Abstract
Background
Metaplastic carcinoma of the breast is an extremely rare subtype of breast cancer with an incidence of <1% of all breast neoplasms. Metaplastic carcinoma with chondroid differentiation is the rarest among all histologic subtypes of breast cancer. We report a case of infiltrating ductal carcinoma with metaplastic features of chondroid differentiation.
Case presentation
A 58-year-old-woman presented to our clinic with a 4-month history of a lump in her right breast. On examination, a firm non-tender mass measuring 2×2 cm was noted in the right upper outer quadrant. It was not attached to the underlying structures. Mammography revealed a dense irregular mass in the axillary tail and a circumscribed nodule in the 6 O'clock periareolar region. This was a new development compared to the patient's most recent screening mammogram performed 2 years and 6 months previously. Ultrasound demonstrated a lobulated solid mass in the axillary tail and a simple cyst in the 6 O'clock periareolar region. Biopsy of the areolar region of the right breast revealed atypical duct hyperplasia. Fine needle aspiration cytology of the right breast axillary tail revealed a poorly differentiated invasive carcinoma consistent with mammary duct origin. On histopathological examination, it was an infiltrating ductal carcinoma with metaplastic features of chondroid differentiation. The tumor was estrogen receptor, progesterone receptor, and HER-2 negative with 0% nuclear staining. Ki-67 index was 52% with strong nuclear staining. The overall ELSTON grade of invasive carcinoma was grade 3. The patient received adjuvant chemotherapy with AC-T (adriamycin, cytoxan, and taxol) and is currently undergoing surveillance for recurrent disease.
Conclusion
Metaplastic breast cancer is an extremely rare subtype of breast carcinoma. Initial management of localized disease consists of wide excision with clear surgical margins followed by radiation or mastectomy and sentinel lymph node biopsy. Although standard breast chemotherapy regimens such as AC-T are routinely used in metaplastic breast cancer in both adjuvant and metastatic settings, outcomes are significantly inferior to other breast subtypes. Further studies are required to explore targeted treatment to achieve better outcomes in this patient population.
Metaplastic carcinoma of the breast was identified as a separate histologic subtype of breast cancer in 2000 (Citation1). It represents 0.2–1% of breast cancer and is typically composed histologically of a poorly differentiated infiltrating ductal carcinoma coexisting with areas of squamous or mesenchymal differentiation (Citation2). We report a case of infiltrating ductal carcinoma with metaplastic features of chondroid differentiation.
Case report
A 58-year-old-woman presented to clinic with a 4-month history of a lump in her right breast close to the axilla. There was no history of nipple discharge or any skin changes. Her most recent screening mammogram was unremarkable and had been performed approximately 2 years and 6 months prior to presentation. There was no family history of breast or ovarian cancer. On examination, a firm non-tender mass measuring 2×2 cm was noted in the right upper quadrant of the breast and the axillary tail, which was not attached to the underlying structures. Mammography revealed a dense irregular mass in the axillary tail and a circumscribed nodule in the 6 O'clock periareolar region. An asymmetric density was also seen in the central right breast. There was no evidence of axillary lymphadenopathy. Ultrasound demonstrated a lobulated solid mass in the axillary tail measuring 2.2×1.8×1.5 cm, and a simple cyst measuring 1.1×0.8×0.7 cm in the peri-areolar region at 6 O'clock. Normal appearing lymph nodes were seen in the right axilla.
Fine needle aspiration cytology of the right breast axillary tail revealed a poorly differentiated invasive carcinoma consistent with mammary duct origin. Biopsy of the areolar region of the right breast revealed a typical duct hyperplasia in a background of dense stromal fibrosis. The patient underwent right-sided total mastectomy with sentinel lymph node biopsy.
