Abstract
Background
IL-32 was recently found to be elevated in the tissue of rheumatoid arthritis and inflammatory bowel disease. Periodontitis is a chronic inflammatory disease caused by polymicrobial infections that result in soft tissue destruction and alveolar bone loss. Although IL-32 is also thought to be associated with periodontal disease, its expression and possible role in periodontal tissue remain unclear. Therefore, this study investigated the expression patterns of IL-32 in healthy and periodontally diseased gingival tissue. The expression of IL-32 in cultured human gingival fibroblasts (HGF) as well as effects of autocrine IL-32 on IL-8 production from HGF were also examined.
Methods
Periodontal tissue was collected from both healthy volunteers and periodontitis patients, and immunofluorescent staining was performed in order to determine the production of IL-32. Using real-time PCR and ELISA, mRNA expression and protein production of IL-32 in HGF, stimulated by Porphyromonas gingivalis (Pg), were also investigated.
Results
Contrary to our expectation, the production of IL-32 in the periodontitis patients was significantly lower than in the healthy volunteers. According to immunofluorescent microscopy, positive staining for IL-32 was detected in prickle and basal cell layers in the epithelium as well as fibroblastic cells in connective tissue. Addition of fixed Pg in vitro was found to suppress the otherwise constitutive expression of IL-32 mRNA and protein in HGF. However, recombinant IL-32 in vitro inhibited the expression of IL-8 mRNA by HGF stimulated with Pg. Interestingly, anti-IL-32 neutralizing antibody upregulated the IL-8 mRNA expression in non-stimulated HGF, indicating that constitutive expression of IL-32 in HGF suppressed IL-8 mRNA expression in the absence of bacterial stimulation.
Conclusion
These results indicate that IL-32 is constitutively produced by HGF which can be suppressed by Pg and may play a role in the downregulation of inflammatory responses, such as IL-8 production, in periodontal tissue.
Periodontitis is a chronic inflammatory and infectious disease characterized by the interaction between periodontal tissue and periodontopathogenic bacteria such as Porphyromonas gingivalis Citation1. Such Gram-negative bacteria are known to produce pathogenic factors, such as outer membrane protein (Omps), leukotoxin, and protease, to induce inflammation in gingival tissue Citation2. These bacteria also possess lipopolysaccharide (LPS) in the outer membrane as a modulator of inflammation Citation3. The immune response to periodontopathogenic bacteria plays an essential role in the breakdown of connective tissue and alveolar bone Citation4 Citation5. Gingival fibroblasts, the major component of periodontal connective tissue, are involved in the inflammatory processes in response to bacterial challenge. In particular, gingival fibroblasts produce proinflammatory cytokines, including IL-1β, IL-6, IL-8 and TNF-α Citation6 Citation7. These cytokines produced from gingival fibroblasts are involved in the activation of monocytes, macrophages, dendritic cells, neutrophils and lymphocytes Citation8. As a consequence of upregulation of acquired cellular immune responses caused by fibroblast-derived proinflammatory cytokines, the progression of periodontal disease is promoted Citation8.
IL-32, a recently characterized cytokine, has six isoforms and is produced by natural killer (NK) cells, T-cells, epithelial cells and monocytes Citation9 Citation10 Citation11 . IL-32 is also upregulated in response to TNF-α and IL-1β in inflammatory bowel disease, Crohn's disease and rheumatoid arthritis Citation12 Citation13. Therefore, it is thought that IL-32 functions as a proinflammatory cytokine. Since IL-32 is involved in the upregulation of the proinflammatory cytokine production in some diseases, it was hypothesized that IL-32 might also be associated with periodontal disease by upregulation of proinflammatory cytokines. Therefore, in this study, the expression of IL-32 in healthy subjects and in the inflamed periodontal tissue collected from patients was compared. The expression patterns of IL-32 were then analyzed in cultured human gingival fibroblasts (HGF). The effect of IL-32 on the proinflammatory cytokine expression by HGF was also investigated.
