Abstract
Exosomes, membrane vesicles of 40–100 nm in diameter, are derived from endosomes in various cells. The bioactive molecules specifically packed into exosomes can be horizontally transferred into recipient cells changing their biological properties, by which tumour cells continuously modify their surrounding microenvironment and distant target cells favouring cancer metastasis. It has been suspected for a long time that exosomes participate in the whole process of tumour metastasis. Although there is much unknown and many controversies in the role of cancer exosome, the major contribution of tumour-associated exosomes to different steps of cancer metastasis are demonstrated in this review. Mainly because these exosomes are easily accessible and capable of representing their parental cells, exosomes draw much attention as a promising biomarker for tumour screening, diagnosis and prognosis. Currently, researchers have found numerous biomarkers in exosomes with great potential to be utilized in personalized medicine. In this article, we summarize the roles of biomarkers, which are validated by clinical samples. Even though many conundrums remain, such as exosome extraction, large multicentre validation of biomarkers and data interpretation, exosomes are certain to be used in clinical practice in the near future as the field rapidly expands.
The majority of deaths from cancer are ascribed to metastasis, making it the most fearful aspect of cancer. Metastasis consists of a series of successive and interrelated steps mainly including invasion into surrounding tissue, intravasation, circulation, adhesion to and extravasation from capillaries in target organs, proliferation and establishment of micrometastasis illustrated in (Citation1).
Fig. 1. The promotion of exosome to cancer metastasis. Tumour-associated exosomes influence other cells and modulate microenvironment, involving the key steps in cancer metastasis cascade. 1) In primary site, tumour cells secrete exosomes to induce EMT and degrade the matrix. The Wnt pathway in cancer cells is activated by exosomes during the migration. 2) As intravasation, endothelium is disturbed directly by tumour-secreted exosomes and indirectly by macrophages activated by exosomes derived from tumour cells. 3) Both circulating tumour cells (CTCs) and tumour-activated platelets secrete exosomes affecting the immune cells and CTCs. 4) Adhesive molecules on endothelial cells are upregulated by exosomes from the adherent tumour cell. 5) Disseminated tumour cells will proliferate forming a micrometastasis in appropriate niche, which is remoulded by exosomes from primary site.
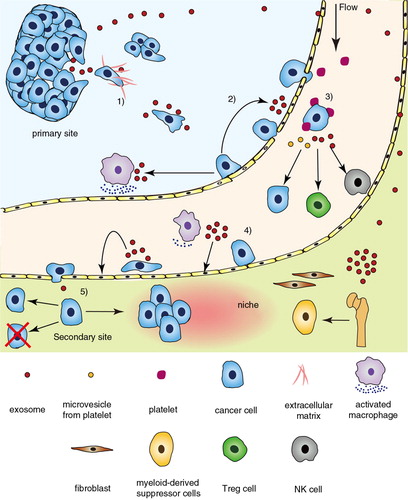
To communicate with the stroma cells in the microenvironment, not only soluble but also membrane-associated factors, namely extracellular vesicles (EVs) are secreted from the tumour cells. EVs consist of exosomes, up to 100 nm in diameter that originate from the fusion of multivesicular bodies with the plasma membrane and microvesicles of 50 nm to 1 µm that directly bud from the plasma membrane (Citation2, Citation3). It seems that exosomes are released in both constitutive (Citation4) and controlled manners (Citation5), regulated by intercellular calcium and Rab GTPases (Citation6–Citation8). Because of a specific molecule sorting mechanism in cells (Citation8), proteins, lipids and RNAs in exosome are different from their parental cells. Ubiquitination associated with Endosomal Sorting Complex Required for Transport, lipids and tetraspanins are proposed as mediators in this sorting mechanism (Citation9). A common set of content as well as an individual subset of component associated with specific cell functions representative of its parental cells are contained in exosome (Citation2). As a carrier, exosomes participate in different stages of cancer progression by horizontally transferring bioactive molecules to recipients (Citation4). Their cargoes are well protected from proteases and nucleases by their phospholipid bilayer. According to Cocucci’ hypotheses (Citation10), part of the exosome can transmit the endothelium to the circulation via transcellular and paracellular routes and enter into a variety of body fluids such as saliva, urine, blood, ascites, breast milk and cerebrospinal fluid (Citation11).
In terms of their easy access and rapid response to stimuli, exosomes have been considered as a platform for personalized diagnostics. Some markers in exosomes are associated with tumour stratification (Citation12, Citation13) and prognosis (Citation14), which are highly likely to be exploited as biomarkers of personalized diagnostics. Although large sample size validation is required, many companies have started to develop commercial diagnostics kits based on exosomes.
In this review, exosomes participation in key steps of cancer metastasis is considered. In the latter part, the feasibility and advantages of exosomes in personalized diagnostics are summarized, as well as current situation and future challenges in the development of exosome diagnostics.
The role of exosomes in carcinogenesis
It has been well known that in tumour development, cells require a long-term, multistage process of carcinogenesis to accumulate alterations at the genetic and/or epigenetic level which ultimately reprogramme a cell to undergo uncontrolled proliferation and metastasis. The expression of initial mutations depends not only on the internal interaction between oncogenes but also on external factors such as exosome, which could change the patterns of specific gene expression temporarily (Citation15). As an intercellular communicative vector, exosomes facilitate the tumorigenesis by transferring both oncogene and oncogenic factors. Melo et al. found that exosomes derived from cells and sera of patients with breast cancer lead normal epithelial cells to form tumours in a Dicer-dependent way. Then, exosomes derived from cancer cell further induce surrounding normal cells to become tumorigenic (Citation16). Furthermore, Lee et al. showed that oncogenic H-ras promotes the emission of exosomes containing H-ras DNA and that exosomes may affect the viability and proliferation of cancer cells through the histones, nucleosomes and chromatin enclosed within them (Citation17). Furthermore, Abd Elmageed et al. revealed exosomes to contain various oncogenic factors, such as H-ras and K-ras transcripts, oncogenic miRNAs and ras superfamily of GTPases, and that prostate cancer cell exosomes cause neoplastic reprogramming of adipose stem cells in vivo (Citation18). Their finding implicates the function of prostate cancer cell-derived exosome in tumour clone expansion. Based on the critical role in carcinogenesis, exosomes possess the potential to indicate the presence of cancer as a biomarker and great efforts has been devoted to the application of exosome.