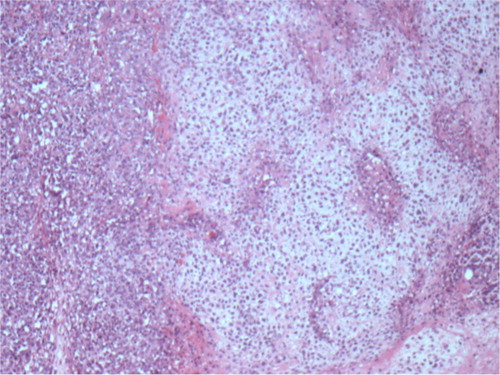
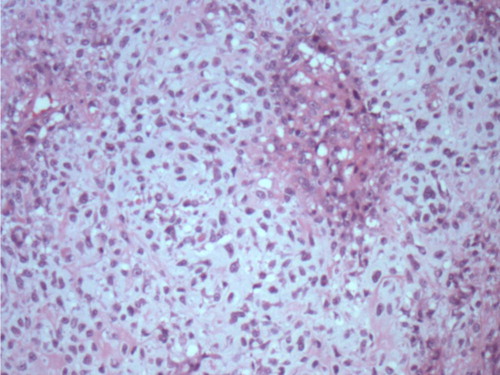
On histopathological examination, the specimen revealed a tumor measuring 2.6 cm in greatest dimension, with resection margins negative for tumor. Skin and nipple were negative for tumor. It was an infiltrating ductal carcinoma with metaplastic features of chondroid differentiation ( and ). The tumor was estrogen receptor (ER), progesterone receptor (PR), and HER-2 negative with 0% nuclear staining. Immunohistochemical stains for pan cytokeratin on the sentinel lymph nodes were negative. Tumor cells showed some degree of anisocytosis with a nuclear pleomorphism score of 3, and mitotic figures in tumor cells were frequent with an average of eight mitoses or more per square mm. The overall ELSTON grade of invasive carcinoma was grade 3. Ki-67 index was 52% with strong nuclear staining. Adjuvant chemotherapy with AC-T (Adriamycin, Cytoxan, and Taxol) was initiated. The patient tolerated chemotherapy well initially but developed taxol-induced sensory neuropathy leading to early discontinuation of taxol. She is currently undergoing surveillance with no evidence of recurrent disease.
Discussion
The lifetime incidence of breast cancer in the United States is one in eight women, and breast cancer is the second most common cause of cancer death (Citation3). Globally, however, breast cancer is the leading cause of cancer deaths among women (Citation3).
Metaplastic breast cancer is an extremely rare subtype of breast cancer, representing 0.2–1% of breast cancer. It generally falls under the broader basal-like subtype group of breast cancers. Basal-type breast cancers are typically ER, PR, and HER-2 receptor negative with a high Ki-67 score and tend to have a worse prognosis than other types of breast cancer. Histologically, metaplastic breast cancer typically consists of a poorly differentiated infiltrating ductal carcinoma coexisting with areas of squamous or mesenchymal differentiation (Citation2).
The World Health Organization (WHO) classifies MBC into (Citation1) epithelial type and (Citation2) mixed type. Epithelial-type MBC is classified into adenocarcinoma with spindle cell differentiation and adenosquamous carcinoma. Mixed-type MBC is classified into (Citation1) carcinoma with chondroid metaplasia (Citation2), carcinoma with osseous metaplasia, and (Citation3) carcinosarcoma (Citation4).
The underlying molecular pathogenesis of metaplastic breast cancer remains unclear. Mutations in oncogenes, proto-oncogenes, and tumor suppressor genes play a significant role in carcinogenesis pathways (Citation2). BRCA1, BRCA2, and p53 mutations are recognized as important tumor suppressor genes in breast cancer (Citation2). The mitogen-activated protein kinase/tyrosine kinase signaling pathway, epithelial growth factor receptor pathway, phosphatidylinositol 3-kinase/protein kinase-B/mammalian target of rapamycin pathway, and the epithelial–mesenchymal transition pathway are thought to be potential therapeutic targets for novel agents directed against metaplastic breast cancer (Citation2).
The clinical presentation of MBC is similar to other invasive breast cancers. It typically presents with a palpable firm breast mass; however, it is less frequently associated with axillary lymph node involvement (Citation5). The median age of MBC ranges from 48 to 59 years compared to other invasive breast cancers, with a median age of 61 years (Citation5). It generally presents as larger size, greater than 2 cm, grows rapidly, and 20% of cases fix to the underlying skin (Citation5). Histopathology remains the definitive diagnosis.
