Abstract
Cells secrete extracellular vesicles (EVs) by default and in response to diverse stimuli for the purpose of cell communication and tissue homeostasis. EVs are present in all body fluids including peripheral blood, and their appearance correlates with specific physiological and pathological conditions. Here, we show that physical activity is associated with the release of nano-sized EVs into the circulation. Healthy individuals were subjected to an incremental exercise protocol of cycling or running until exhaustion, and EVs were isolated from blood plasma samples taken before, immediately after and 90 min after exercise. Small EVs with the size of 100–130 nm, that carried proteins characteristic of exosomes, were significantly increased immediately after cycling exercise and declined again within 90 min at rest. In response to treadmill running, elevation of small EVs was moderate but appeared more sustained. To delineate EV release kinetics, plasma samples were additionally taken at the end of each increment of the cycling exercise protocol. Release of small EVs into the circulation was initiated in an early phase of exercise, before the individual anaerobic threshold, which is marked by the rise of lactate. Taken together, our study revealed that exercise triggers a rapid release of EVs with the characteristic size of exosomes into the circulation, initiated in the aerobic phase of exercise. We hypothesize that EVs released during physical activity may participate in cell communication during exercise-mediated adaptation processes that involve signalling across tissues and organs.
To access the supplementary material to this article, please see Supplementary files under ‘Article Tools’.
Extracellular vesicles (EVs) comprise a diverse group of membrane-surrounded particles secreted by cells into the extracellular space (Citation1, Citation2). EVs recently gained interest with regard to their involvement in intercellular communication as they can specifically interact with target cells over long distances and mediate horizontal transfer of biologically active molecules, including enzymes, mRNAs and small RNAs. According to their mode of release, EVs are classified into apoptotic bodies (>1 µm), microvesicles (MVs) shed from the plasma membrane (>100 nm) and smaller exosomes (around 100 nm) secreted from multivesicular endosomes (Citation3, Citation4). All body fluids contain a heterogeneous mixture of these vesicles derived from various cells of the body. In addition to a set of common EV proteins utilized for their characterization, EVs carry a pattern of biomolecules related to their mother cell. Their content includes biologically active enzymes (e.g. the chaperone Hsp70), as well as nucleic acids, largely in the form of small RNAs. MV and exosome secretion occurs in constitutive and regulated fashion, controlled by Ca2+ signalling in response to extracellular signals such as ATP (monocytes), neurotransmitters (oligodendrocytes), depolarization (neurons), thrombin receptor activation (platelets), lipopolysaccharides (dendritic cells) or by cell stress (Citation5–Citation13).
Peripheral blood contains a substantial amount of circulating EVs and their amount, composition and molecular profile reflects the physiological and pathophysiological condition of the body. Thus, EVs received recognition as potential biomarkers in semi-invasive diagnostics (Citation14). Proteomic profiling of plasma EVs revealed high variability between individuals in quantitative and qualitative terms, indicating that EVs in the circulation are contributed from multiple cell types across tissues and are highly dynamic (Citation15). The vast majority of plasma EVs originate from platelets and erythrocytes, which shed MV and secrete exosomes during their activation and maturation, respectively (Citation13, Citation16) (Citation17). Furthermore, endothelial cells exposed to shear stress shed EVs that appear elevated under several pathological conditions (Citation18–Citation21).
Physical exercise is associated with immediate changes in physiological parameters [such as heart rate, blood pressure, respiration, lactate levels and circulating cell-free DNA (cfDNA)] and elicits an acute stress response. Furthermore, regular exercise initiates long-term adaptation processes of muscle metabolism, the cardiovascular system as well as immune modulatory effects that are largely considered beneficial (Citation22–Citation25). Several reports examined the effect of various exercise protocols on circulating EVs, mainly focussing on larger particles (>500 nm) reflecting apoptotic bodies and MVs. These studies revealed that platelet-derived pro-coagulant particles, as well as monocyte-derived particles transiently increased in response to strenuous exercise (Citation26–Citation31), while the levels of endothelial-derived MVs were unchanged (Citation30, Citation32) (Citation33) or only elevated after moderate endurance exercise (Citation30). However, the impact of physical activity on the level of small EVs with characteristic features of exosomes in the circulation has never been addressed. Here, we analyzed the dynamics of EVs below the size of 200 nm detected in blood plasma during the acute phase of exercise and the early recovery period. We found that the levels of small EVs increased in response to cycling exercise and dropped during the early recovery phase. Intriguingly, the dynamics of small EVs in peripheral blood differed between cycling and running exercise protocols. The results suggest that small EVs such as exosomes may be implicated in long distance signalling during exercise-mediated adaptation processes.
Materials and methods
Ethical approval
All experimental procedures were approved by the Human Ethics Committee Rhineland-Palatinate in agreement with the standards of the declaration of Helsinki of the World Medical Association. We informed all subjects orally and in written form about the procedures and the purpose of the study, and they confirmed in writing to take part.