Exosomes are involved in tumour metastasis
Invasion
Cancer cells at the margin of a tumour, and far from tumour blood vessels, are often in a hypoxic condition and likely to invade into surrounding tissue to obtain more oxygen and nutrients (Citation19). In the beginning, cancer cells lose their cell–cell adhesion and detach from the primary tumour, a process called epithelial–mesenchymal transition (EMT), which is generally considered the hallmark of metastasis. During EMT, the epithelial cells deconstruct their cell junction, lose their polarity and gain mesenchymal-cell-like migratory and invasive properties. Tauro et al. found that, compared with control, the exosome derived from H-ras transformed MDCK cells containing proteases, annexins and integrins, which may induce the EMT of a recipient cell (Citation20). Since intercellular communication can be mediated by the horizontal transfer of information molecules in exosome, it is tempting to suspect that exosomes can induce and maintain the EMT (Citation21, Citation22). Recently, Josson et al. co-cultured prostate cancer cells with the exosomes derived from prostate stromal cells overexpressing miR-409 resulting in morphologically and biochemically defined EMT. This is the first study suggesting that stromal-derived exosomal miRNA can induce EMT in cancer (Citation23).
Once tumour cells leave the primary site, they may be trapped by the extracellular matrix (ECM) surrounding the tumour, which is composed by collagen, laminin, fibronectin and elastin (Citation24). Various metalloproteinases are secreted by cancer cells to degrade the ECM, such as matrix metalloproteinases, ADAM and ADAMTS, which have been detected in exosomes by proteomic analysis. Furthermore, it has been clearly proven that there is a positive correlation between the quantity of exosomes, the amount of lytic enzymes and in vitro invasive capability (Citation25).
Subsequently, tumour cells undergo chemotaxis, which is recognized as an essential part of metastasis. Cell migration is a continuous cycle of several interdependent steps including polarization, elongation, extending the pseudopod attaching to ECM substrate and, finally, contracting the cell body and trailing edge (Citation24). Increasing numbers of experiments indicate that exosomes are associated with tumour cell migration. For instance, Lin et al. determined that exosomes derived from adipose mesenchymal stem cells promote the migration of MCF-7, a breast cancer cell line, and that the Wnt/β-catenin signalling pathway was stimulated (Citation26). Luga et al. found that Wnt/PCP (planar cell polarity), which can be activated by CD81 on fibroblast-secreted exosomes, also plays an important role in breast cancer cell protrusion and invasive migration (Citation27). On the other hand, it was determined that exosomes from prostate cancer cells could transfer integrins to recipient cells thereby mediating the adhesion of tumour cells to ECM (Citation28). Noticeably, although tumour cells migration across 2D substrate depends on adhesion, in the 3D situation, cells move in a cadherin-independent manner akin to amoeboid migration (Citation24). Additionally, tumour cells can migrate collectively in several modes (Citation29). Thus, there is a need to further explore the role of exosomes on the migration of tumour cells during metastasis (Citation30).
Intravasation
Intravasation as a limiting step in metastasis refers to the process whereby locally invasive tumour cells enter lymphatic or blood vessels. To obtain more nutrients and/or follow chemokine gradients, tumour cells migrate rapidly towards nearby vessels. In the vicinity of capillaries, tumour cell may slow down and enter a stage of inactivity, active intravasation or dormancy. Once tumour cells pass through the basement membrane, they will adhere to the vascular endothelial cells (EC) moving along the vessel wall (Citation31). At some place, tumour cells undergo transendothelial migration (TEM) to make a way to the capillary lumen. Not only the properties of the tumour cell but also other factors such as macrophages and cytokines in microenvironment influence the intravasation (Citation32).
As a carrier, exosomes plays an important function in the interaction between tumour cells, EC and macrophages. For one thing, tumour-derived exosomes can promote macrophage secretion of several kinds of pro-inflammatory cytokines, through the NF-κB pathway. These pro-inflammatory cytokines, such as IL-6, TNF-α and CCL2, increase the permeability of the endothelial barrier in favour of TEM (Citation33). Macrophages can also be activated by MFG-E8 in exosomes derived from prostate cancer (Citation34). Moreover, exosomes derived from tumour cells can trigger EC migration, the hallmark of intravasation. For instance, vascular endothelial growth factor (VEGF) positive tumour exosomes may promote vascular endothelial cadherin (VE-cadherin), endocytosis, increase vascular permeability and induce the EC retraction and matrix degradation (Citation35) as was similarly observed by Taverna et al. (Citation36). Besides VEGF, other important factors such as transforming growth factor beta (TGF-β) and TNF-α harboured in exosomes derived from various tumours can also facilitate the transmigration (Citation37). Additionally, Asangani et al. discovered that miR-21 possesses the potential of inducing cancer cell's intravasation through reducing Pdcd4 (Citation38). The increase of miR-21 has been found extensively in exosomes derived from various types of tumours such as laryngocarcinoma (Citation39), hepatocellular carcinoma (Citation40) and breast cancer (Citation41). Zhou et al. characterized that miR105-rich exosomes derived from metastatic breast cancer cell decrease the tight junction protein ZO-1 with an impairment of the endothelium integrity (Citation42).
Circulation
Upon intravasation into vessel, the circulating tumour cell (CTC) has to face many threats like shear force and immune attack.
One of the principal strategies to combat these is the absorption of platelets onto cancer cells via the interaction between P-selectin and its ligand mucin. The formed emboli prevent the shear force as a physical shield. Meanwhile, the cancer cell activates adherent platelets (Citation43) and secretes a great deal of EVs including microvesicles (50 nm to 1 µm) and exosomes (40—to 100 nm). Janowska-Wieczorek et al. have proven that platelet microvesicles (PMV) play multiple functions in metastasis. For instance, PMV can increase tumour cells’ chemotaxis to target organs through the transfer of the CD41 integrin onto cancer cells (Citation44). Zhu et al. observed a similar phenomenon that human bone marrow mesenchymal stem cell secretes exosomes upregulating VEGF and CXCR4 expression on tumour cells through ERK1/2 and p38 MAPK pathways (Citation45).
