Abstract
Background : Type 2 diabetes mellitus (T2DM) is prevalent in people with obesity. It has been proposed that these conditions are related to specific features of the microflora of the mouth and lower gastrointestinal (GI) tract. Hyperglycemia often resolves quickly after Roux-en-Y gastric bypass (RYGB) but the role of the GI microflora cannot be examined easily because of reduced intestinal mobility. We propose that the study of microorganisms present in the mouth of patients undergoing RYGB will contribute to our understanding of the role of bacteria in the pathogenesis of T2DM.
Objective : To conduct a feasibility study to examine differences in oral microbes in obese patients with and without T2DM and to determine whether it is feasible to measure changes after gastric bypass surgery.
Methods : Individuals with morbid obesity (n=29), of whom 13 had T2DM, were studied. Oral rinses, stool samples, and blood samples were obtained before RYGB, and oral rinses and blood samples were obtained at 2 and 12 weeks postsurgery.
Results : Prior to surgery, participants with T2DM had slightly higher total levels of oral bacteria than those without diabetes. Those with HbA1c > 6.5% had rather lower levels of Bifidobacteria in the mouth and stool. At 2 weeks post-RYGB, patients with T2DM were able to reduce or discontinue their hypoglycemic medications. Stool samples could not be obtained but oral rinses were readily available. The levels of oral Bifidobacteria had increased tenfold and levels of circulating endotoxin and tumor necrosis factor-alpha had decreased.
Conclusions : The study of oral bacteria before and after RYGB is feasible and should be tested in larger patient populations to increase our understanding of the role of microorganisms in the pathogenesis of obesity and T2DM.
Obesity is increasing in prevalence and has now reached epidemic proportions in the United States Citation1. Obesity is associated with type 2 diabetes mellitus (T2DM), periodontal disease, and other comorbidities Citation2. Although the mechanism of these associations is not entirely clear, an increasing body of evidence implicates the microbiota of the gastrointestinal (GI) tract.
Obesity and T2DM can be induced in mice by a high fat diet, but only if the mice have a normal GI microflora Citation3. If antibiotics change the microflora, weight loss and improved glycemic control are observed Citation4. These effects are associated with elevated plasma levels of endotoxin Citation5, which can stimulate elevations in circulating levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) Citation6, causing insulin resistance Citation7. Although no specific GI pathogens have been discovered that induce obesity or diabetes in mice, the conditions are associated with reduced abundance of Bifidobacteria Citation8 Citation9.
Human obesity and T2DM show several similarities to the animal model. Obese individuals have differences in their GI microflora from non-obese individuals and the flora changes when weight is lost Citation10. The oral cavity of obese individuals has higher levels of various bacteria Citation11–Citation13, whereas patients with T2DM have fewer Bifidobacteria in the intestine than control subjects Citation14. In addition to the bacterial features, obesity, T2DM, and periodontal disease are associated with elevated circulating levels of inflammatory markers such as endotoxin and TNF-α Citation15 Citation16.
Although the clinical association between obesity and its comorbidities is consistent with the changes in the microflora, the relevant mechanisms remain obscure. The interpretation of data is complicated by the fact that patients gain and lose weight slowly. Consequently, changes in adiposity, glycemic control, the microbial flora, and the inflammatory markers all occur within the same time frame. One approach to separating these effects is to study individuals who are undergoing Roux-en-Y gastric bypass surgery (RYGB), which results in improved glycemic control within days, before significant weight loss has occurred Citation17. The reasons for this sudden improvement are unknown and also the role of microorganisms has not been assessed because there is a postsurgical loss of intestinal mobility, which prevents the collection of stool samples. The current study investigated the feasibility of studying differences in oral microflora in obesity with and without T2DM.
Methods
Consecutive patients with morbid obesity who had been selected to undergo RYGB at SUNY Upstate Medical University between August and December 2010 were invited to participate. All participants met the criteria for bariatric surgery established by the NIH Consensus Conference Citation18. Exclusion criteria included current smokers and those having received antibiotics within the previous 6 months. The project was approved by the Institutional Review Board for the Protection of Human Subjects and all participants provided signed informed consent.
