Aim
Find the periodontal treatment that best maintained clinical results over time evaluated by changes in pocket depth (PD) and clinical attachment level (CAL).
Methods
229 patients with chronic periodontitis from USA (n=134) and Sweden (n=95) were randomly assigned to eight groups receiving Citation1 scaling+root planing (SRP) alone or combined with Citation2 surgery (SURG)+systemic amoxicillin (AMOX)+systemic metronidazole (MET); Citation3 SURG+local tetracycline (TET); Citation4 SURG; Citation5 AMOX+MET+TET; Citation6 AMOX+MET; Citation7 TET; and Citation8 SURG+AMOX+MET+TET. Antibiotics were given immediately after SRP. Plaque, gingival redness, bleeding on probing, suppuration, PD, and CAL were recorded at baseline and after 3, 6, 12, 18, and 24 months. Treatment effects were evaluated by linear multilevel regression and logistic multilevel regression models. We considered only data from sites with a baseline PD of at least 5 mm of 187 patients completing the study.
Results
Surgically treated patients experienced most CAL loss. Adjunctive therapy including SURG was most effective in reducing PD. Combining SURG with AMOX, MET, and TET gave significant clinical benefits. Past and current smoking habits were significant predictors of deeper PD. Only current smoking was a significant predictor of CAL loss. Bleeding, accumulation of plaque, gingival redness, and suppuration were significant predictors of further CAL loss and deeper PD.
Conclusions
Both surgical and non-surgical therapies can be used to arrest chronic periodontitis. SURG+AMOX+MET+TET gave best maintenance of clinical results.
Chronic periodontitis in mild to moderate forms is considered the most common form of periodontitis occurring worldwide with prevalences from 13 to 57% in different populations depending upon oral hygiene and socioeconomic status Citation1. Treatment combinations are often recommended for periodontitis. Such combinations include removal of tooth-borne bacterial plaque and calculus often in conjunction with periodontal surgery (SURG). Addition of antibiotics aims at suppressing pathogenic microorganisms and allowing recolonization with those compatible with health Citation2.
In the current study, the effectiveness of eight different therapies including combinations of therapies on two established markers of periodontitis, pocket depth (PD) and clinical attachment level (CAL), was compared. The therapies were scaling and root planing (SRP), SURG, and systemic and local antibiotics.
The necessity for pocket elimination by SURG has been challenged. The main objective of SURG is to contribute to long-term preservation of the periodontium by promoting plaque removal and infection control. However, it has been suspected that SURG may initiate loss of CAL through a detrimental effect on osteoblasts. Subantimicrobial doses of doxycycline reduce gingival collagenolytic activity by inhibiting neutrophil matrix metalloproteinases Citation3–Citation8. The present study assessed whether loss of CAL associated with SURG could be diminished by combining SURG with antibiotics such as systemic amoxicillin (AMOX) and systemic metronidazole (MET) and locally delivered tetracycline (TET).Studies on periodontal treatment have generally indicated that it immediately improves clinical parameters of periodontitis. Usually, adjunctive systemic antibiotic treatment provides better results than SRP alone, particularly in terms of PD reduction and gain of CAL Citation9–Citation11.
Several studies have tested the effect of local application of TET in the management of periodontitis Citation12–Citation16. MET has been used alone Citation11 Citation17 Citation18 or in combination with AMOX Citation18–Citation20. AMOX has been used to suppress periodontal pathogens and increase the gain of clinical attachment Citation21. MET+AMOX have been applied successfully in the treatment of adult periodontitis, especially in Aggregatibacter actinomycetemcomitans-associated infections Citation22 Citation23. Even though systemically administered adjunctive antibiotics may provide better clinical results than SRP alone, little is known if any of these agents are more effective than others when long-term clinical effects are considered.
The effectiveness of a therapy is usually masked by risk factors for health such as smoking habits. Responses after periodontal treatment are thought to be modified by cigarette consumption. Current smokers experience poorer clinical responses than former or never smokers Citation24–Citation26. A therapy may also work well in one ethnic group and fail to reproduce similar results in a different ethnic group. Differences have been demonstrated in the prevalence of periodontitis between and across continents Citation27 Citation28. In the present study, white Americans, Swedes, African Americans, and Asians were examined to see how they maintained over time the beneficial clinical status achieved by different periodontal therapies. There are no clear inherent differences between males and females in their susceptibility to periodontitis, but men have worse periodontal health than women in multiple studies from different populations Citation28–Citation30.