In circulation, tumour cells lose the immunologic suppressive protection of the microenvironment in the primary tumour and become vulnerable to immune attack. Besides the platelet armour, the CTC secretes a bulk of exosomes modifying the immune system to escape immunologic surveillance. CTC inhibits the number and the cytotoxic activity of NK cells through some exosome-associated mechanisms such as inhibition of perforin release, reducing CD3 and NKG2D (Citation46) and inactivating the Jak3 pathway (Citation47). In addition, T cells are inhibited through similar mechanisms like the apoptosis induced by FasL and TRAIL-specific exosomes (Citation48). Moreover, with inhibiting anti-tumour effector lymphocytes, tumour cells also activate the regulatory immune cells such as Treg and myeloid-derived suppressor cells (MDSC). It has been proven that exosomes in patient serum could transform CD4+CD25-T cells to CD4+CD25+Foxp3 + Treg cells via TGF-D4 independent mechanisms (Citation49). Furthermore, in breast cancer, melanoma and colonal cancer, researchers observed that exosomes can influence the maturation of DC skewing them to an immunologically suppressive state like MDSC (Citation50).
Extravasation
If the CTC survive, they will eventually reach a blood vessel wall and a location of the metastatic site, which mainly depends on the blood flow (the mechanical hypothesis) and cell adhesion (the seed and soil hypothesis). To colonize in the target organ, the tumour cell has to extravasate from the capillary. This process includes initially transient attachment, subsequent stable adhesion and lastly transmigration.
As the mechanical hypothesis describes, extravasation often occurs in small capillaries where the diameter is less than that of CTC. As CTC encounters and rolls along the vessel wall, there is a slipping motion between tumour cell and endothelium, in a similar way to leucocyte, which provides the possibility for receptor–ligand bonding between tumour cell and EC (Citation51). If ECs of specific organs express sufficient adhesion molecules and provide enough adhesive strength, the tumour cell will initiate a transient weak adhesion. The selectin on EC, especially E-selectin, mediates the homing of tumour cells (Citation52). In fact, the E-selectin in quiescent EC is at an extremely low level, and it can be induced by inflammatory cytokines such as TNF-α and IL-1 (Citation53). It has been proven that exosomes derived from tumour cells can stimulate immune cells to release more inflammatory cytokines (Citation33), and exosomes themselves can act as a vector transferring TNF-α (Citation54). However, a detailed investigation into the effect of cancer exosomes on E-selectin expression on EC has not yet been conducted, although E-selectin is an important factor in cancer metastasis. In addition to the E-selectin, Al-Nedawi et al. observed that the MAPK pathway will be activated when EC takes up EGFR-containing exosomes derived from cancer cells (Citation55). It has been proven that in inflammatory disease, MAPK activation can facilitate cell extravasate from the vessel (Citation56). Research into the effect of cancer exosomes on selectin expression of EC is quite sparse for the moment.
Shortly, after the initial rolling stage, there is a stable, firm attachment between elongated tumour cells and activated EC. This is mainly mediated by the interaction of tumour cell integrins and immunoglobulin superfamily members like ICAM-1, and VCAM-1 (Citation57). It seems that the integrinβ1 is constantly activated without stimuli (Citation58) and, by contrast, the increase of integrin ligand on activated EC controls this stable adhesion. Taverna et al. found in chronic myelogenous leukaemia that, at the beginning of stable adhesion, the tumour exosome could induce VCAM-1 and ICAM-1 expression in EC (Citation36); after a long period of arrest, tumour exosomes will then downregulate VCAM-1-promoting tumour cells to seek a site with more chemoattractants.
During the adhesion stage, tumour cell driven by chemoattractants crawls along the blood vessel wall to a preferred place of transmigration where the level of adhesive molecules and its ligands is high enough to initiate the transmigration. In this process, the tumour cell turns from round to elongate and, further, extends the protrusions into the gap resulting in the opening of the junction and eventual transmigration. Since the tumour cell is twice as big as the leucocyte, it cannot transmigrate in the same way as the leucocyte without impairing the endothelium (Citation51). Specifically, the tumour cell disrupts the adhesion junction and induces EC retraction, even apoptosis (Citation59), which thus shares many similarities with intravasation. The only clue about exosomes causing EC apoptosis comes from the research on platelet-derived exosomes in sepsis (Citation60), and the act of tumour exosome on EC apoptosis in extravasation needs more researches. The above-mentioned process mainly refers to paracellular transmigration which involves the tumour cell squeezing between adjacent ECs. Transcellular transmigration which allows tumour cell transmigration through the EC directly still remains an enigma (Citation61).
Proliferation
After extravasation, the disseminated tumour cells may die, or enter a state of dormancy of proliferation, which is determined by the tumour cells and the microenvironment at the secondary site, namely the niche (Citation62). As the primary site, the niche is a crucial part of tumour progression in the metastatic organ. Their functions include facilitating CTCs homing, promoting the extravasation, fostering an inflammatory milieu as the primary tumour, deregulating the immune system, providing survival and proliferative signals, keeping “stemness” properties of cancer stem cells and maintaining dormancy. It has been determined that, in fact, the pre-metastatic niche has been changed by factors secreted by primary tumours prior to the arrival of CTCs (Citation63).
Castellana et al. show that exosomes have the same systemic effects on the remodelling of the pre-metastatic niche as the soluble factors in primary tumours (Citation64). It seems that exosome is an essential companion for soluble factors’ initiating pre-metastatic niche (Citation65, Citation66). Exosomes derived from tumour cells can transform the host cell to a pre-metastatic phenotype favouring tumour cell survival and proliferation. For instance, exosomes can change genes expression associated with the formation of the pre-metastatic niche in the lymph node stroma cells and lung fibroblasts in metastatic rat adenocarcinoma (Citation67). In addition to directly changing the stroma cells, exosomes can educate and recruit bone marrow derived cells (BMDCs), which are an important constituent in the pre-metastatic niche. Peinado et al. observed this and further showed that BMDC mobilization is caused by horizontal transfer of the MET oncoprotein by exosomes (Citation68).
If the niche is not suitable, tumour cells will enter dormancy waiting for an appropriate condition. This dormancy can be considered as a protective mechanism of cancer cells. Exosomes derived from bone marrow mesenchymal stem cells can transfer miR-23b and downregulate MARCKS gene expression promoting the dormancy (Citation69). It is highly possible that exosomes regulate cancer cell's dormancy, although the dormancy is not yet fully understood. A biomarker indicating the dormancy is of great meaning for recurrence and drug resistance.