Subjects and exercise setting
Twelve healthy, physically active (physical activity more than 3 h per week), male volunteers participated in this study. If subjects had signs of infection or other diseases, injuries, were taking prescription drugs or anticoagulant medicine (e.g. Aspirin), they were excluded. Since physical activity influences physiological pathways, the participants abstained from exercise 24 h before the test. The subjects had a standardized breakfast, and all tests were executed at 9:00 a.m. This ensured that all parameters were at baseline levels when starting. In the first exercise setting, the subjects performed an incremental cycling test on an ergometer (ergoselect 200; Ergoline, Bitz, Germany) starting at 50 W and increasing power by 50 W every 3 min until exhaustion. In the second setting, the subjects performed an incremental test on a motorized treadmill (HP Cosmos, Traunstein, Germany) with starting speed at 6 km/h and increasing velocity by 2 km/h every 3 min with a constant incline of 1.5% until exhaustion. During both tests, heart rate, ventilatory and metabolic responses were monitored. Thirty millilitres of blood was taken from the medial cubital vein using a Safety-Multifly® needle (0.8×19 mm) (Sarstedt, Nümbrecht, Germany) and collected in tripotassium-EDTA covered Monovettes® (Sarstedt) before (pre), immediately after (post), 90 min after (90+) and 360 min (360+, treadmill running only) after the test. To analyse the kinetics of EV release, blood samples (7.5 ml) were collected after each increment. For lactate analysis, 20 µl of capillary blood was collected from the earlobe prior to the run, at the end of each exercise step, directly after the run and after 10, 30 and 90 min of recovery. The samples were measured with the lactate analyser Biosen 5130 (EKF Diagnostics, Magdeburg, Germany). The individual anaerobic threshold (IAT) was calculated for cycling determining the point of 1 mmol lactate increase above baseline and for running 1.5 mmol increase above baseline (Citation34).
EV isolation
Purification of EVs was achieved by differential centrifugation as described before with modifications (Citation35). Directly after blood draw (maximum 5 min delay), blood was centrifuged at 1,600×g for 10 min at 4°C in an Eppendorf FA-45-6-30 rotor (Eppendorf, Hamburg, Germany). Plasma was always kept on ice. The plasma fraction was transferred carefully, and 2 ml of the supernatant was centrifuged at 10,000×g for 30 min at 4°C in a fixed angle rotor (220.78, Hermle, Wehingen, Germany). The remaining pellet was washed 2 times with PBS to remove soluble proteins (MV pellet) and solved in 150 µl standard SDS–PAGE sample buffer (4×100 mM dithiothreitol). Two millilitres of the 10,000×g supernatant was filtered through 0.2 µm syringe filters (Millex-GP; Merck Millipore, Darmstadt, Germany), and 1.4 ml of the filtrate was centrifuged for 2 h at 47,000 rpm [RCF (avg) 98,963, RCF (max) 130,000, k-factor 90.4] and 4°C in a Beckman TLA-55 rotor (Beckman Coulter, Krefeld, Germany) using 1.5 ml Beckman Polyallomer tubes to pellet small EVs. For the analysis of cfDNA, 100,000×g supernatants were retained (release kinetics). EV pellets were stored at −20°C before analysis. For Western blotting, 100,000×g pellets were washed 2 times with PBS and subsequently resuspended in 20 µl SDS–PAGE sample buffer (4×). For nanoparticle tracking (NTA) analysis, 100,000×g pellets were dissolved in 200 µl PBS.
Western blot analysis
The following antibodies were used: mouse anti-Hsp70 (SC-24; Santa Cruz, Heidelberg, Germany, 1:1,000), rabbit anti-Flotillin-1 (F1180; Sigma–Aldrich, Taufkirchen, Germany, 1:1,000), mouse anti-Tsg101 (4A10; GeneTex, Irvine, CA, USA), mouse anti-Integrin αIIb (SC-59923; Santa Cruz, 1:1,000) and HRP-coupled secondary antibodies (Goat-anti-Mouse-HRP, 115-035-003, 1:10,000; Goat-anti-Rabbit-HRP, 111-035-003, 1:10,000; Dianova, Hamburg, Germany).
EV pellets dissolved in sample buffer were subjected to SDS–PAGE (4–12% Bis-Tris gel) and Western blotting (NuPAGE; Life Technologies, Darmstadt, Germany). For analysis of 10,000×g and 100,000×g pellets, 20 µl was loaded on the gel. Proteins were blotted onto a PVDF membrane. Next, the membrane was blocked with 4% milk powder, 0.1% Tween in PBS and incubated sequentially with primary and HRP-coupled secondary antibodies. Proteins were detected with chemiluminescence reagents (Luminata Crescendo, Merck Millipore, Darmstadt, Germany) and X-ray films. X-ray films were scanned and signal intensities were measured using ImageJ 1.44 h (National Institutes of Health, Bethesda, MD, USA).