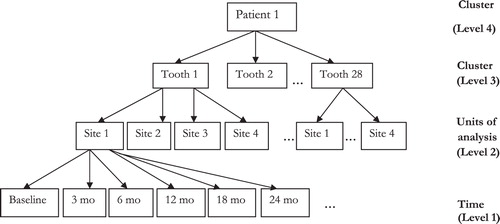
When analyzing clustered longitudinal clinical data on periodontal disease, tooth sites are nested in teeth, which are in turn nested within patients and share a common feature of correlated observations. Because of this hierarchical structure (), analysis assuming independence of observations is rendered inappropriate. Any method of analysis that attempts to aggregate the data at patient level results in loss of valuable information and may not explicitly reflect the site-specific nature of periodontal disease Citation31 Citation32. Furthermore, it is reasonable to assume that based on the severity of the disease, periodontal therapies are likely to vary not only between patients but also between tooth sites in subjects.
The use of random effects in multilevel modeling is one common and convenient way to model such grouping structure Citation33 Citation34. It is therefore appropriate to consider ‘site within tooth’ and ‘tooth within patient’ to be random factors. We used this approach that takes into account the clustering effect of the data and provides reasonable explanations for how therapeutic effects change over time.
The main aim of the present study was to compare the clinical effectiveness of eight different therapies in periodontitis. We examined changes in PD and CAL to assess treatment effects. In particular, we were interested in comparing if patients receiving SURG alone maintained their improved clinical status for a shorter period than patients treated with SURG combined with systemic or local antibiotics. The hypothesis was that SURG combined with systemic or local antibiotics gives better long-term clinical results than SURG alone. All treatments were compared to SRP (control).
The study also aimed at determining whether treatment reduced PD in sites or subjects by>1.5 mm or if it could produce CAL gain of >1.5 mm. We also examined the effects on the periodontal treatment of underlying explanatory variables such as age, smoking habits, race, nationality and gender. The current study, based on previously published data Citation35, is an attempt to mathematically model infectious disease using periodontitis as a basis.
Materials and methods
Patient population, inclusion and exclusion criteria, periodontal treatment, and clinical monitoring have been described in detail previously Citation35. Only a brief version is given here.
Study group
A total of 229 (109 females and 120 males) American (n=134) and Swedish (n=95) patients aged 20–79 years (mean 48) were originally recruited for this 2-year follow-up study. The Americans were collected at the Forsyth Institute and at Boston University, Boston, and the Swedes at the University of Göteborg. In the Swedish population, 98% of the subjects were white versus 60% in the US population. Current smokers constituted 57% of Swedish subjects and 25% in US subjects. Patients were in good general health and had at least 15 natural teeth including minimum one molar in each quadrant and at least 4 teeth with PD≥5 mm and 8 teeth with CAL>3 mm at baseline. Males and females of any race were accepted to participate. Participants, of whom 187 completed the study, were followed up for 2 years and the data on different clinical measurements recorded from all teeth except third molars. The experiments were undertaken with the understanding and written consent of each subject. The study design was independently reviewed and approved by the Review Boards of the Forsyth Institute, Boston University, and the Ethics Committee at the University of Göteborg.
Exclusion criteria were known allergies to the antibiotics to be used, antibiotic or periodontal treatment last 3 months, pregnancy, nursing, or systemic conditions that would influence the course of periodontal treatment, or allergy to amoxicillin, metronidazole, tetracycline, lidocaine, or chlorhexidine.
Experimental design and treatment
The patients were stratified into current smokers and non-smokers and randomly assigned to permuted blocks of eight different treatment combinations. A clinical co-coordinator assigned the patients to each group. summarizes the baseline characteristics of the patients in the eight groups. All patients had equal chances of being assigned to any of the therapies. The treatments were Citation1 SRP alone; Citation2 SRP+SURG+AMOX+MET; Citation3 SRP+SURG+TET; Citation4 SRP+SURG; Citation5 SRP+AMOX+MET+TET; Citation6 SRP+AMOX+MET; Citation7 SRP+TET; and Citation8 SRP+SURG+AMOX+MET+TET (). Subjects were monitored at baseline and at 3, 6, 12, 18, and 24 months.