Others
Four characteristic features in tumour progression and metastasis, inflammation, immunity, hypoxia and angiogenesis, are highly interrelated and together influence the development of tumour. They accompany tumour cell development and are not limited to a certain step of metastasis. The influences on metastasis mediated by exosomes are thus demonstrated separately in the following section.
It has long been recognized that there is an inextricable connection between chronic inflammation and tumour development. It promotes tumour development by diverse pro-inflammatory factors, which could perturb cell signalling pathways and increase the expression of growth-promoting genes, such as cytokines and interleukins. As a container of various pro-inflammatory factors, exosome is an important part of inflammation associated with metastasis (Citation70). Fabbri et al. show that miR-21 and miR-29a in tumour-derived exosome can reach, bind to and activate the Toll-like receptor (TLR) such as murine TLR7 and human TLR8 in the intracellular endosomes of immune cells. The activated TLRs can trigger a pro-metastatic inflammatory response via NF-κB signalling pathway, and ultimately lead to tumour growth and metastasis (Citation71).
Local cancer-associated inflammation can also induce a severe immunosuppression. Aberrant nucleic acids and other products in tumours can recruit and activate immune cells related to inflammation. This immune response elicited by the tumour is generally unable to eradicate the tumour, and, on the contrary, it will remodel the microenvironment in the primary site and select the genetically fittest cancer cells that could evolve into aggressive malignant tumours. The tumour-specific antigens harboured in tumour-derived exosomes can initiate tumour immunity and participate in tumour-associated immunity. Clayton et al. reported that the NKG2D ligands in tumour-derived exosome can inhibit the cytotoxic CD8 + T cells and nature killer cells inducing an immunosuppression (Citation72). Compared with directly influencing cytotoxic T cell, exosome can also impact the immune system via regulatory cells. For example, TGF-β1 present at the exosome surface can increase the activity and number of Foxp-3 positive Treg, which inhibit the proliferation response of CD8+ T cells to interleukin-2 (Citation73). In addition, the antigen in tumour exosomes can modulate dendritic cells towards a more suppressive cell phenotype in favour of the escape of tumour cells (Citation74).
Since the high proliferation rate of tumour cells surpasses that of vasculature expansion, the majority of the tumour is hypoxia. Furthermore, the new blood vessels in the tumours are abnormal, leaky or non-functional. For the increasing genomic instability induced by hypoxia, cancer cells mutate and adapt to the tumour microenvironment rapidly. Consequently, cancer cells with low apoptotic potential and high aggressive property are screened out by the hypoxia (Citation75). Additionally, much necrotic debris derived from hypoxia comprises pro-inflammatory factors, which can recruit a host of inflammatory cells (Citation76). For example, King et al. showed that hypoxia-mediated activation of HIF-1α enhances the release of tumour-derived exosomes, which promotes cancer cell survival and invasion in a breast model (Citation77).
When a solid tumour grows beyond 1–2 mm in diameter, it has to develop its own blood vessel networks to supply nutrients and carry away wastes (Citation78). Angiogenesis not only allows further growth of the tumour but also provides an avenue for metastatic tumour cells to intravasate into the circulation and establish distant metastases (Citation79). It is increasingly clear that cancer-derived EV exerts complex effects on angiogenesis-associated cells contributing to vessel formation through delivery of angiogenic proteins and nuclear material (Citation80). For example, Hood et al. showed that exosomes derived from melanoma can promote endothelial spheroid formation in a dose-dependent manner (Citation81). Nazarenko et al. reported that tumour-derived tetraspanin Tspan8 positive exosomes can efficiently induce angiogenesis in tumours by increasing levels of von Willebrand factor, VEGF, VEGF-R2 and other factors, which could drive EC proliferation, migration and sprouting (Citation82).
Exosome as a biomarker in personalized diagnostics
The potential of the exosome as a biomarker
As a complicated disease with numerous genetic abnormalities, a prominent feature of cancer is its high heterogeneity which exists not only amongst different lesions in 1 patient, but also cells in the same tumour mass. It is not unusual that patients with an identical type of cancer and stage have variations in genotype and/or phenotype. This fingerprint-like variability results in patients’ different responses to antitumor therapies, for example, some active drugs have no effects on certain patients, or even have adverse effects. The practice of “one medicine for all patients with the same diseases” cannot stand any more. Since Kewal K. Jain first put forward the concept of personalized medicine in 1998, it has revolutionized our approach to the cancer therapy (Citation83). Personalized medicine suggests that the tumour therapy should be tailored according to individual characteristics and responses to the specific treatment. In personalized medicine, the customized treatment depends on information about the molecular characteristics of the cancer signature, namely the personalized diagnostics. Biomarkers in personalized diagnostics can be divided into several subgroups according to their application: screening, early diagnosis, prognosis, prediction, monitoring and companion diagnostics. Prognostic markers aim to predict the likely outcome of cancer patients overall, while the predictive markers predict the likelihood of a response to certain therapies, both of which often cannot be distinguished, especially in recurrence. Companion diagnostics, firstly approved by the FDA in 2013, come along with the development of personalized medicine, which identifies potential patients responding to a particular therapy.
Although traditional biomarkers have been widely and maturely utilized in personalized medicine, their inherent limitations cannot be neglected. First, the most common method to detect pharmacogenomically relevant sequences is fluorescence in situ hybridization, which depends on the sample from biopsy. As Gerlinger pointed out, about two-thirds of mutations in a single biopsy cannot be detected uniformly throughout the whole sampled region of the same tumour (Citation84). Thus, a single biopsy is incapable of representing the landscape of genomic abnormalities in 1 tumour, which impede doctors’ understanding the global situation. Secondly, tissue biopsy, the best method of diagnosis, is invasive and dangerous, and is unable to be applied to repeated diagnosis. Thirdly, the low specificity of serum biomarkers in present clinical diagnosis may contribute to a low efficiency of diagnosis.
As mentioned above (illustrated by Table and ), exosomes act as communicative vectors between tumour cells and the carcinogenesis and metastasis microenvironment. They possess great potential as cancer biomarkers in personalized medicine. Firstly, the bioactive molecules transferred by exosomes have high specificity, which can represent the identity and state of their parental cells. Besides, exosomes bear multifunction in tumour development, such as promoting carcinogenesis, transforming cancer cells’ phenotype and modifying the cancer microenvironment. Additionally, these exosomes represent sensitive and rapid responder towards environmental stimuli. The amount and composition of molecules in exosomes are associated with tumorigenesis, tumour progression and alleviation, for example, the number of exosomes increasing with aggravation in gastric cancer (Citation13, Citation14).