Materials and methods
Collection of human gingival tissue samples
After obtaining approval from the Ethical Committees of The Forsyth Institute and Harvard School of Dental Medicine (HSDM, Boston, MA, USA), both healthy and inflamed gingival tissue samples were collected in the Periodontology Department laboratories at HSDM. Healthy gingival tissue was characterized by the lack of bleeding on probing (gingival pocket depth ≤ 3 mm; n=5, 2 males and 3 females, aged 29–43 years) and collected from the healthy volunteer who signed informed consent prior to enrollment. Periodontal disease was divided into three categories by the degree of swelling of the site collected from the patients. Since there was a limitation among the small number of samples to discuss the correlation between the production of IL-32 and three different categories, these were all grouped as one category ‘inflamed’. Inflamed gingival tissues were obtained from patients with periodontal disease at flap surgery. The periodontally diseased sites of patients who donated the gingival tissue samples were also characterized by radiological bone resorption and bleeding on probing (gingival pocket depth ≥ 5 mm; n=7, 4 males and 3 females, aged 20–55 years) ().
Table 1. Clinical parameters
Cell culture
Human gingival fibroblasts (HGF) were obtained from healthy volunteers as described previously Citation14. HGF were cultured to confluence in a 35 mm diameter dish with Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY, USA), 100 units/ml penicillin, 100 µg/ml streptomycin and 1 µg/ml amphotericin B. When HGF cells formed a confluent monolayer, cells were harvested with 0.05% trypsin and 0.02% EDTA and transferred to a 100 mm diameter plastic culture dish. Fourth passage HGF cultures were used in the following experiments.
Detection of interleukin-32 and IL-8 by enzyme-linked immunosorbent assay (ELISA)
In order to examine the production of IL-32 in the gingival tissue, gingival tissues were homogenized in lysis buffer Citation15. IL-32 from the gingival tissue homogenate or the supernatant of HGF was detected by ELISA, as previously described Citation16. Briefly, anti-IL-32 mouse monoclonal antibody (2 µg/ml, #513501; BioLegend, San Diego, CA, USA; sodium bicarbonate buffer, pH 9.7) was coated onto a 96-well ELISA plate (BD Falcon, Franklin Lakes, NJ, USA). After blocking each well with 0.5% BSA in PBS supplemented with 0.05% Tween 20 (PBST), either a homogenate of gingival tissue samples or a cultured medium supernatant was applied into each well, followed by purified anti-IL-32 rabbit IgG (2 µg/ml, #IMX-5871; IMGENEX, San Diego, CA, USA). The IL-8 ELISA Development kit (#900-K18; Peprotech, Rocky Hill, NJ, USA) was used to detect IL-8, following the manufacturer's protocol. Each well was then reacted with anti-rabbit IgG-conjugated horseradish peroxidase (Roche, Indianapolis, IN, USA). Colorimetric reactions were developed with o-Phenylenediamine (Sigma, Tokyo, Japan) in the presence of 0.02% H2O2. Color development was paused with H2SO4 (2 N) and measured by an ELISA reader (OD 490 nm). The actual concentration of IL-32 was calibrated by referring to a standard curve prepared by serial dilution of recombinant human IL-32γ (BioVision, Mountain View, CA, USA) or γ (R and D Systems, Inc., Minneapolis, MN, USA). Each sample was examined in triplicate wells of a 96-well ELISA plate.
Immunofluorescent microscopic observation
Frozen sections of each tissue (two healthy and three inflamed tissues) were fixed with Acetone-alcohol at 4°C for 1 min. After blocking with 0.5% BSA in PBST, each section was incubated with anti-IL-32 mouse monoclonal antibody (2 µg/ml, #513501; BioLegend) or anti-IL-8 mouse monoclonal antibody (2 µg/ml, #500-M8; Peprotech) in PBS at 4°C for 12 hours. Then, Alexa594-labeled anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) was applied for 30 min at room temperature. The Alexa594 signals in the sections were observed using fluorescent microscopy (model BZ-9000; Keyence, Osaka, Japan).