Nanoparticle tracking
Pellets of 100,000×g resulting from 1.4 ml blood plasma were resuspended in 200 µl PBS and 1:10 dilutions were analyzed using the Nanosight LM10 system (camera model Hamamatsu C11440-50B/A11893-02) equipped with the green laser (532 nm) and the syringe pump and the Nanosight 2.3 software (Malvern, Herrenberg, Germany) at 23°C (temperature controlled). The following settings were used: camera control in standard mode (camera level 16), particle detection in standard mode (screen gain 16, detection threshold 6 and minimum expected particle size auto). Script control was used (Repeatstart, Syringeload 500, Delay 5, Syringestop, Delay 15, Capture 30 and Repeat 4). Five 30 s videos were recorded, particles were tracked (batch process) and average values were formed. Particle measurements were verified utilizing silica microspheres (Polysciences, Warrington, PA, USA) with a size of 100 and 300 nm as described in Gardiner et al. (Citation36).
Quantification of cfDNA
DNA was purified from 700 µl of the 100,000×g supernatants using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. DNA was eluted in 70 µl of the kit's AVE elution buffer, and samples were stored at −20°C. Concentrations were measured within a maximum of 1 week.
Quantification of cfDNA was based on the amplification of human long terminal repeat sequences as described before (Citation37). Samples were analyzed by quantitative real-time PCR (qPCR) with an iCycler MyIQ Detection System (Bio-Rad, Munich, Germany) under the following conditions: 5 min incubation at 95°C, followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 61°C for 30 s and extension at 80°C for 30 s. The experiments were performed in triplicates with a final volume of 15 µl per reaction including 5 µl of template DNA, 7.5 µl PCR master mix containing 0.1 U/µl HotStarTaq Plus Polymerase (Qiagen), 2×PCR buffer (Qiagen), 1 mM MgCl2 (Qiagen), 0.4 mM dNTPs (Carl Roth, Karlsruhe, Germany), 0.28×SYBR green (Sigma–Aldrich), 5 nM FITC (Sigma–Aldrich) and 2.5 µl primer mix of a final concentration of 312 nM. Non-template controls and positive controls for interplate calibration were also analyzed in triplicates. Formation of the expected PCR product was confirmed by melting curve analysis. The qPCR data were captured with the MyIQ5 Optical System Software, Version 2.4 (Bio-Rad, Munich, Germany).
Statistical analysis
Data revealed by ImageJ for the various Western blot analyses were neither normally distributed as determined by Wilk–Shapiro W-test, nor homogenous in variance as indicated by the Levene test. Box–Cox transformation of data succeeded to achieve normal distributed values with equal variances between groups only for the running but not for the cycling condition. Values relative to resting levels in the cycling setting were, therefore, statistically compared using Kruskal–Wallis testing. For the running condition, Box–Cox-transformed data were compared using paired Student's t-test. For both parametric and non-parametric testing, data revealing a significant global test across all points in time followed by a respective significant post-hoc test on an alpha level of 0.05 and smaller were considered to be significant. We corrected the p-value of the post hoc testing for the multiple comparisons by applying the Bonferroni correction. Statistical analysis was performed with JMP 11 (SAS Institute, Inc., Cary, NC, USA).
Results
Cycling exercise triggers rapid release of small EVs
To investigate the influence of a single bout of physical activity on the levels of circulating EVs in plasma, we recruited 8 healthy, physically active men [mean (SD) age, 41.1 (±14.9) years; IAT, 181.1 (±77.8) W] performing an incremental cycling test until exhaustion [mean (SD) time 16.6 (±5) min]. We collected venous blood samples (EDTA-anticoagulated blood) before (pre), immediately after (post) and 90 min after cessation of exercise (90+) and prepared plasma. To analyse the levels of EVs in the plasma samples, we performed differential centrifugation at 10,000×g pelleting larger vesicles including platelet remnants and apoptotic bodies (here defined as crude MVs), followed by filtration of the supernatant and ultracentrifugation at 100,000×g to collect small vesicles of a size below 200 nm, which include exosomes (here defined as small EVs). Pellets of 100,000×g derived from 2 study participants (age >30 years) were analyzed by NTA revealing particles with a mean size of 120 (SEM±3.6) nm in diameter (a). The total amount of particles increased in average 2.7 times (SEM±0.2) directly after exercise and returned to baseline levels after 90 min. Next, we investigated 100,000×g pellets of 6 subjects by Western blotting with antibodies against the EV marker proteins Flot1, Hsp/Hsc70 and Tsg101 (b) and quantified signal intensities (c–f). The numbers of these 3 universal EV marker proteins increased on average 5.2 times (SEM±1.3) after exercise. Moreover, we detected integrin αIIb found on platelets indicating the presence of platelet-derived small EVs (b and f). The level of this protein rose 2.9 times (SEM±1.2) upon exercise. Samples taken in the early recovery phase after exercise (90+) revealed a decrease in the amounts of EV proteins, but the levels were not fully reaching the baseline as determined by NTA. A statistical analysis using the Wilcoxon–Kruskal–Wallis test revealed that the detected associations between release of small EVs and exercise were significant for Flot1 (p=0.0125) and Hsp70 (p=0.002) on a global level across all 3 points in time (pre, post and 90 min after exercise) with post hoc testing revealing significant increases pre to post (Flot1, p=0.0021 and Hsp/Hsc70, p=0.0021), respectively (c–f). Variances in the amount of small EVs released during exercise between individual subjects were noticeable and may depend on the condition of the test person and age. Individuals above the age of 30 appear to respond less intensely to exercise (n=2, highlighted by red symbols in b–f), although this observation certainly requires further in-depth analysis.