Table 1. Baseline characteristics of the study subjects
SRP
At baseline, all subjects received SRP performed under local anesthesia and usually finished in four weekly visits. During SRP treatment, subjects rinsed twice daily with 0.1% chlorhexidine.
Systemic antibiotics
Systemic antibiotics were given as 14 days of medication (metronidazole 250 mg×3 and amoxicillin 500 mg×2). This therapy started immediately after the first session of SRP and was administered parallel to local tetracycline fiber application. Compliance was reported by the patients.
Local tetracycline
Local antibiotics were given as tetracycline fibers (Actisite®, Proctor and Gamble, Cincinnati, OH) in pockets≥5 mm immediately after mechanical instrumentation at the SRP visits. The fibers were removed after 7 days, i.e. at the next appointment for quadrant-wise SRP.
Surgery
Surgery was performed at the 3-month monitoring visit following SRP. Subjects who had residual PDs≥5 mm and bleeding on probing (BOP) received modified Widman flap surgery at weekly intervals as needed. Chlorhexidine, 0.1%, was used as a mouth rinse for 1 min twice daily during the surgical phase and 2 weeks following the last surgical session.
Home care
Each subject was provided with a powered toothbrush and triclosan-containing toothpaste and instructed to brush twice daily and to perform daily interdental cleaning with dental floss, toothpicks, and/or interdental brushes.
Post-treatment care
At each follow-up visit, the patients’ oral hygiene standard was checked and reinforced when indicated. Furthermore, at the 12-month recall, all sites with PD≥5 mm and BOP were subjected to subgingival mechanical debridement.
Clinical assessments
At each visit, visible plaque, gingivitis, BOP, suppuration, probing depth, and CAL were recorded at four sites per tooth (mesiobuccal, distobuccal, distolingual, and mesiolingual). Full-mouth computations included only approximal sites measuring≥5 mm at baseline. At each study visit, PD and CAL measurements were repeated and the means of the pairs of each measurement were used to determine changes in these parameters. A North Carolina periodontal probe (Hu-Friedy, Chicago, IL, USA) was used for PD and CAL recordings. The same clinician did measurements at all visits for a given subject, but the clinician making the measurements did not perform treatment on that subject. Each clinician examined or treated the same number of patients in each group. The calibration of the performance of clinicians was done as described by Haffajee et al. Citation10. Correlation within examiner was 0.82 for PD and 0.78 for CAL and between examiners pairwise correlations were 0.75 for PD and 0.82 for CAL. Correlations between centers was controlled by calibration sessions.
Data structure
Clinical parameters were measured at baseline and at 3, 6, 12, 18, and 24 months for each tooth site with PD≥5 mm. The patients were at the highest level of the structural data hierarchy (level 4), the 28 teeth excluding the third molars in each patient were in level 3, and level 2 comprised the four tooth sites. The bottom level of the data hierarchy had the time variables at baseline and the five monitoring time points. All the variables indicating the units of analysis (level 2) and the clusters of units (levels 3 and 4) were assumed to be random factors in the analysis.
Multilevel modeling
We considered a four-level model with random intercept and random effect of time on patients at level 3, random intercept for teeth at level 2, and tooth sites at level 1. The PD for any given patient at time t corresponding to the baseline visit and the 3, 6, 12, 18, and 24 months visits on site i, in tooth j nested within patient k denoted PDtijk, is given by model 1:
The parameter estimates β0 through to β13 represent fixed effects associated with the intercept, time effect, treatment, gender, age, race, nationality, smoking habits, baseline PD, plaque accumulation, BOP, suppuration, gingival redness, and the interaction of treatment with time. For the random part, α0k and α1k represent the patient random effect associated with the intercept and time slope, respectively. α0jk is the random effect of a tooth nested within a patient, α0ijk the random effect of a site nested in a tooth, which in turn is nested in patients, and ɛtijk is the random error, which is assumed to be homogeneous and uncorrelated with mean 0 and variance σ2.
The model parameter estimates were obtained in the statistical software R using the restricted maximum likelihood method (REML). We also considered a similarly structured model for CAL loss.