Stimulation of HGF
The confluent culture of HGF was stimulated with formalin fixed P. gingivalis W83 (107 CFU/ml) for 12 hours to examine the expression of mRNA and for 24 hours to detect the production of IL-32 or IL-8 by ELISA.
Real-time PCR analysis
After the stimulation of HGF, total RNA was purified and analyzed by the two-step LightCycler® 480 Real-Time PCR System Citation17. Briefly, one microgram of total RNA was used for reverse transcription by the First Strand cDNA Synthesis Kit for RT-PCR (Roche Diagnostics, Tokyo, Japan). Then, one microliter of cDNA was used for real-time PCR. The amplified condition was described previously Citation17. The primers used in this study are listed in .
Table 2. Primers
Statistical analysis
Differences between the two groups were analyzed by Student's t-test.
Results
Detection of IL-32 and IL-8 in human gingival tissue homogenate
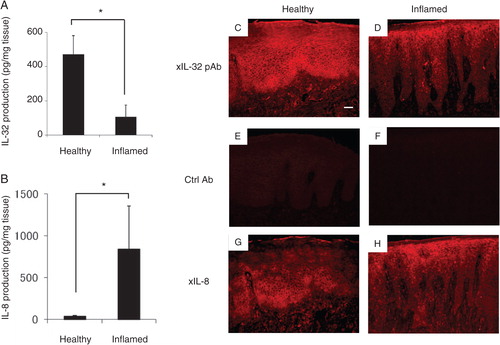
The production of total IL-32 in human healthy or inflamed gingival tissues was examined by ELISA (A). Although IL-32 was detected in both healthy and inflamed tissues, the amount of IL-32 in the inflamed gingival tissue was lower than that observed in the healthy tissue (average healthy tissue: 471.6±108.7 pg/mg tissue; inflamed tissue: 106.1±69.7 pg/mg tissue). Proinflammatory cytokine IL-8 production in the gingival tissues was also measured by ELISA (B). The amount of IL-8 in periodontally diseased tissue was higher than in healthy gingival tissue (average healthy tissue: 38.6±11.4 pg/mg tissue; inflamed tissue: 840.5±513.6 pg/mg tissue), suggesting that the gingival tissues sampled from patients with periodontal disease were inflamed. Immunohistostaining of IL-32 showed the presence of many positively stained cells in the healthy gingival tissue section (C). In particular, prickle and basal cell layers in the epithelium and fibroblastic cells in connective tissue were heavily stained. On the other hand, there were fewer positive cells in the inflamed gingival tissue (D). Neither healthy nor diseased gingival tissue was stained in the control non-immunized mouse IgG (E, F). On the other hand, the production of IL-8 in healthy tissue was weak, whereas positive staining of IL-8 expression was found in the inflamed gingival tissues, especially epithelium and connective tissue (G, H).
Fig. 1. Analyses of IL-32 and IL-8 expressed in healthy and inflamed human gingival tissue. (A) In order to determine the production of IL-32 by ELISA, both healthy gingival tissue (gingival pocket depth ≤ 3mm; n = 5, two males and three females, aged 29–43 years) and inflamed gingival tissue (gingival pocket depth > 4mm; n = 5, four males and two females, aged 20–55 years) were collected from volunteer patients. The gingival tissue was homogenized for the sample. IL-32 present in the gingival tissue homogenates was measured using ELISA. (B) IL-8 production was measured by ELISA Development kit in accordance with the manufacturer's instructions. The values represent the means and standard deviations of triplicate experiments. *Significantly higher than healthy gingival tissue, by Student's t-test (P<0.01). (C–H) Immunohistochemical analysis of human IL-32 and IL-8 production in the gingival tissue: IL-32 production in human healthy (C, E) and inflamed (D, F) gingival tissues was determined using immunohistochemical staining, following the previously published protocol. For IL-32-positive staining, mouse monoclonal anti-IL-32 was utilized to detect the production of IL-32 (C, D). As a negative control, non-immune mouse IgG was used (E, F). In order to show the inflammation of the tissue, IL-8 was stained following the published procedure. In the positively stained samples, arrows indicate IL-32- and IL-8-positive cells. Original magnification is 200 times. The white bar corresponds to 50 µm.