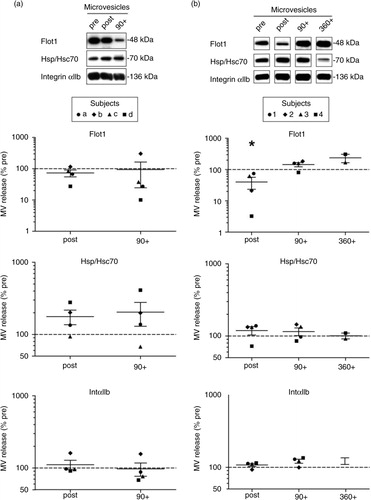
Fig. 1. Effect of cycling exercise on plasma small EVs. Plasma samples were collected pre, post and 90 min post (90+) ergometer exercise, and 100,000×g pellets were prepared. Particle size and concentration were measured by NTA [(a) subjects a and b, age above 30] and protein content was analyzed by Western blotting [(b) subjects 1–6]. Western blot signals of Flot1 [(c) pre/post: n=6, 90+: n=5], Hsp/Hsc70 [(d) pre/post: n=6, 90+: n=5], Tsg101 [(e) pre/post: n=3, 90+: n=2; not detectable in all experiments] and IntαIIb [(f) pre/post: n=6, 90+: n=5] were quantified (**p<0.01, Wilcoxon–Kruskal–Wallis test). Subjects older than 30 years are marked in red (subject 2, 38 years; subject 5, 58 years).
![Fig. 1. Effect of cycling exercise on plasma small EVs. Plasma samples were collected pre, post and 90 min post (90+) ergometer exercise, and 100,000×g pellets were prepared. Particle size and concentration were measured by NTA [(a) subjects a and b, age above 30] and protein content was analyzed by Western blotting [(b) subjects 1–6]. Western blot signals of Flot1 [(c) pre/post: n=6, 90+: n=5], Hsp/Hsc70 [(d) pre/post: n=6, 90+: n=5], Tsg101 [(e) pre/post: n=3, 90+: n=2; not detectable in all experiments] and IntαIIb [(f) pre/post: n=6, 90+: n=5] were quantified (**p<0.01, Wilcoxon–Kruskal–Wallis test). Subjects older than 30 years are marked in red (subject 2, 38 years; subject 5, 58 years).](/cms/asset/b03aff9f-f88b-4eb8-af8e-0ae9bc074224/zjev_a_11815559_f0001_ob.jpg)
Western blot analysis of 10,000×g pellets (including MVs) revealed fluctuations in the levels of Flot1, Hsp/Hsc70 and Integrin αIIb across the different time points of analysis; however, no significant correlation with physical activity was observed (n=4, Wilcoxon–Kruskal–Wallis global analysis: Flot1, p=0.32; Hsp/Hsc70, p=0.38; IntαIIb, p=0.23, a). Taken together, acute bicycle exercise triggers the release of small EVs with characteristic features of exosomes into the circulation followed by a fast decline in the early recovery phase, while MVs seem to be less affected.
Fig. 2. Effect of exercise on plasma microvesicles (MV). Semi-quantitative Western blot analysis of Flot1, Hsp70 and IntαIIb in 10,000×g MV pellets isolated from plasma after incremental ergometer cycling (a, n=4) or treadmill running exercise (b, n=4). Plasma samples were collected pre, post and 90 min post-cycling (a) and in addition 360 min postrunning exercise (b). Overall, no significant correlation between the detected MV markers and physical activity was observed, with the exception of Flot1, which revealed lower signals immediately after running exercise (*p<0.05, Wilcoxon–Kruskal–Wallis test).
Sustained elevation of plasma small EVs after running exercise
To examine whether stimulation of small EV/exosome release by physical activity is dependent on the mode of exercise, we modified the exercise setting and performed treadmill running, which has a higher eccentric component and is commonly associated with higher heart rates at the same level of subjective load or rate of perceived exertion. Four healthy, physically active men [mean (SD) age, 27 (±2.1) years; IAT, 11.8 (±0.8) km/h] performed an incremental treadmill test until exhaustion [mean (SD), 14.7 (±0.9) min]. Analogous to the cycling experiments, venous blood was collected (pre, post and 90+) with the implementation of a longer recovery period of 6 h (360+) in 2 subjects, followed by isolation of crude MVs and EVs below 200 nm. Analysis of MV pellets by Western blotting revealed no major changes of MV levels related to exercise with the exception of Flot1 (b, p=0.029), which decreased immediately after exercise. The 100,000×g pellets were analyzed by NTA (a) and Western blotting (b–e). The amount of small EVs appeared elevated directly after running exercise [in average 1.5 (SEM±0.2) times more particles detected by NTA, mean size 164 (SEM±5.5) nm], although to a lower extent as observed in the cycling setting. The minor rise may be partly explained by higher baseline levels of small EVs present already at the pre-exercise stage in some subjects (see a, subject b). While the EV marker Flot1 was elevated after exercise (p=0.029), Hsp/Hsc70 and IntαIIb showed a similar trend (Tsg101 was not detectable in these experiments). During the early recovery phase, signals for small EVs were varying but in general stayed elevated 90 min post exercise (90+, b–e, see also Supplementary Fig. 1, progression curves). In 2 participants, we assessed a prolonged follow-up at 6 and 24 h, which indicated a return to baseline of data assessed by NTA as well as by Western blots. In summary, running exercise is associated with different dynamics of small EV/exosome release, characterized by a more moderate increase in EV levels that are maintained through the early recovery phase.