A therapy that reduces PD by>1.5 mm or results in gain of CAL of >1.5 mm would be considered clinically effective. Such binary response variables were also of interest. The binary responses were modeled by logistic multilevel regression given by model 2:
Y
tijk∼Binomial(1, p
tijk)
Y
tijk is 1 if patient k at time t on site i nested in tooth j had a decrease in PD of >1.5 mm and 0 otherwise. The parameter estimates are defined as in model 1. The random effects α0k, α1k, α0jk, and α0ijk are assumed to be normally distributed with mean 0 and variances ,
,
, and
, respectively. ɛtijk has mean 0 and variance σ2. However, interest was on fixed effects, particularly changes in treatment effect over time. Random effects only aided in describing variation in the population under study. We considered a similarly structured model for CAL gain of>1.5 mm. These generalized linear mixed models were estimated by Laplace approximations through the lmer routine in R.
Results
Dropout rate of the US subjects (32% at the Forsyth Institute, 24% at Boston University) was much greater than in the Swedish material (7%). Those in the SURG group dropped out more often than those receiving antibiotics. During the active treatment phase, 25 teeth were extracted, 41 during post-therapy monitoring. SURG increased the number of extracted teeth whereas systemic antibiotics gave reduced tooth loss. During follow-up, 66 teeth were extracted in 48 subjects.
There were no significant baseline differences between patients in the control group (SRP) and those receiving other treatments ().
Pocket depth
gives mean changes in PD for the effect of all treatments over time when compared to SRP obtained by fitting model 1. As expected, SURG was more effective in PD reduction after 6 months but combining it with systemic AMOX and MET gave better long-term clinical improvement. Positive estimates represent mean PD increase whereas negative estimates mean PD decrease at various time points. For example, the estimated effect of AMOX+MET+TET produced significant negative estimates after 6 months, suggesting that patients treated with AMOX+MET+TET had significant PD reductions over time compared to SRP. However, SURG+AMOX+MET+TET had larger significant estimates in absolute terms after 6 months, suggesting that there were more PD decrease for patients on this combination therapy than for any other therapy across the study period when compared to SRP.
Table 2. Results from fitting a linear multilevel regression model for changes in treatment effect on PD over time on sites that had a baseline PD of at least 5 mm using the restricted maximum likelihood estimation (REML)
Fig. S1 in the Appendix section is a plot of the mean PD at each study time point. It shows that sites treated with SURG+AMOX+MET+TET had the least mean PD while sites that were treated with SRP and TET had the largest mean PD. A plot of the mean difference in PD from the mean PD at the previous visit also shows similar trends with SURG+AMOX+MET+TET producing the least PD (Fig. S3). In Fig. S2, sites that were treated by SRP and with TET reverted to baseline conditions after 6 months.
Fixed effects on PD
shows the fixed effects on PD from fitting model 1. The overall estimated effect of age on PD was significant [0.01(0.01, 0.03)]. This means that, for every year increase in age, the PD was expected to increase by 0.01 mm. However, the effects of gender and nationality were not significant. The overall effect of site-level covariates (accumulation of plaque, BOP, gingival redness, and suppuration) on PD was highly significant.
Table 3. Results from fitting a linear multilevel regression model for treatment effect and other fixed effects on PD and CAL on sites that had a baseline PD of at least 5 mm using the restricted maximum likelihood estimation (REML)
Smoking habits
Both past [0.19 (0.02, 0.35)] and present [0.23 (0.08, 0.38)] smoking habits increased PD significantly by 0.19 and 0.23 mm, respectively.
We also fitted a model with a third-order interaction between treatment, time, and smoking status in order to investigate to what extent treatment effect varied with smoking status (not shown). A significant third-order interaction was found with regard to both PD and CAL. This means that the effect of treatment on either PD or CAL differed significantly between smokers and non-smokers. The difference was clearly expressed for the reference group (SRP), in favor of the non-smokers, while for the other treatment groups, the difference was somewhat smaller.
Pairwise treatment comparison on PD
We carried out a Tukey's all-pairwise comparison of the treatments on PD to establish those statistically significant from each other at specific time points. The results are given in Table S1 and include only comparisons that had significant outcome. After 3 months, SURG+TET were the least effective treatment when compared to AMOX+MET, TET, and SURG+AMOX+MET+TET. After 6 months, the effect of SRP waned and the following therapies produced significant results when compared to SRP; SURG+AMOX+MET, SURG+TET, SURG, and SURG+AMOX+MET+TET, which were also significantly effective when compared to TET. From the 12th months visit through to the 24th months visit, the effectiveness of all therapies leveled off (see also Fig. S1). After 12 months, SURG+AMOX+MET+TET would be a better choice than TET (p<0.01), better than SRP at 18 months (p<0.01), and at 24 months, it was better than SRP and TET (p <0.01).