IL-32 mRNA expression and production in HGF stimulated with P. gingivalis
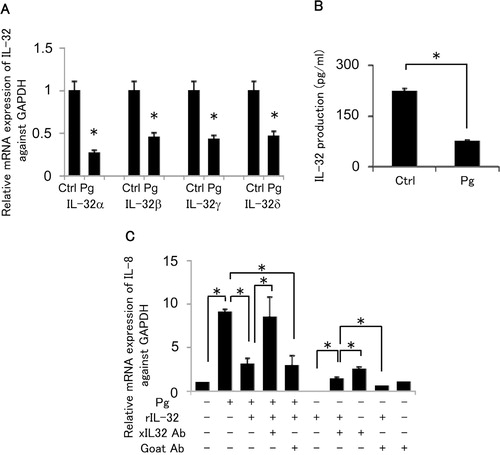
Four isoforms of IL-32 mRNA are well known: IL-32α, β, γ and δ Citation10. To determine the mRNA expression of IL-32α, β, γ and δ in HGF stimulated by P. gingivalis W83, real-time PCR was performed by using specific primer and the LightCycler system. Each IL-32 mRNA isoform was detected in unstimulated HGF. However, stimulation of HGF with P. gingivalis downregulated the mRNA expression of each IL-32 isoform, respectively (A). A similar result was obtained in the total IL-32 production at the protein level in the HGF culture supernatant (66% reduction) (B).
Fig. 2. In vitro evaluation of IL-32 and IL-8 expression in human gingival fibroblasts. (A) Effects of Porphyromonas gingivalis stimulation on the production of IL-32 mRNA in human gingival fibroblasts: Primary culture of human gingival fibroblasts was stimulated with formalin fixed P. gingivalis (108 cells/ml) for 12 hours, and total RNA was extracted to perform the quantitative RT-PCR for the four different isoforms of IL-32. The mRNA expression of IL-32 was standardized by the ratio against Glyceraldehyde 3 – phosphate dehydrogenase (GAPDH). The values represent the means and standard deviations of triplicate experiments. *Significantly higher than medium control alone without bacteria by Student's t-test (P <0.01). (B) Effect of Porphyromonas gingivalis on IL-32 protein production from HGF: The production of IL-32 in contact with P. gingivalis for 24 hours was monitored using an IL-32 ELISA. The values represent the means and standard deviations of triplicate experiments. *Significantly higher than control without bacteria (the far left bar with #) by Student's t-test (P<0.01). (C) The effects of IL-32 on expression of IL-8 mRNA in HGF: In order to determine the effect of IL-32 in HGF, recombinant human IL-32γ (10 ng/ml) was applied to the culture medium with and without P. gingivalis stimulation. Anti-IL-32 polyclonal antibody (2 µg/ml) or normal goat IgG (2 µg/ml) was also added into the medium for neutralization of IL-32. Total RNA was extracted for quantitative RT-PCR to analyze the IL-8 mRNA expression after 12 hours of stimulation by P. gingivalis. The values represent the means and standard deviations of triplicate experiments. *Significantly higher than control without bacteria (the far left bar with #) by Student's t-test (P<0.01).
The effect of IL-32 on expressions of IL-8 mRNA in HGF
In an attempt to examine the anti-inflammatory effect of IL-32, recombinant IL-32γ was added into the HGF culture with or without fixed P. gingivalis. Bacterial stimulation induced the mRNA expression of IL-8 (C). However, the addition of IL-32γ resulted in the suppression of IL-8 mRNA expression (66% reduction). Furthermore, anti-IL-32 goat IgG partially reversed the suppression of IL-8 mRNA caused by P. gingivalis. Interestingly, recombinant IL-32γ alone without P. gingivalis also showed inhibitory effect on IL-8 mRNA expression in HGF (35% reduction). Finally, anti-IL-32 neutralizing antibody upregulated IL-8 mRNA expression in the non-stimulated HGF, indicating that constitutively expressed IL-32 appeared to suppress IL-8 mRNA expression. These results suggested that IL-32 may play a role in the downregulation of IL-8 mRNA expression in HGF and that the periodontal pathogen P. gingivalis can suppress IL-32 production from HGF.
