196(Suppl 2): S364–71.
- Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. Br Med J. 1992; 304: 1491–4.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, etal. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Control Clin Trials. 1996; 17: 1–12.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, etal. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in Epidemiology (MOOSE) group. JAMA. 2000; 283(15): 2008–12.
- Stuart A, Ord JK. Kendall's advanced theory of statistics. 1994; 6th edn, London: Edward Arnold.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7: 177–88.
- Deeks JJ, Egger M, Smith GD, Altman DG. Systematic reviews of evaluations of diagnostic and screening tests. Systematic reviews in health care. Meta-analysis in context . 2001; London: BMJ Books. 157–62.
- Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2005; 25(20): 3443–57.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50: 1088–101.
- Sterne JAC, Egger M, Davey-Smith G. Investigating and dealing with publication and other biases in meta-analysis. Br Med J. 2001; 323: 101–5.
- Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001; 54: 1046–55.
- Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, etal. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015; 372: 40–7.
- World Health Organization. Outbreak(s) of Ebola haemorrhagic fever, Congo and Gabon, October 2001–July 2002. Wkly Epidemiol Rec. 2003; 78(26): 223–5.
- Okware SI, Omaswa FG, Zaramba S, Opio A, Lutwama JJ, Kamugisha J, etal. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002; 7(12): 1068–75.
- Georges AJ, Leroy EM, Renaud AA, Benissan CT, Nabias RJ, Ngoc MT, etal. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: Epidemiologic and health control issues. J Infect Dis. 1999; 179: S65–75.
- Khan AS, Tshioko FK, Heymann DL, Le Guenno B, Nabeth P, Kerstiëns AS, etal. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J Infect Dis. 1999; 179: S76–86.
- Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011; 204(Suppl 3): S810–16.
- Jeffs B. A clinical guide to viral hemorrhagic fevers: Ebola, Marburg and Lassa. Trop Doct. 2006; 36(1): 1–4.
- Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, etal. Inflammatory responses in Ebola virus infected patients. Clin Exp Immunol. 2002; 128(1): 163–8.
- Leroy EM, Baize S, Debre P, Lansoud-Soukate J, Mavoungou E. Early immune responses accompanying human asymptomatic Ebola infections. Clin Exp Immunol. 2001; 124(3): 453–60.