The surgical approach in the management of breast cancer patients depends on tumor size. Breast conservation therapy with adjuvant radiation can be considered if the tumor size is less than 5 cm. If the tumor size exceeds 5 cm, total mastectomy is suitable (Citation1). The relative chemoresistance of MBC is well documented, with inferior results to other histologic subtypes of breast cancer (Citation1). AC-T is a standard adjuvant regimen for invasive ductal carcinoma (IDC) and current National Comprehensive Cancer Network chemotherapy guidelines do not differentiate between IDC and MBC. Therefore, treatment protocols in these guidelines do not vary by histology. In a single institution study of 18 women with MBC, one-third of the patients presented with metastatic disease, and chemotherapy was given to 17 patients. The most common regimens were AC (adriamycin, cyclophosphamide) and FAC (5-FU, adriamycin, cyclophosphamide). A total of 4–6 cycles were administered. The median follow-up period was 31.3 months. Cancer relapse was confirmed in 50% of the study patients, with a median time to relapse of 17.5 months (Citation6).
Gene expression profiling of a patient's tumor holds great promise for the future. A Phase I clinical trial in the treatment of advanced malignancies, including metaplastic breast cancer, demonstrated that the combination of liposomal doxorubicin, bevacizumab, and temsirolimus was tolerable. A partial or complete remission was obtained in 5 of 12 patients with metaplastic breast cancer enrolled in the study, resulting in a 42% response rate. Eleven out of 28 patients with PIK3CA mutation and or a PTEN mutation or loss achieved a partial or complete remission (Citation7). A case report of a 59-year-old woman with metaplastic breast cancer and both PIK3CA and TP53 mutations on gene expression profiling described the patient's partial response to liposomal doxorubicin, bevacizumab, and temsirolimus (Citation8). Ongoing clinical trials with these three agents for MBC may lead to promising future treatments for this disease.
In conclusion, further studies are required to explore targeted treatments to achieve better outcomes in patients with metaplastic breast cancer.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Schwartz TL, Mogal H, Papageorgiou C, Veerapong J, Hsueh EC. Metaplastic breast cancer: Histologic characteristics, prognostic factors and systemic treatment strategies. Exper Hematol Oncol. 2013; 2: 31.
- Ostad SN, Parsa M. Mehmet Gunduz. Breast cancer – Focusing tumor microenvironment, stem cells and metastasis. Breast cancer from molecular point of view: Pathogenesis and biomarkers . 2011. Available from: http://www.intechopen.com/books/breast-cancer-focusing-tumor-microenvironment-stem-cells-andmetastasis/breast-cancer-from-molecular-point-of-view-pathogenesis-and-biomarkers [cited 14 December 2011]..
- DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics 2013. CA Cancer J Clin. 2014; 64(1): 52–62.
- Fritz A, Percy C, Jack A, Solin LH. International classification of diseases of oncology. 2000; 3rd edn, Geneva: World Health Organization.
- Al Sayed AD, EL Weshi AN, Tulbah AM, Rahal MM, Ezzat AA. Metaplastic carcinoma of the breast clinical presentation, treatment results and prognostic factors. Acta Oncol. 2006; 45: 188–95.
- Nowara E, Drosik A, Samborska-Plewicka M, Nowara EM, Stanek-Widera A. Metaplastic breast carcinomas-analysis of prognostic factors in a case studies. Contemp Oncol. 2014; 18(2): 116–19.
- Moroney JW, Fu S, Moulder S, Falchook G, Helgason T, Levenback C, etal. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biological activity. Clin Cancer Res. 2012; 18: 5796–805.
- Agarwal R, Koenig K, Rohren E, Subbiah V. Combined antiangiogenic and mammalian target of rapamycin inhibitor targeted therapy in metaplastic breast cancer harboring a PIK3CA mutation. J Breast Cancer. 2014; 17(3): 287–90.