Discussion
In this study, we investigated the production pattern of IL-32 in periodontal tissue with or without periodontal disease. The production of IL-32 was lower in the tissue from periodontitis patients compared with the tissue of healthy volunteers (). An in vitro study showed that IL-32 was constitutively expressed in HGF, but suppressed by P. gingivalis stimulation. Recombinant IL-32γ exhibited an anti-inflammatory effect by suppressing the IL-8 expression in HGF stimulated with P. gingivalis. Furthermore, antibody-mediated neutralization of IL-32 in non-stimulated HGF increased IL-8 production. Taken together, these results suggested that IL-32 appeared to play an anti-inflammatory role in healthy gingival tissue, while suppression of IL-32 by the periodontal pathogen P. gingivalis may lead to the upregulation of inflammatory response, which promotes the progression of periodontal disease.
Previous reports show that IL-32 was originally cloned as a transcript (NK4) that is produced in NK- or T-cells Citation11. In addition, IL-32 is produced by mononuclear, endothelial and epithelial cells after stimulation by IL-1β, IL-2, IL-18, TNF-α or IFN-γ Citation9 Citation18 Citation19 Citation20 . DNA microarray analysis shows that P. gingivalis-derived LPS induced the expression of IL-32 in monocytes (THP-1 cells) Citation3. However, very little is known about the expression of IL-32 in the gingival fibroblasts which constitute majority of tissue supporting cells in lamina propria of periodontal tissue. Immunohistochemistry showed strong staining patterns in subgingival fibroblastic cells as well as gingival epithelial cells (C, D), and such expression of IL-32 in the gingival tissue was lower in the diseased gingival tissue compared with the healthy ones (A). Although some cytokines are known to be influenced by genetic background or single-nucleotide polymorphism (SNP), the small number of subjects used in this study is not suitable to compare the correlation between healthy and periodontitis tissue Citation21. Furthermore, the production of IFN-g and IL-13 in whole blood cell from smokers is reported to be higher than non-smoker Citation22. Therefore, it is very significant to analyze the production of IL-32 in many subjects including multiple genetic background. Our in vitro experiment showed that the addition of P. gingivalis suppressed the otherwise constitutively expressed IL-32 mRNA and protein in HGF (A, B). These results indicated that down-regulatory effects of IL-32 on inflammatory response can be interrupted by periodontal pathogen, P. gingivalis, in the context of periodontal disease.
It has been reported that the production of IL-32 is increased in rheumatoid arthritis (RA), an inflammatory bone resorptive disease, which shares several pathogenic characteristics in common with periodontal disease Citation23. Therefore, these results were unexpected. However, one of the major differences between RA and periodontal disease is the phenomenon of polymicrobial infection. A number of virulent factors are reported to be produced from periodontal pathogens such as P. gingivalis Citation24 Citation25 Citation26 . Therefore, it is plausible that some bacterial factors, including LPS, outer membrane protein, and protease, might have acted on HGF and suppressed IL-32 production by generating a negative signal to reduce IL-32 mRNA expression. On the other hand, IL-32β has been reported to induce the production of anti-inflammatory cytokine IL-10 Citation27. More specifically, IL-32 was found to promote the expression of IL-10 in monocyte linage cells upon stimulation with phorbol 12-myristate 13-acetate (PMA) or lipopolysaccharide (LPS) Citation27. While immune cells are accepted as the major cellular source of IL-10 in the context of inflammation Citation28 Citation29, it is also true that HGF produce IL-10 in response to bacterial challenge Citation30. Therefore, it is conceivable that IL-10 may intervene in the bacteria-mediated suppression of IL-32 production from HGF.