Fig. 3. Effect of running exercise on plasma small EVs. Plasma samples were taken pre, post, 90 post (90+) and 360 post (360+) treadmill exercise. Pellets of 100,000×g were analyzed for particle size and concentration by NTA [(a) subjects a and b] and for protein composition by Western blotting [(b) subjects 1–4]. Signal intensities of Flot1 (c), Hsp/Hsc70 (d) and IntαIIb (e) were quantified (pre/post/90+: n=4, 360+: n=2, p<0.05, Student's t-test).
![Fig. 3. Effect of running exercise on plasma small EVs. Plasma samples were taken pre, post, 90 post (90+) and 360 post (360+) treadmill exercise. Pellets of 100,000×g were analyzed for particle size and concentration by NTA [(a) subjects a and b] and for protein composition by Western blotting [(b) subjects 1–4]. Signal intensities of Flot1 (c), Hsp/Hsc70 (d) and IntαIIb (e) were quantified (pre/post/90+: n=4, 360+: n=2, p<0.05, Student's t-test).](/cms/asset/592e7d4b-0f71-4e91-b4fb-57c1e8bb40eb/zjev_a_11815559_f0003_ob.jpg)
Release of small EVs is initiated in the aerobic phase of exercise
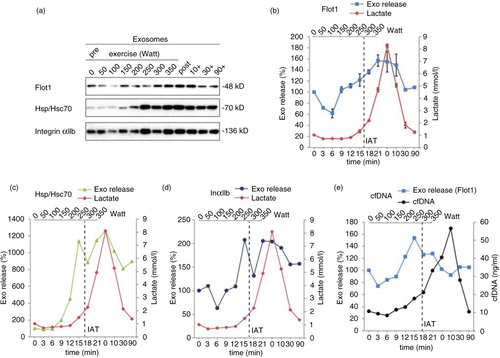
To assess the release kinetics of small EVs/exosomes during exercise, we collected blood samples after every increment of cycling exercise and purified small EVs as described above (1 subject and 2 independent experiments). The cycling protocol was chosen, since it allows convenient blood extraction at the increments and revealed robust release of small EVs in the previous experiments. By Western blotting, 100,000×g pellets were analyzed for the EV proteins Flot1, Hsc/Hsp70 and platelet-derived IntαIIb (a). The obtained signals were quantified and plotted against time and power. EV amounts started to increase after 9 min (150 W), rose more or less constantly to reach the maximum between 15 and 21 min (250–300 W) and started to decline 10 min post exercise (b–d). In addition, we determined lactate concentrations and the levels of cfDNA, which have been shown to rise during exercise with release kinetics similar to lactate (Citation38, Citation39). Lactate levels began to elevate after 15 min (250 W), and the IAT was reached at 289 (±8) W, demonstrating that small EV release is initiated before the IAT and the accumulation of lactate and acidification of blood (b–e). cfDNA concentrations started to rise after 15 min (250 W) and reached the maximum 10 min after cessation of exercise (e), providing evidence that secretion of small EVs and cfDNA release is 2 independent mechanistic events. Thus, small EV release is triggered early during exercise and occurs before lactate and cfDNA release.
Fig. 4. Kinetic analysis of small EV release during incremental cycling exercise. Kinetics of exosome, lactate (a–d) and cfDNA (e) release were recorded during and after an incremental cycling exercise (n=2, same subject). Plasma samples taken after each increment and post exercise were subjected to differential centrifugation, and small EVs were analyzed by Western blot (a). Western Blot signals were quantified for Flot1 (b, n=2), Hsp/Hsc70 (c, n=1) and IntαIIb (d, n=1). The individual anaerobic threshold (IAT) is indicated as vertical dashed line.