Table I. The contribution of EV to key steps in tumour metastasis
Compared with traditional biomarkers, exosomes bear some other advantages potentially. Firstly, exosomes can travel across the endothelium into the circulation allowing serum detection. In contrast to invasive tissue biopsy, exosomes are effective biomarkers in the diversified diagnosis of personalized medicine. Secondly, exosomes are akin to vessels enriched with much information about the parental cells, and the cargoes in exosomes are protected by the phospholipid bilayer from degradation by proteinases and nucleases. Consequently, biomarkers at a relatively low expression are much easier to be detected through isolating exosomes. For instance, some biomarkers such as PCA3 and TMPRSS2 are mRNAs not easily detected in body fluids, but appear in exosomes in prostate cancer (Citation86). Thirdly, analysing the fraction of exosomes can effectively weaken the serum matrix effect and reduce the dynamic range. Fourthly, exosomes are constituently secreted by various cells and the number of exosomes increases obviously in the serum of cancer patients (Citation87). Accordingly, it is reasonable to assume that the representativeness of exosomes is better than fine needle biopsy (Citation12), which is of great meaning for the accuracy of personalized diagnostics.
By now, a huge amount of effort has been devoted to the utilization of exosome as a cancer biomarker. Some promising biomarkers that are found in clinical studies with patients’ body fluid are summarized in Table . Although the sample size and standard operating procedures need optimization, these studies throw light upon some important potential biomarkers. As novel biomarkers, some clinical validated traditional molecules are also found in exosome-like prostate-specific antigen (PSA). These traditional molecules in exosomes possess higher specificity and relevance to disease than their total amount in serum or plasma (Citation88, Citation89). It seems the combination of traditional and novel biomarkers may achieve the highest prediction value (Citation132). Additionally, the biomarkers in exosome of ovarian cancer (Citation12), melanoma (Citation68) and pancreatic adenocarcinoma (Citation90) may be used in cancer stratification as they change regularly in antitumor therapy. Exosomes may be exploited as a companion diagnostic to identify the susceptible population and eligible patients for drugs. In the research of Kahlert et al. the mutated KRAS in exosome is associated with poor therapeutic response and has the great possibility to be utilized as a companion diagnostic for Erbitux (Citation91, Citation92). In Thakur's research, the mutated BRAFV600E in exosome can be considered as a companion diagnostic biomarker for Vemurafenib, a melanoma drug (Citation133).
Table II. Molecules in EV from body fluids of patients with cancer as potential markers for personalized medicine
The technologies in exosome diagnosis
At present, the isolation of exosomes mainly depends on non-specific physicochemical properties such as particle size, density and solubility, all of which cannot separate exosome from EVs thoroughly. Differential centrifugation, as the earliest and most classical method, is widely used in the exosome research. However, differential centrifugation is unable to separate exosomes from other EVs based on density. It is difficult to avoid aggregations of exosomes, contamination from co-precipitated lipoprotein and protein complexes and the shear forces on exosomes. It is also an operator-dependent, low-throughput and low yield method. Because of these flaws, centrifugation is unsuitable for routine clinical practice. In recent years, researchers have developed various methods to overcome these shortcomings, such as exosome precipitation, magnetic beads and size-exclusion chromatography. Their advantages and limitations are listed in Table , which in summary indicate that exosomes isolated by commercialized exosome precipitation reagent have a greater purity and quantity than the other 3 methods (Citation134–Citation136). However, it remains far from satisfactory due to low purity, which impairs downstream analysis. As high quality exosomes are critical for downstream analyses, there is an urgent need for a standard, efficient and reliable method for both exosome basic research and in clinical practice.
Table III. Isolation strategy of EV
During the last 2 decades, nucleic acid testing technologies have progressed tremendously allowing us to detect exosomal nucleic acid derived from diverse body fluids. Several platforms have been applied to exosome RNA analysis, such as microassay, digital polymerase chain reaction (PCR) and next-generation sequencing, and each has its own advantages. The digital PCR provides a higher sensitivity and precision with similar methodology to quantitative PCR. Chen et al. were able to reliably detect the mRNA mutations in isocitrate dehydrogenase (IDH1) derived from exosomes in patients’ CSF and which has a high concordance with biopsy (Citation137). Next-generation sequencing can provide thousands of gene sequences at a time, presenting the diseases relevant gene mutations and aberrant miRNA expression. Jenjaroenpun et al. obtained a different transcriptome in exosomes derived from highly and low malignant breast cancer by next-generation sequencing (Citation138). Like the detection of other material, the consistency and the transformation between data from different technologies are perturbing problems, which need further investigation.
In the detection of exosome proteins, great efforts are being devoted to specific antibody based technologies, which may directly analyse biomarkers in a cluster of exosomes from body fluids without isolation. These technologies mainly consist of ELISA, flow cytometry (Citation139), protein microarray (Citation140), handheld diagnostic magnetic resonance (Citation141), nanoplasmonic exosome technology (Citation142) and Exoscreen (Citation143). In converse, mass spectrometry has been also utilized to analyse proteins in exosomes. Although mass spectrometry can provide an accurate concentration of proteins in exosomes (Citation144), its application to clinical practice is obviously in the primary stage. Challenges that these technologies have to face include low sample throughput, the variability and complexity of body fluids and complicated sample preparation.
Challenges and perspectives
Although the studies of vesicles has been performed for decades since the first observation in 1946 (Citation145), the utilization of exosomes in diagnosis is a relatively new field. There remain lots of challenges to overcome in exosome diagnostics. Isolation of exosomes, undoubtedly, is the biggest problem, including not only standardizing the protocol in research but also creating an efficient, stable and clinically feasible extraction method. Additionally, the huge amounts of data from the developing detection technologies provide a chance not only to find the key molecules but also to render a problem of ascertaining the valuable marker amongst numerous molecules. Moreover, present potential biomarkers, the majority of which are derived from small-sample studies, need large multicentre validation. The accurate correlation between these potential markers and clinical practice also requires deeper and more rigorous studies. Last but not the least, the knowledge of the basic properties of exosomes is insufficient ranging from the factors affecting exosome synthesis, secretion and transfer to storage conditions of exosomes, which is crucial to the accuracy of exosome diagnosis.