Decreases in PD of >1.5 mm
and S2 show estimates obtained from the analysis of model 2. The effectiveness of therapies including SURG in reducing PD by >1.5 mm over time is clearly seen in . SURG combined with AMOX, MET, and TET was significantly more likely to achieve PD reduction of >1.5 mm than any other therapy. The odds of former smokers gaining on PD more than 1.5 mm was significantly reduced by 36% and was also reduced significantly by 39% in patient who were smokers compared to patients who had never smoked before.
Table 4. Odds ratios and their confidence intervals from a multilevel logistic regression model on PD reduction of more than 1.5 mm for sites that had a baseline PD of at least 5 mm
CAL loss/gain
presents mean changes in CAL for the effect of all treatments over time when compared to SRP obtained by fitting model 1. Negative estimates imply that there was gain of CAL and positive estimates represent further loss of CAL. SURG increased further loss of CAL compared to SRP at the 12-month visit. Sites that were surgically treated had lost more CAL than their baseline level conditions. However, when SURG was combined with AMOX+MET+TET, significant gain of CAL was observed. AMOX+MET produced significant gain of CAL from the 6-month visit.
Table 5. Results from fitting a linear multilevel regression model for changes in treatment effect over time on CAL in sites that had a baseline probing depth of at least 5 mm using the restricted maximum likelihood estimation (REML)
In Fig. S4, sites that were treated with SURG+ AMOX+MET had the least mean loss in clinical attachment. As shown in Fig. S5, only sites that had been treated with AMOX+MET+TET, AMOX+MET, and SURG+TET had slight gains in clinical attachment compared to their baseline conditions after 6 months. However, sites that were treated with AMOX+MET+TET gained most on clinical attachment but SURG treated sites gained less (Fig. S6).
Fixed effects on CAL
The fixed effects on CAL from fitting model 1 are shown in . All tooth site-level covariates like visible plaque, BOP, gingival redness, and suppuration produced highly significant positive estimates, thus were strong predictors for further loss of CAL. We did not find any effect of past smoking on loss of CAL but current smokers had higher risk of further loss of CAL.
However, we did not find other significant predictors, which either reduced or increased chances of sites gaining CAL by >1.5 mm in the logistic multilevel model (results not shown).
Pairwise treatment comparison on CAL
We also carried out Tukey's all-pairwise comparison of the treatments on CAL and the results given in Table S3 include only comparisons that had significant outcomes. After 3 months, sites that were treated with SURG+AMOX+MET were on average 1.39 mm deeper than sites that were treated with AMOX+MET+TET. Surgically treated sites were on average 1.31 mm deeper after 18 months and 1.26 mm deeper after 24 months compared to sites that were treated with AMOX+MET+TET.
Discussion
A multilevel approach to study factors influencing the outcome of periodontal treatment has been used previously Citation36–Citation39. The advantage of this approach over the traditional methods of generalized linear models is that it takes into account the relatedness of observations measured over time.
The results of the present study revealed that, for sites with a baseline PD of at least 5 mm, SURG and any therapy combined with SURG significantly reduced PD. When combined with AMOX, MET, and TET, SURG produced highly significant clinical benefits, which were maintained throughout the 2-year study period. Our results indicate that, if non-surgical therapies are to be used in arresting periodontitis, then the best therapy would be AMOX+MET+TET. These antibiotics must be used as a combination therapy since using them on their own does not produce long-lasting clinical benefits. On the other hand, if surgical treatment is preferred, then the best choice would be to combine SURG with AMOX, MET, and TET. Actually, this study revealed that SURG+AMOX+MET+TET produced more clinical benefits than any other treatment. Also Goodson et al. Citation35 found that AMOX+MET provided CAL gain and PD reduction.
We did not find any effect of gender, race, or nationality on PD. However, site level effects such as baseline PD, accumulation of visible plaque, gingival redness, BOP, and suppuration were significant predictors of PD increase. The effect of past and present smoking was also highly significant, and each year increase in age was significantly associated with an increase in PD.