Discussion
Exercise is associated with a number of immediate physiological responses as well as long-range homeostatic processes. EVs emerge as comprehensive signalling entities mediating adaptive responses over large distances with widespread implications in physiology (Citation40, Citation41). In this study, we asked whether physical activity affects the level of EVs in the circulation with focus on small EVs. The results indicate that a single bout of exhaustive exercise triggers the release of EVs with the size and marker profile of exosomes, which are cleared from the circulation during the early recovery period after cycling, but stay elevated after running exercise. Increasing levels of larger platelet and endothelial cell-derived microparticles have been described after strenuous as well as moderate exercise, focussing on aspects of blood haemostasis (Citation26, Citation28) (Citation30, Citation31). A recent study demonstrated that the dynamic release and clearance of microparticles during pharmacologically induced cardiac stress was part of a physiological response, which was diminished in individuals with vascular disease (Citation42). Looking at a total population of larger EVs by semi-quantitative means, we did not find evidence for increased total levels of large EVs; however, this analysis is probably not powerful enough to resolve the dynamics of EV subpopulations derived from distinct cells.
We utilized differential centrifugation and filtration to separate circulating EVs in 2 fractions of particles, designated here as MVs and small EVs/exosomes, respectively. While the MV fraction remained more or less constant in response to exercise, significant changes were observed in the exosome fraction by semi-quantitative evaluation of distinct exosome markers as well as by single particle analysis (NTA). Consistent with previous reports, we found that the content of plasma of exosomes in healthy individuals is low, in particular under resting conditions, and some genuine exosome markers are difficult to uncover from the background of prominent plasma components (Citation15). With regard to the ongoing discussion in the field about MV and exosome definition (Citation43), it is important to note that the present study does not provide information on the origin of these small EVs, whether they derive from endosomal stores, or stem from shedding plasma membrane, or represent a mixture of both. The study adheres to the current best practice of EV classification (Citation43, Citation44), which relies on different criteria referring to the presence of a combination of EV markers as well as the size distribution profile of the vesicle fraction and, thus, we propose to use the term exosomes to describe these small EVs. Quantification of particles in the exosome fraction by NTA most likely also displays co-isolated lipoprotein particles (LDL, HDL), which were reported of being moderately increased after exercise as well (Citation45). However, the increase in NTA particle count in the exosome fraction was confirmed by increased representation of genuine EV and exosome markers in the biochemical analysis.
Examination of the αIIb integrin subunit indicated that platelet-derived vesicles are among exosomes released in response to physical activity. Platelets are known to produce pro-coagulant EVs including exosomes as a result of different activation stimuli (Citation13, Citation46). It should be noted that plasma processing affects platelet vesiculation and fractionation, which may contribute to the recovery of platelet EVs in MV and small EV fractions (Citation47, Citation48). However, the pre-analytical parameters were identical for all subjects and time points compared in this study. Most likely, other cells such as endothelial cells, red blood cells, monocytes and neutrophils release exosomes into the circulation during physical activity. Shear stress is a hallmark of exercise and has been suggested to stimulate secretion and transfer of exosomes from endothelial cells (HUVECs) to smooth muscle cells, mediating atheroprotective functions (Citation49). Further detailed studies are required to decipher the cellular source and the full complexity of exosomes released during physical activity. Since EVs are dynamic structures known to modulate their content in response to cell stress (Citation12), it is likely that circulating exosomes after exercise differ from those before exercise not only in amount but also in their origin and their individual composition.
Which factors trigger the discharge of EVs into the circulation? It appears conceivable that the physiological activation state of the body during exercise provokes an acute stress response, which facilitates exosome release. A reliable marker of the acute stress response known to increase in the circulation depending on exercise is Hsp70 (also designated Hsp72), although the mode of Hsp70 release was still unclear (Citation50–Citation52). A link between the acute stress response and exosome-dependent release of Hsp70 into plasma was recently found in rats challenged by tail shock (Citation53). Our study provides evidence that during exercise, Hsp70 is released into the human circulation in association with exosomes. Intriguingly, the release kinetics of exosomes (and Hsp70) during incremental exercise indicated that exosomes are already liberated during the aerobic phase of exercise before the IAT and, thus, independent of metabolic stress associated with lactate accumulation and blood acidification. The kinetics of exosome release is also faster compared with that of the catecholamine stress hormones epinephrine and norepinephrine (Citation54–Citation56) or the rise of cfDNA levels, which were suggested to correlate with physical exhaustion (Citation25). Hence, cfDNA release is definitely not coupled to exosome release, as also suggested by qPCR determination of DNA-levels in pellet and supernatant fractions, which was conducted in parallel (Citation57). Our study thus indicates that physical activity-triggered exosome release occurs independently of metabolic stress and the induction of the acute stress response. Potential factors initiating exosome release likely act already during the aerobic phase of exercise.