As a carrier encapsulating a variety of molecular information, the potential and feasibility of exosome diagnosis is supported by an increasing number of publications. Many markers in cancer exosomes are associated with the state of tumours. To exploit exosomes as a biomarker, it is essential to determine the applied range of novel biomarkers and illustrate their relations with clinical validated markers. Besides, a reference standard for exosome molecule profile is needed, like the referenced genomic DNA used in present personalized medicine. For instance, Huang et al. have applied next-generation sequencing to characterize the profiles of exosome RNA in 3 healthy human plasma samples (Citation146). Furthermore, researchers have assumed that surface markers will distinguish tumour-derived exosomes from host exosomes. Unfortunately, many surface markers are shared by exosomes derived from various cancer cell lines and between tumour and non-tumour tissues. Exosome membrane proteins are of significant meaning to exosome capture. It provides a chance to improve the specificity of exosome diagnosis. The Exosome protein database such as ExoCarta constructed by ISEV, as a powerful instrument, will assist researchers to explore the synthesis mechanism and function of content in exosome (Citation144) as well as Vesicle pedia (Citation147).
Conclusion
With the development of recent research, exosomes are no longer recognized as merely part of a physiological process to remove unwanted cellular debris, but an important tool of intercellular communication, which can transport bioactive molecules and influence recipient cells. In tumour progression, exosomes are secreted by cancer cells to modify the tumour itself and its microenvironment and to participate in every step of metastasis. Since the profile of exosome contents is origin specific and because of the many other advantages, exosomes are promising cancer biomarkers in personalized medicine. Although the isolation of exosomes is a troubling issue, exosomes have the potential to be a kind of liquid biopsy with a higher sensitivity and accuracy.
Conflict of interest and funding
The authors declare that there are no relevant conflicts of interest. This work was supported by the National Natural Science Foundation of China (81371901) and Guangzhou Science and Technology Project (201510010097).
Acknowledgements
We thank the members of the Laboratory Medicine Center in Nanfang Hospital for helpful discussions and comments on this manuscript. We apologize to all researchers whose primary papers were not cited due to space constraints.
References
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003; 3: 453–8. [PubMed Abstract].
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006; 172: 923–35. [PubMed Abstract] [PubMed CentralFull Text].
- Inal JM, Ansa-Addo EA, Stratton D, Kholia S, Antwi-Baffour SS, Jorfi S et al. Microvesicles in health and disease. Arch Immunol Ther Exp (Warsz). 2012; 60: 107–21. [PubMed Abstract].
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011; 81: 1171–82. [PubMed Abstract].
- Saunderson SC, Schuberth PC, Dunn AC, Miller L, Hock BD, MacKay PA et al. Induction of exosome release in primary B cells stimulated via CD40 and the IL-4 receptor. J Immunol. 2008; 180: 8146–52. [PubMed Abstract].
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006; 31: 642–8. [PubMed Abstract].
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010; 189: 223–32. [PubMed Abstract] [PubMed CentralFull Text].
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010; 12: 19–30; sup pp. 1–13. [PubMed Abstract].
- Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011; 39: 559–62. [PubMed Abstract].
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009; 19: 43–51. [PubMed Abstract].
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012; 1820: 940–8. [PubMed Abstract].
- Szajnik M, Derbis M, Lach M, Patalas P, Michalak M, Drzewiecka H. Exosomes in plasma of patients with ovarian carcinoma: potential biomarkers of tumor progression and response to therapy. Gynecol Obstet (Sunnyvale). 2013 Suppl 4 3.
- Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003; 39: 184–91. [PubMed Abstract].
- Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010; 59: 841–50. [PubMed Abstract].
- Gao Q, Zhao YJ, Wang XY, Guo WJ, Gao S, Wei L et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology. 2014; 146: 1397–407. [PubMed Abstract].
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014; 26: 707–21. [PubMed Abstract].
- Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem Biophys Res Commun. 2014; 451: 295–301. [PubMed Abstract].
- Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014; 32: 983–97. [PubMed Abstract] [PubMed CentralFull Text].
- Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y, Kato Y, Shinto O et al. Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFbeta signaling. PLoS One. 2013; 8: e62310. [PubMed Abstract] [PubMed CentralFull Text].
- Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial–mesenchymal transition. Mol Cell Proteomics. 2013; 12: 2148–59. [PubMed Abstract] [PubMed CentralFull Text].
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654–9. [PubMed Abstract].
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013; 32: 623–42. [PubMed Abstract].
- Josson S, Gururajan M, Sung SY, Hu P, Shao C, Zhau HE et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2014; 34: 2690–9. [PubMed Abstract].
- Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012; 24: 277–83. [PubMed Abstract] [PubMed CentralFull Text].
- Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog. 2013 doi: 10.1002/mc.22124. [Epub ahead of print].
- Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013; 383: 13–20. [PubMed Abstract].
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012; 151: 1542–56. [PubMed Abstract].
- Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJ, Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles. 2013; 2 [PubMed CentralFull Text].
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003; 3: 362–74. [PubMed Abstract].
- Kriebel PW, Barr VA, Rericha EC, Zhang G, Parent CA. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J Cell Biol. 2008; 183: 949–61. [PubMed Abstract] [PubMed CentralFull Text].
- Wong AD, Searson PC. Live-cell imaging of invasion and intravasation in an artificial microvessel platform. Cancer Res. 2014; 74: 4937–45. [PubMed Abstract].
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007; 67: 2649–56. [PubMed Abstract].
- Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013; 288: 36691–702. [PubMed Abstract] [PubMed CentralFull Text].
- Soki FN, Koh AJ, Jones JD, Kim YW, Dai J, Keller ET et al. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J Biol Chem. 2014; 289: 24560–72. [PubMed Abstract].
- Taraboletti G, D'Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi D et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006; 8: 96–103. [PubMed Abstract] [PubMed CentralFull Text].
- Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012; 130: 2033–43. [PubMed Abstract] [PubMed CentralFull Text].
- Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011; 186: 2219–28. [PubMed Abstract].
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008; 27: 2128–36. [PubMed Abstract].
- Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M et al. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014; 31: 148. [PubMed Abstract].
- Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014; 2014: 864894. [PubMed Abstract] [PubMed CentralFull Text].
- Corcoran C, Friel AM, Duffy MJ, Crown J, O'Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin Chem. 2011; 57: 18–32. [PubMed Abstract].