In terms of CAL loss, major improvements in the clinical parameters occurred from 6 months in subjects who were treated with AMOX+MET+TET, AMOX+MET, and SURG+AMOX+MET+TET. Sites from patients who had been surgically treated experienced an increase in CAL loss. This might be due to a detrimental effect on osteoblasts through SURG. CAL loss was common in all sites that had been surgically treated only. Although SURG increased CAL loss, the effect could be improved if SURG was combined with antibiotics. We concluded that combination therapies with SURG were better than SURG at maintaining post-treatment effects on attachment.
In the present study, we did not find the effect of race and past smoking habits on loss of attachment in treated patients although current smokers and males were at a higher risk of CAL loss. This is in concordance with previous observations that responses after periodontal treatment are modified by cigarette smoking Citation24–Citation26. However, accumulation of visible plaque, BOP, gingival redness, and suppuration were significant predictors of further CAL loss.
Our study revealed that sites of the Swedish subjects experienced significant CAL loss after treatment compared to sites of the American subjects. In a study of 79 Swedish and 79 US citizens with a healthy periodontium or minimal periodontal disease, Haffajee et al. Citation40 found differences in the subgingival microbiota of the two populations. After adjusting for multiple comparisons five species (Actinomyces naeslundii genospecies 1, Streptococcus sanguinis, Eikenella corrodens, Tannerella forsythia, and Prevotella melaninogenica) were more abundant in the Swedish than in the American patients. How the microbiota relates to clinical changes in the present study groups remains to be investigated.
Periodontal diseases are common infections in man in which the supporting tissues of teeth are attacked resulting in CAL loss. They are common even in developed countries and a leading cause of tooth loss Citation1. Despite the fact that different periodontal therapies are being used, continued CAL loss is common in some patients. The current study aimed to investigate the maintenance over time of clinical effects after different combinations of treatments of periodontitis. The major objective was to find a treatment that could arrest periodontal breakdown and maintain the beneficial effects of treatment during a 2-year follow-up period. We have shown that while standard periodontal treatments such as SRP or SURG may improve periodontal conditions, the use of adjunctive systemic or local antibiotic treatments would be a better choice especially if used as combination therapies. If SURG is to be used, it should be combined with AMOX+MET+TET to maintain the best clinical results.
The present study is part of a series of studies in modeling of infectious disease using periodontitis as basis where we try to combine concepts and techniques from different disciplines. The use of multilevel analysis is on the frontier in periodontal research. However, available software for analyzing such models sometimes gives different results, and if the data are huge and depending on the hierarchy of the model to be fitted, they tend to converge slowly and in some cases never converge at all.
Conflict of interest and funding
J. Max Goodson developed the tetracycline fibers used in this study between 1976 and 1994 when they were introduced. They have not been on the market since 2003. No other participants have any conflict of interest with the products tested in this study. This work was supported by The Faculty of Dentistry, University of Oslo, and by Grant No. DE 12861 from the National Institute of Dental and Craniofacial Research.
Supporting information
Additional Supporting Information can be found in the online version of this article:Appendix S1. This appendix contains multiple comparisons of treatment effect on mean PD and mean CAL for sites that had a baseline PD of at least 5 mm (Tables S1 and S3, respectively) and estimated multilevel logistic regression parameters, standard errors, and odds ratios for reduction in PD of more than 1.5 mm on sites that had a baseline PD of at least 5 mm (Table S2). Figs. S1–S6 are also included. Fig. S1. Mean PD at each study time point. Fig. S2. Mean difference in PD from mean baseline PD. Fig. S3. Difference in mean PD at previous visit. Fig. S4. Mean CAL at each study point. Fig. S5. Mean difference in CAL from mean baseline CAL. Fig. S6. Differences in mean CAL from mean CAL at previous visit.
Please note: COACTION is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Acknowledgements
We acknowledge the Faculty of Dentistry, University of Oslo, and the National Institute of Dental and Craniofacial Research for financial support. Dr. Anna Bogren, Dr. Jan Lindhe, and Dr. Jan L. Wennstr?m, Institute of Odontology, The Sahlgrenska Academy at G?teborg University, G?teborg, Sweden, are kindly acknowledged for clinical samples and help with the manuscript.