A remarkable observation was that exosome levels appeared sustained through the early recovery period after running, while exosomes after cycling exercise were cleared from the circulation more rapidly. This difference could be due to continued exosome generation or restrained clearance after cessation of treadmill running. Although the mean age differed significantly between cycling and running groups, age was not responsible for the observed divergence. Individuals below the age of 30 years (as analyzed in treadmill running) exhibited a robust EV release in response to cycling exercise, which diminished 90 min after exercise. In contrast to cycling, running is associated with higher heart rates and includes higher eccentric burden for the muscle, which may be a stronger challenge of tissue homeostasis requiring a more pronounced cascade of repair. Whether sustained levels of exosomes after running are somehow linked to these attributes remains to be addressed. Clearance of EVs from the circulation is most likely mediated by uptake in target organs. The present study provides information about the in vivo clearance kinetics of EVs endogenously released into the circulation of humans. EV turnover appears to relate to the nature of the EVs and the body conditions. In rodents, which have a small blood volume compared to humans, clearance of ectopically injected EVs occurs fast within 30 min (Citation58, Citation59) and depends on the presence of macrophages (Citation60). Phagocytosing cells such as macrophages and monocytes may be the primary target of circulating EVs and mediate various immune modulatory functions. Human body fluid-derived exosomes were shown to simulate TLR-signalling in monocytic cells leading to downstream activation of NFκB and STAT3 pathways mediating production of pro-inflammatory cytokines (Citation61).
What may be the physiological relevance of EVs released during exercise? Activity-induced platelet-derived MVs were shown to promote coagulation and, hence, were correlated with an increased risk of thrombosis after exercise (Citation26, Citation30). In general, increased levels of EV in the circulation may reflect the physiological activation state of the body during exercise. EV discharge into the circulation could aid the disposal of cellular waste products generated under stress conditions and help the cells to maintain homeostasis. Furthermore, exosomes have been recognized as powerful signalling vehicles that play versatile roles in immune modulation (Citation62–Citation65), inflammatory responses activating tissue repair (Citation52, Citation66) and vascular biology including angiogenesis and cardioprotection (Citation67–Citation69). In view of the health-protective effects of regular exercise, it is tempting to speculate that EVs (in particular exosomes) released into the circulation deliver signals that are instrumental in regulating physiological adaptation processes in response to physical activity. Further studies deciphering the cellular source, targets and signalling components of exercise-induced EVs are required to reveal their potential role as mediators of health-promoting effects associated with physical activity.
Authors’ contributions
CF, PS and EMKA conceived and designed experiments. CF and SH performed experiments. CF, EMKA, SH and PS collected and analyzed data. ST wrote ethical approval and recruited subjects. EMKA and CF wrote the manuscript.
Conflict of interest and funding
The authors declare no conflict of interest. This study was supported by a DFG grant to EMKA (KR3668/1-1).
Acknowledgements
The authors are grateful to the volunteers participated in the study. Wen Ping Kuo is thanked for critical comments on the manuscript. EMKA and CF thank Jacqueline Trotter for general support.
Notes
To access the supplementary material to this article, please see Supplementary files under ‘Article Tools’.
References
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–83.
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Ann Rev Cell Dev Biol. 2014; 30: 255–89.
- Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014; 29: 116–25.
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015; 25: 364–72.
- Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003; 278: 20083–90.
- Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004; 173: 1202–8.
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013; 11: e1001604.
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011; 46: 409–18.
- Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998; 91: 2313–25.
- Obregon C, Rothen-Rutishauser B, Gitahi SK, Gehr P, Nicod LP. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006; 169: 2127–36.
- Nolte-‘t Hoen EN, van der Vlist EJ, de Boer-Brouwer M, Arkesteijn GJ, Stoorvogel W, Wauben MH. Dynamics of dendritic cell-derived vesicles: high-resolution flow cytometric analysis of extracellular vesicle quantity and quality. J Leukoc Biol. 2013; 93: 395–402.
- Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010; 5: e15353.
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999; 94: 3791–9.
- Revenfeld AL, Baek R, Nielsen MH, Stensballe A, Varming K, Jorgensen M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther. 2014; 36: 830–46.
- Bastos-Amador P, Royo F, Gonzalez E, Conde-Vancells J, Palomo-Diez L, Borras FE et al. Proteomic analysis of microvesicles from plasma of healthy donors reveals high individual variability. J Proteomics. 2012; 75: 3574–84.
- Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013; 200: 367–71.
- Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet S et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014; 12: 614–27.
- Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009; 101: 439–51.
- VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003; 59: 277–87.
- Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, Loirand G et al. Shear stress regulates endothelial microparticle release. Circ Res. 2013; 112: 1323–33.
- Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009; 335: 143–51.
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013; 17: 162–84.
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011; 17: 6–63.
- Walsh NP, Gleeson M, Pyne DB, Nieman DC, Dhabhar FS, Shephard RJ et al. Position statement. Part two: maintaining immune health. Exerc Immunol Rev. 2011; 17: 64–103.
- Breitbach S, Tug S, Simon P. Circulating cell-free DNA: an up-coming molecular marker in exercise physiology. Sports Med. 2012; 42: 565–86.
- Chen YW, Chen YC, Wang JS. Absolute hypoxic exercise training enhances in vitro thrombin generation by increasing procoagulant platelet-derived microparticles under high shear stress in sedentary men. Clin Sci. 2013; 124: 639–49.
- Chen YW, Chen JK, Wang JS. Strenuous exercise promotes shear-induced thrombin generation by increasing the shedding of procoagulant microparticles from platelets. Thromb Haemost. 2010; 104: 293–301.
- Chaar V, Romana M, Tripette J, Broquere C, Huisse MG, Hue O et al. Effect of strenuous physical exercise on circulating cell-derived microparticles. Clin Hemorheol Microcirc. 2011; 47: 15–25.