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014; 25: 501–15. [PubMed Abstract] [PubMed CentralFull Text].
- Egan K, Crowley D, Smyth P, O'Toole S, Spillane C, Martin C et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011; 6: e26125. [PubMed Abstract] [PubMed CentralFull Text].
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005; 113: 752–60. [PubMed Abstract].
- Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012; 315: 28–37. [PubMed Abstract].
- Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011; 6: e16899. [PubMed Abstract] [PubMed CentralFull Text].
- Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013; 41: 245–51. [PubMed Abstract] [PubMed CentralFull Text].
- Cereghetti DM, Lee PP. Tumor-derived exosomes contain microRNAs with immunological function: implications for a novel immunosuppression mechanism. Microrna. 2014; 2: 194–204. [PubMed Abstract].
- Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010; 5: e11469. [PubMed Abstract] [PubMed CentralFull Text].
- Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012; 22: 342–9. [PubMed Abstract].
- Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008; 25: 305–24. [PubMed Abstract].
- Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011; 108: 3725–30. [PubMed Abstract] [PubMed CentralFull Text].
- Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS Jr, Gimbrone MA. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987; 84: 9238–42. [PubMed Abstract] [PubMed CentralFull Text].
- Soderberg A, Barral AM, Soderstrom M, Sander B, Rosen A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med. 2007; 43: 90–9. [PubMed Abstract].
- Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009; 106: 3794–9. [PubMed Abstract] [PubMed CentralFull Text].
- Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999; 5: 439–47. [PubMed Abstract].
- Reymond N, Im JH, Garg R, Vega FM, Borda dAB, Riou P et al. Cdc42 promotes transendothelial migration of cancer cells through beta1 integrin. J Cell Biol. 2012; 199: 653–68. [PubMed Abstract] [PubMed CentralFull Text].
- Barthel SR, Hays DL, Yazawa EM, Opperman M, Walley KC, Nimrichter L et al. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 2013; 73: 942–52. [PubMed Abstract] [PubMed CentralFull Text].
- Brandt B, Heyder C, Gloria-Maercker E, Hatzmann W, Rotger A, Kemming D et al. 3D-extravasation model – selection of highly motile and metastatic cancer cells. Semin Cancer Biol. 2005; 15: 387–95. [PubMed Abstract].
- Gambim MH, do CAO, Marti L, Verissimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce EC apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007; 11: R107. [PubMed Abstract] [PubMed CentralFull Text].
- Reymond N, d'Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013; 13: 858–70. [PubMed Abstract].
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009; 9: 285–93. [PubMed Abstract] [PubMed CentralFull Text].
- Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006; 66: 11089–93. [PubMed Abstract] [PubMed CentralFull Text].
- Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009; 69: 785–93. [PubMed Abstract].
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005; 438: 820–7. [PubMed Abstract] [PubMed CentralFull Text].
- Jung T, Castellana D, Klingbeil P, Cuesta HI, Vitacolonna M, Orlicky DJ et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009; 11: 1093–105. [PubMed Abstract] [PubMed CentralFull Text].
- Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013; 15: 281–95. [PubMed Abstract] [PubMed CentralFull Text].
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012; 18: 883–91. [PubMed Abstract] [PubMed CentralFull Text].
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014; 7: ra63. [PubMed Abstract].
- Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol. 2014; 28: 51–7. [PubMed Abstract].
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012; 109: E2110–16. [PubMed Abstract] [PubMed CentralFull Text].
- Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005; 34: 206–13. [PubMed Abstract].
- Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007; 67: 7458–66. [PubMed Abstract].
- Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011; 6: e22517. [PubMed Abstract] [PubMed CentralFull Text].
- Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996; 56: 5754–7. [PubMed Abstract].
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140: 883–99. [PubMed Abstract] [PubMed CentralFull Text].
- King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012; 12: 421. [PubMed Abstract] [PubMed CentralFull Text].
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002; 29(Suppl 16): 15–18. [PubMed Abstract].
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990; 348: 555–7. [PubMed Abstract].
- Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014; 20: 385–93. [PubMed Abstract].
- Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011; 71: 3792–801. [PubMed Abstract].
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010; 70: 1668–78. [PubMed Abstract].
- Jain KK. Personalized medicine: the impact of pharmacogenetics on drug development. 1998; Waltham, MA: Decision Resources. v, 44 leaves p.
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012; 366: 883–92. [PubMed Abstract].
- Ginestra A, La Placa MD, Saladino F, Cassara D, Nagase H, Vittorelli ML. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998; 18: 3433–7. [PubMed Abstract].
- Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009; 100: 1603–7. [PubMed Abstract] [PubMed CentralFull Text].
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012; 64: 676–705. [PubMed Abstract].
- Mizutani K, Terazawa R, Kameyama K, Kato T, Horie K, Tsuchiya T et al. Isolation of prostate cancer-related exosomes. Anticancer Res. 2014; 34: 3419–23. [PubMed Abstract].
- Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z et al. Can urinary exosomes act as treatment response markers in prostate cancer?. J Transl Med. 2009; 7: 4. [PubMed Abstract] [PubMed CentralFull Text].
- Que R, Ding G, Chen J, Cao L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J Surg Oncol. 2013; 11: 219. [PubMed Abstract] [PubMed CentralFull Text].
- Yamashita T, Kamada H, Kanasaki S, Maeda Y, Nagano K, Abe Y et al. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharmazie. 2013; 68: 969–73. [PubMed Abstract].
- Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014; 289: 3869–75. [PubMed Abstract] [PubMed CentralFull Text].
- Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, Ashok A et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One. 2012; 7: e46737. [PubMed Abstract] [PubMed CentralFull Text].
- Lu Q, Zhang J, Allison R, Gay H, Yang WX, Bhowmick NA et al. Identification of extracellular delta-catenin accumulation for prostate cancer detection. Prostate. 2009; 69: 411–18. [PubMed Abstract] [PubMed CentralFull Text].
- Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012; 106: 768–74. [PubMed Abstract] [PubMed CentralFull Text].
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008; 105: 10513–18. [PubMed Abstract] [PubMed CentralFull Text].
- Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One. 2009; 4: e6229. [PubMed Abstract] [PubMed CentralFull Text].
- Helley D, Banu E, Bouziane A, Banu A, Scotte F, Fischer AM et al. Platelet microparticles: a potential predictive factorof survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol. 2009; 56: 479–84. [PubMed Abstract].