References
- Rylev M, Kilian M. Prevalence and distribution of principal periodontal pathogens worldwide. J Clin Periodontol. 2008; 35(Suppl 8): 346–61. 10.3402/jom.v4i0.17535.
- Van Winkelhoff AJ, Winkel EG. Systemic antibiotic therapy in severe periodontitis. Curr Opin Periodontol. 1997; 4: 35–40.
- Golub LM, Wolff M, Roberts S, Lee HM, Leung M, Payonk GS. Treating periodontal diseases by blocking tissue-destructive enzymes. J Am Dent Assoc. 1994; 125: 163–9.
- Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, et al.. Treatment with subantimicrobial dose doxyxycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000; 71: 521–32. 10.3402/jom.v4i0.17535.
- Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, et al.. Subantimicrobial dose doxycycline as an adjunct to scaling and root planing: post-treatment effects. J Clin Periodontol. 2001; 28: 782–9. 10.3402/jom.v4i0.17535.
- Emingil G, Atilla G, Sorsa T, Luoto H, Kirilmaz L, Baylas H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinases-8 levels in chronic periodontitis. J Periodontol. 2004; 75: 106–15. 10.3402/jom.v4i0.17535.
- Gürkan A, Cinarcik S, Hüseyinov A. Adjunctive subantimicrobial dose doxycycline: effect on clinical parameters and gingival crevicular fluid transforming growth factor-beta levels in severe, generalized chronic periodontitis. J Clin Periodontol. 2005; 32: 244–53. 10.3402/jom.v4i0.17535.
- Tüter G, Kurti( B, Serdar M, Aykan T, Okyay K, Yücel A, et al.. Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol. 2007; 34: 673–81. 10.3402/jom.v4i0.17535.
- Herrera D, Sanz M, Jepsen S, Needleman T, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002; 29(Suppl 3): 136–59. 10.3402/jom.v4i0.17535.
- Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003; 8: 115–81. 10.3402/jom.v4i0.17535.
- Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1-year results. J Clin Periodontol. 2007; 34: 243–53. 10.3402/jom.v4i0.17535.
- Aimetti M, Romano F, Torta I, Cirillo D, Caposio P, Romagnoli R. Debridement and local application of tetracycline-loaded fibres in the management of persistent periodontitis: results after 12 months. J Clin Periodontol. 2004; 31: 166–72. 10.3402/jom.v4i0.17535.
- Gilbert A. Local tetracycline is an effective adjunct in the treatment of chronic periodontitis. Evid Based Dent. 2004; 5: 67. 10.3402/jom.v4i0.17535.
- Liu DZ, Chen WP, Lee CP, Wu SL, Wang YC, Chung TW. Effects of alginate coated on PLGA microspheres for delivery of tetracycline hydrochloride to periodontal pockets. J Microencapsul. 2004; 21: 643–52. 10.3402/jom.v4i0.17535.
- Rodrigues RM, Gonçalves C, Souto R, Feres-Filho EJ, Uzeda M, Colombo AP. Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J Clin Periodontol. 2004; 31: 420–7. 10.3402/jom.v4i0.17535.
- Romano F, Torta I, Debernardi C, Aimetti M. Debridement and local application of tetracycline in the management of persistent periodontitis. Clinical and microbiological results after 12 months. Minerva Stomatol. 2005; 54: 43–51.
- Söder B, Nedlich U, Jin LJ. Longitudinal effect of non-surgical treatment and systemic metronidazole for 1 week in smokers and non-smokers with refractory periodontitis: a 5-year study. J Periodontol. 1999; 70: 761–71. 10.3402/jom.v4i0.17535.
- Xajigeorgiou C, Sakellari D, Slini T, Baka A, Konstantinidis A. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. J Clin Periodontol. 2006; 33: 254–64. 10.3402/jom.v4i0.17535.
- Matarazzo F, Figueiredo LC, Cruz SE, Faveri M, Feres M. Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2008; 35: 885–96. 10.3402/jom.v4i0.17535.
- Cionca N, Giannopoulou C, Upolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J Periodontol. 2009; 80: 364–71. 10.3402/jom.v4i0.17535.
- Nowzari H, Matian F, Slots J. Periodontal pathogens on polytetra-fluoroethylene membrane for guided tissue regeneration inhibit healing. J Clin Periodontol. 1995; 22: 469–74. 10.3402/jom.v4i0.17535.