- Sossdorf M, Otto GP, Claus RA, Gabriel HH, Losche W. Release of pro-coagulant microparticles after moderate endurance exercise. Platelets. 2010; 21: 389–91.
- Sossdorf M, Otto GP, Claus RA, Gabriel HH, Losche W. Cell-derived microparticles promote coagulation after moderate exercise. Med Sci Sports Exerc. 2011; 43: 1169–76.
- Maruyama K, Kadono T, Morishita E. Plasma levels of platelet-derived microparticles are increased after anaerobic exercise in healthy subjects. J Atheroscler Thromb. 2012; 19: 585–7.
- Wahl P, Jansen F, Achtzehn S, Schmitz T, Bloch W, Mester J et al. Effects of high intensity training and high volume training on endothelial microparticles and angiogenic growth factors. PLoS One. 2014; 9: e96024.
- Guiraud T, Gayda M, Juneau M, Bosquet L, Meyer P, Theberge-Julien G et al. A single bout of high-intensity interval exercise does not increase endothelial or platelet microparticles in stable, physically fit men with coronary heart disease. Can J Cardiol. 2013; 29: 1285–91.
- Vogt S, Heinrich L, Schumacher YO, Blum A, Roecker K, Dickhuth HH et al. Power output during stage racing in professional road cycling. Med Sci Sports Exerc. 2006; 38: 147–51.
- Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons?. Proteomics Clin Appl. 2007; 1: 1446–61.
- Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles. 2013; 2: 19671. doi: http://dx.doi.org/10.3402/jev.v2i0.19671.
- Tug S, Helmig S, Deichmann ER, Schmeier-Jurchott A, Wagner E, Zimmermann T et al. Exercise-induced increases in cell free DNA in human plasma originate predominantly from cells of the haematopoietic lineage. Exerc Immunol Rev. 2015; 21: 164–73.
- Beiter T, Fragasso A, Hudemann J, Niess AM, Simon P. Short-term treadmill running as a model for studying cell-free DNA kinetics in vivo. Clin Chem. 2011; 57: 633–6.
- Breitbach S, Sterzing B, Magallanes C, Tug S, Simon P. Direct measurement of cell-free DNA from serially collected capillary plasma during incremental exercise. J Appl Physiol. 2014; 117: 119–30.
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015; 4: 27066, . doi: http://dx.doi.org/10.3402/jev.v4.27066.
- Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012; 44: 11–5.
- Augustine D, Ayers LV, Lima E, Newton L, Lewandowski AJ, Davis EF et al. Dynamic release and clearance of circulating microparticles during cardiac stress. Circ Res. 2014; 114: 109–13.
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013; 2: 20389. doi: http://dx.doi.org/10.3402/jev.v2i0.20389.
- Lötvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014; 3: 26913. doi: http://dx.doi.org/10.3402/jev.v3.26913.
- Sondergaard E, Poulsen MK, Jensen MD, Nielsen S. Acute changes in lipoprotein subclasses during exercise. Metabolism. 2014; 63: 61–8.
- Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014; 3: 24692. doi: http://dx.doi.org/10.3402/jev.v3.24692.
- White JG, Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967; 30: 625–35.
- Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012; 10: 437–46.
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012; 14: 249–56.
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001; 6: 386–93.
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005; 26: 552–7.
- Beninson LA, Fleshner M. Exosomes: an emerging factor in stress-induced immunomodulation. Semin Immunol. 2014; 26: 394–401.
- Beninson LA, Brown PN, Loughridge AB, Saludes JP, Maslanik T, Hills AK et al. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142-5p and miR-203). PLoS One. 2014; 9: e108748.
- Schneider DA, McGuiggin ME, Kamimori GH. A comparison of the blood lactate and plasma catecholamine thresholds in untrained male subjects. Int J Sports Med. 1992; 13: 562–6.
- Chmura J, Nazar K, Kaciuba-Uscilko H. Choice reaction time during graded exercise in relation to blood lactate and plasma catecholamine thresholds. Int J Sports Med. 1994; 15: 172–6.
- Weltman A, Wood CM, Womack CJ, Davis SE, Blumer JL, Alvarez J et al. Catecholamine and blood lactate responses to incremental rowing and running exercise. J Appl Physiol. 1994; 76: 1144–9.
- Helmig S, Frühbeis C, Krämer-Albers EM, Simon P, Tug S. Release of bulk cell free DNA during physical exercise occurs independent of extracellular vesicles. Eur J Appl Physiol. 2015. in press.
- Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, van den Bos AG et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005; 105: 2141–5.
- Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013; 165: 77–84.
- Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015; 4: 26238. doi: http://dx.doi.org/10.3402/jev.v4.26238.
- Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013; 288: 36691–702.
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009; 9: 581–93.
- Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015; 6: 13718–30.
- Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol. 2014; 28: 51–7.
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014; 14: 195–208.
- Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014; 10: 356–64.
- De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014; 5: 608.
- Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015; 1852: 1–11.
- Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014; 5: 370.