- Tavoosidana G, Ronquist G, Darmanis S, Yan J, Carlsson L, Wu D et al. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci U S A. 2011; 108: 8809–14. [PubMed Abstract] [PubMed CentralFull Text].
- Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009; 9: 244. [PubMed Abstract] [PubMed CentralFull Text].
- Keller S, Konig AK, Marme F, Runz S, Wolterink S, Koensgen D et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009; 278: 73–81. [PubMed Abstract].
- Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007; 107: 563–71. [PubMed Abstract].
- Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004; 64: 7045–9. [PubMed Abstract].
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008; 110: 13–21. [PubMed Abstract].
- Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011; 32: 1976–83. [PubMed Abstract].
- Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA et al. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004; 31: 114–21. [PubMed Abstract].
- Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010; 28: 1721–6. [PubMed Abstract].
- Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN et al. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013; 8: 1156–62. [PubMed Abstract] [PubMed CentralFull Text].
- Rodriguez M, Silva J, Lopez-Alfonso A, Lopez-Muniz MB, Pena C, Dominguez G et al. Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes Chromosomes Cancer. 2014; 53: 713–24. [PubMed Abstract].
- Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009; 10: 42–6. [PubMed Abstract].
- Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012; 18: 1835–40. [PubMed Abstract] [PubMed CentralFull Text].
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008; 10: 1470–6. [PubMed Abstract] [PubMed CentralFull Text].
- Mao X, Sun Y, Tang J. Serum miR-21 is a diagnostic and prognostic marker of primary central nervous system lymphoma. Neurol Sci. 2014; 35: 233–8. [PubMed Abstract].
- Manterola L, Guruceaga E, Gallego P-LJ, Gonzalez-Huarriz M, Jauregui P, Tejada S et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro-Oncology. 2014; 16: 520–7. [PubMed Abstract] [PubMed CentralFull Text].
- Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S et al. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013; 8: e78115. [PubMed Abstract] [PubMed CentralFull Text].
- Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010; 5: 13515.
- Rupp AK, Rupp C, Keller S, Brase JC, Ehehalt R, Fogel M et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011; 122: 437–46. [PubMed Abstract].
- Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012; 227: 658–67. [PubMed Abstract].
- Arscott WT, Camphausen KA. EGFR isoforms in exosomes as a novel method for biomarker discovery in pancreatic cancer. Biomark Med. 2011; 5: 821. [PubMed Abstract].
- Lau C, Kim Y, Chia D, Spielmann N, Eibl G, Elashoff D et al. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J Biol Chem. 2013; 288: 26888–97. [PubMed Abstract] [PubMed CentralFull Text].
- Choi DS, Park JO, Jang SC, Yoon YJ, Jung JW, Choi DY et al. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics. 2011; 11: 2745–51. [PubMed Abstract].
- Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014; 9: e92921. [PubMed Abstract] [PubMed CentralFull Text].
- Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012; 51: 409–18. [PubMed Abstract].
- Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009; 4: e5219. [PubMed Abstract] [PubMed CentralFull Text].
- Smalley DM, Sheman NE, Nelson K, Theodorescu D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J Proteome Res. 2008; 7: 2088–96. [PubMed Abstract].
- Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis?. J Thromb Haemost. 2007; 5: 520–7. [PubMed Abstract].
- Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013; 119: 1159–67. [PubMed Abstract].
- Tanaka M, Oikawa K, Takanashi M, Kudo M, Ohyashiki J, Ohyashiki K et al. Down-regulation of miR-92 in human plasma is a novel marker for acute leukemia patients. PLoS One. 2009; 4: e5532. [PubMed Abstract] [PubMed CentralFull Text].
- Wang W, Li H, Zhou Y, Jie S. Peripheral blood microvesicles are potential biomarkers for hepatocellular carcinoma. Cancer Biomark. 2013; 13: 351–7. [PubMed Abstract].
- Aushev VN, Zborovskaya IB, Laktionov KK, Girard N, Cros MP, Herceg Z et al. Comparisons of microRNA patterns in plasma before and after tumor removal reveal new biomarkers of lung squamous cell carcinoma. PLoS One. 2013; 8: e78649. [PubMed Abstract] [PubMed CentralFull Text].
- Fleitas T, Martínez-Sales V, Vila V, Reganon E, Mesado D, Martín M et al. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012; 7: e47365. [PubMed Abstract] [PubMed CentralFull Text].
- Younus S, Rodgers G. Biomarkers associated with cardiometabolic risk in obesity. Am Heart Hosp J. 2011; 9: E28–32. [PubMed Abstract].
- Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014; 24: 766–9. [PubMed Abstract] [PubMed CentralFull Text].
- Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol. 2011; 728: 235–46. [PubMed Abstract].
- Rekker K, Saare M, Roost AM, Kubo AL, Zarovni N, Chiesi A et al. Comparison of serum exosome isolation methods for microRNA profiling. Clin Biochem. 2014; 47: 135–8. [PubMed Abstract].
- Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012; 82: 1024–32. [PubMed Abstract].
- Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA et al. BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic Acids. 2013; 2: e109. [PubMed Abstract] [PubMed CentralFull Text].
- Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013; 1: e201. [PubMed Abstract] [PubMed CentralFull Text].
- Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010; 77: 502–14. [PubMed Abstract] [PubMed CentralFull Text].
- Jorgensen M, Baek R, Pedersen S, Sondergaard EK, Kristensen SR, Varming K. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013; 2
- Shao H, Min C, Issadore D, Liong M, Yoon TJ, Weissleder R et al. Magnetic nanoparticles and microNMR for diagnostic applications. Theranostics. 2012; 2: 55–65. [PubMed Abstract] [PubMed CentralFull Text].
- Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014; 32: 490–5. [PubMed Abstract] [PubMed CentralFull Text].
- Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014; 5: 3591. [PubMed Abstract] [PubMed CentralFull Text].
- Inal JM, Kosgodage U, Azam S, Stratton D, Antwi-Baffour S, Lange S. Blood/plasma secretome and microvesicles. Biochim Biophys Acta. 2013; 1834: 2317–25. [PubMed Abstract].
- Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946; 166: 189–97. [PubMed Abstract].
- Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013; 14: 319. [PubMed Abstract] [PubMed CentralFull Text].
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012; 10: e1001450. [PubMed Abstract] [PubMed CentralFull Text].