Our in vivo and in vitro results showed that IL-32 is produced constitutively in healthy conditions. Hence, it was hypothesized that IL-32 might have an anti-inflammatory effect in periodontal tissue. IL-8 is a chemokine produced by gingival epithelial cells and upregulated in the presence of periodontopathogenic bacterial stimulation Citation31. IL-8 induces the chemoattraction of neutrophils through CXCR1 receptors in the affected lesion, which, in turn, promote inflammatory response by such tissue destructive factors as reactive oxygen species (ROS) from migrating neutrophils Citation32. Recombinant IL-32γ suppressed the induction of IL-8 mRNA in HGF, while anti-IL-32 antibody neutralized the effect of IL-32. Furthermore, the addition of anti-IL-32 antibody into HGF culture without P. gingivalis increased IL-8 mRNA. Thus, IL-32 may function as an autocrine factor to downregulate the expression of IL-8.
It is curious that a putative receptor for IL-32 remains unknown. To date, the only identified IL-32 binding protein is proteinase 3 (PR3) whose catalytic activity partially cleaves IL-32, increasing bioactivity of IL-32 Citation33. The difficulty of identifying an IL-32 receptor was speculated to result from its possibly high molecular weight which is not feasibly separated in SDS-PAGE. Alternatively, the binding affinity of IL-32 could be lower than PR3 Citation33 which is not suitable for affinity-dependent isolation. However, based on the contrary results between present study and the report of RA Citation3 Citation13, two different types of IL-32 receptors can be assumed to induce cell signaling; one type of receptor induces a proinflammatory signal, while another induces an anti-inflammatory signal. Based on the results of this study, it can be hypothesized that HGF express IL-32 that has a receptor of the second type. This theory, however, requires further study to identify the HGF-specific IL-32 receptor and to characterize the properties of the cell signaling elicited by the IL-32 receptor expressed on HGF.
In conclusion, since constitutive production of IL-32 can inhibit IL-8 production, IL-32 might play an important role in regulating inflammation in healthy gingival tissue. On the other hand, it was found that periodontopathogenic bacterial challenge can suppress IL-32 production, leading to the increase of inflammatory response by loss of anti-inflammatory IL-32. Taken together, these findings could help to further define the etiology of periodontal disease, as well as form a basis for novel therapeutic regimens against periodontitis. Further study will be needed to determine the target molecule of IL-32 in periodontitis and the structural component of P. gingivalis that suppresses IL-32 expression.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
Acknowledgements
This research was supported by a Grant-in-Aid for the Encouragement of Young Scientists (B) 22792085 from the Japan Society for the Promotion of Science and by an NIH grant DE-018499 from the NIDCR (Kawai). We would like to thank Mr. Tomoya Matsumoto (Keyence, Osaka, Japan) for his helpful advice and technical support.
References
- Socransky SS, Haffajee AD. Implications of periodontal microbiology for the treatment of periodontal infections. Compend Suppl. 1994: S684–5., 8–93; quiz S714–7.
- Kadowaki T, Nakayama K, Okamoto K, Abe N, Baba A, Shi Y, et al.. Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J Biochem. 2000; 128: 153–9.
- Barksby HE, Nile CJ, Jaedicke KM, Taylor JJ, Preshaw PM. Differential expression of immunoregulatory genes in monocytes in response to Porphyromonas gingivalis and Escherichia coli lipopolysaccharide. Clin Exp Immunol. 2009; 156: 479–87.
- Oshiro T, Shiotani A, Shibasaki Y, Sasaki T. Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. Anat Rec. 2002; 266: 218–25.
- Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, et al.. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000; 106: R59–67.
- Steffen MJ, Holt SC, Ebersole JL. Porphyromonas gingivalis induction of mediator and cytokine secretion by human gingival fibroblasts. Oral Microbiol Immunol. 2000; 15: 172–80.
- Imatani T, Kato T, Okuda K. Production of inflammatory cytokines by human gingival fibroblasts stimulated by cell-surface preparations of Porphyromonas gingivalis. Oral Microbiol Immunol. 2001; 16: 65–72.