- Gordon JM, Walker CB. Current status of systemic antibiotic usage in destructive periodontal disease. J Periodontol. 1993; 64(Suppl 8): 760–71. 10.3402/jom.v4i0.17535.
- Winkel EG, Van Winkelhoff AJ, Timmerman M, Van der Velden U, Van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double-blind placebo-controlled study. J Clin Periodontol. 2001; 28: 296–305. 10.3402/jom.v4i0.17535.
- Papantonopoulos GH. Effect of periodontal therapy in smokers and non-smokers with advanced periodontal disease: results after maintenance therapy for a minimum of 5 years. J Periodontol. 2004; 75: 839–43. 10.3402/jom.v4i0.17535.
- Rieder C, Joss A, Lang NP. Influence of compliance and smoking habits on the outcomes of supportive periodontal therapy (SPT) in a private practice. Oral Health Prev Dent. 2004; 2: 89–94.
- Stavropoulos A, Mardas N, Herrero F, Karring T. Smoking affects the outcome of guided tissue regeneration with bioresorbable membranes: a retrospective analysis of infrabony defects. J Clin Periodontol. 2004; 31: 945–50. 10.3402/jom.v4i0.17535.
- Baelum V, Chen X, Manji F, Luan WM, Fejerskov O. Profiles of destructive periodontal disease in different populations. J Periodontal Res. 1996; 31: 17–26. 10.3402/jom.v4i0.17535.
- Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontology 2000. 2002; 29: 177–206. 10.3402/jom.v4i0.17535.
- Hugoson A, Laurell L, Lundgren D. Frequency distribution of individuals aged 20–70 years according to severity of periodontal disease experience in 1973 and 1983. J Clin Periodontol. 1992; 19: 227–32. 10.3402/jom.v4i0.17535.
- Susin C, Kingman A, Albandar JM. Effect of partial recording protocols on estimates of prevalence of periodontal disease. J Periodontol. 2005; 76: 262–7. 10.3402/jom.v4i0.17535.
- Albandar JM, Goldstein H. Multi-level statistical models in studies of periodontal diseases. J Periodontol. 1992; 63: 690–5. 10.3402/jom.v4i0.17535.
- Gilthorpe MS, Cunningham SJ. The application of multilevel, multivariate modelling to orthodontic research data. Community Dent Health. 2000; 17: 236–42.
- Faraway JJ, editor. Texts in statistical science. Extending the linear model with R. Generalized linear, mixed effects and nonparametric regression models. Chapter 8. Random effects, Chapman & Hall/CRC. Taylor & Francis Group: London: Boca Raton., 2006; p. 153–83.
- Zuur AF, Leno EN, Walke NJ, Saveliv GM, Smith GM editors. Fixed effects models and extensions in ecology. withR. Springer.: New York; 2009.
- Goodson JM, Haffajee AD, Socransky SS, Kent R, Teles R, Hasturk H, et al.. Control of periodontal infections: A randomized controlled trial. I. The primary outcome, attachment gain and pocket depth reduction at treated sites. J Clin Periodontol. (in press).
- Axtelius B, Söderfeldt B, Attström R. A multilevel analysis of factors effecting pocket probing depth in patients responding differently to periodontal treatment. J Clin Periodontol. 1999; 26: 67–76. 10.3402/jom.v4i0.17535.
- D'Aiuto F, Ready D, Parkar M, Tonetti S. Relative contribution of patient-, tooth-, and site-associated variability on the clinical outcomes of subgingival debridement. I. Probing depths. J Periodontol. 2005; 76: 398–405. 10.3402/jom.v4i0.17535.
- Needleman I, Suvan J, Gilthorpe MS, Tucker R, St George G, Giannobile W, et al.. A randomized-controlled trial of low-dose doxycycline for periodontitis in smokers. J Clin Periodontol. 2007; 34: 325–33. 10.3402/jom.v4i0.17535.
- Tomasi C, Leyland AH, Wennström JL. Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol. 2007; 34: 682–90. 10.3402/jom.v4i0.17535.
- Haffajee AD, Japlit M, Bogren A, Kent RL Jr, Goodson JM, Socransky SS. Differences in subgingival microbiota of Swedish and USA subjects who were periodontally healthy or exhibited minimal periodontal disease. J Clin Periodontol. 2005; 32: 33–9. 10.3402/jom.v4i0.17535.