- van Winkelhoff AJ. Antibiotics in periodontics: are we getting somewhere?. J Clin Periodontol. 2005; 32: 1094–5.
- Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005; 22: 131–42.
- Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006; 65 (Suppl 3): iii61–4.
- Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992; 148: 597–603.
- Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, et al.. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007; 149: 480–6.
- Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al.. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006; 103: 3298–303.
- Fujita T, Iwata T, Shiba H, Igarashi A, Hirata R, Takeda K, et al.. Identification of marker genes distinguishing human periodontal ligament cells from human mesenchymal stem cells and human gingival fibroblasts. J Periodontal Res. 2007; 42: 283–6.
- Ueki Y, Lin CY, Senoo M, Ebihara T, Agata N, Onji M, et al.. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 ‘cherubism’ mice. Cell. 2007; 128: 71–83.
- Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, et al.. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006; 146: 218–25.
- Shiba H, Tsuda H, Kajiya M, Fujita T, Takeda K, Hino T, et al.. Neodymium-doped yttrium-aluminium-garnet laser irradiation abolishes the increase in interleukin-6 levels caused by peptidoglycan through the p38 mitogen-activated protein kinase pathway in human pulp cells. J Endod. 2009; 35: 373–6.
- Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, et al.. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005; 102: 16309–14.
- Mun SH, Kim JW, Nah SS, Ko NY, Lee JH, Kim JD, et al.. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the Syk/protein kinase Cdelta/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 2009; 60: 678–85.
- Nold-Petry CA, Nold MF, Zepp JA, Kim SH, Voelkel NF, Dinarello CA. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc Natl Acad Sci USA. 2009; 106: 3883–8.
- Takashiba S, Naruishi K. Gene polymorphisms in periodontal health and disease. Periodontol 2000. 2006; 40: 94–106.
- de Heens GL, van der Velden U, Loos BG. Cigarette smoking enhances T cell activation and a Th2 immune response; an aspect of the pathophysiology in periodontal disease. Cytokine. 2009; 47: 157–61.
- Smolik I, Robinson D, El-Gabalawy HS. Periodontitis and rheumatoid arthritis: epidemiologic, clinical, and immunologic associations. Compend Contin Educ Dent. 2009; 30: 188–90., 92, 94 passim; quiz 98, 210.
- Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. The host cytokine response to Porphyromonas gingivalis is modified by gingipains. Oral Microbiol Immunol. 2009; 24: 11–7.
- Baba A, Kadowaki T, Asao T, Yamamoto K. Roles for Arg- and Lys-gingipains in the disruption of cytokine responses and loss of viability of human endothelial cells by Porphyromonas gingivalis infection. Biol Chem. 2002; 383: 1223–30.
- Pingel LC, Kohlgraf KG, Hansen CJ, Eastman CG, Dietrich DE, Burnell KK, et al.. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol. 2008; 86: 643–9.
- Hong J, Bae S, Kang Y, Yoon D, Bai X, Chan ED, et al.. 2009Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 49: 171–6.
- Colic M, Gazivoda D, Vasilijic S, Vucevic D, Lukic A. Production of IL-10 and IL-12 by antigen-presenting cells in periapical lesions. J Oral Pathol Med. 39: 690–6.
- Conti P, Kempuraj D, Kandere K, Di Gioacchino M, Barbacane RC, Castellani ML, et al.. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003; 86: 123–9.
- Morandini AC, Sipert CR, Ramos-Junior ES, Brozoski DT, Santos CF. Periodontal ligament and gingival fibroblasts participate in the production of TGF-beta, interleukin (IL)-8 and IL-10. Braz Oral Res. 2009; 25: 157–62.
- Uchida Y, Shiba H, Komatsuzawa H, Takemoto T, Sakata M, Fujita T, et al.. Expression of IL-1 beta and IL-8 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans. Cytokine. 2001; 14: 152–61.
- Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992; 307: 97–101.
- Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci USA. 2006; 103: 3316–21.