Prior to surgery, the height and weight of each subject was measured, and each maintained a diet journal in which they recorded all food and drink for 3 consecutive days. An oral examination was performed by the principal investigator to determine the presence of periodontal pockets of 4 mm depth or greater. Before surgery all subjects provided an oral sample by rinsing their mouths for 30 seconds with 10 ml of ice-cold saline and provided a fresh stool sample. A non-fasting blood sample was obtained, the level of HbA1c was determined, and the serum was separated. Oral, stool, and serum samples were immediately frozen and stored at −80°. All participants were assigned study identifier numbers and the staff members performing analyses were blind to group allocation.
On the day of surgery, each subject was given a single prophylactic intravenous dose of cephazolin. Clindamycin was administered to penicillin-allergic patients, as per usual surgical practice. No other antibiotics were given to any subject at any time during the study. The surgical procedure consisted of open or laparoscopic RYGB as previously described Citation19.
At 2- and 12-weeks after surgery, oral rinses and blood samples were obtained as before, and the subjects’ weight was recorded. Again, the subjects provided a diet journal. Those subjects with T2DM continued to monitor their blood glucose levels, and their glucose-lowering medications were reduced or stopped as indicated by their physician.
Markers of inflammation
Serum levels of endotoxin were determined with a Limulus Amebocyte Lysate assay (Associates of Cape Cod Inc., Falmouth, MA). Serum levels of TNF-α were measured with a Quantikine ELISA assay (R&D Systems, Minneapolis MN). Serum levels of CRP were determined using the high-sensitivity C-reactive protein ELISA assay (Calbiotech Inc., Spring Valley, CA).
Microflora
DNA was isolated from 1.5 ml of each oral rinse using the DNeasy Blood and Tissue kit (Qiagen Inc, Valencia, CA) and from 0.2 g of each stool sample using the QIAmp DNA stool kit (Qiagen). A real-time quantitative PCR (RTq-PCR) was used to determine the concentration of ‘All Bacteria’, Firmicutes, Bacteroidites, Bifidobacteria, Bacteroides thetaiotaomicron, Porphyromonas gingivalis and Methanobrevibacter smithii, using previously published primer pairs Citation20–Citation25. The RTq-PCR was calibrated with DNA from cultures of reference strains.
Statistical analysis
Data are expressed as the mean±SEM for each group. Differences between groups were assessed by student's t-test, using the computer program Prism (GraphPad Software Inc., La Jolla, CA). A p-value < 0.05 was taken as indicator of statistically significant difference. To find what sample sizes would be needed to evaluate likely differences between patient groups, a power analysis Citation26 was performed.
Results
Clinical data
Participants (n=29) with morbid obesity were enrolled consecutively over a period of 4 months; 7 males, 22 females, mean age 41 years (range 23–55), and mean body mass index (BMI) 48 (range 37–97). Prior to surgery, 13 participants had been diagnosed as having T2DM by their primary care physician, using standard criteria. All 13 were receiving oral hypoglycemic medications for T2DM, and 3 were also taking insulin. The HbA1c levels of all participants ranged from 5.2% to 11.3% with 8/13 with T2DM having a level > 6.5%.
One subject was edentulous and no oral rinses were obtained from this patient. All other patients had 8 to 30 teeth (mean 24.4±0.9). Two patients had a single periodontal pocket of 4 mm depth and the others had no pockets. None of the patients therefore met any of the current definitions of periodontitis Citation27.
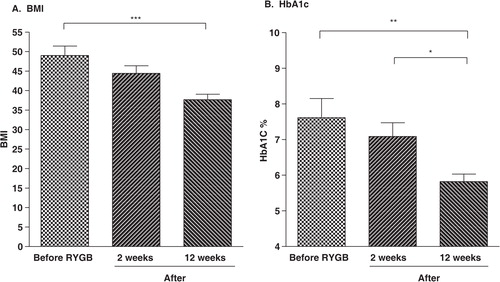
Immediately after surgery, medications for the treatment of diabetes were completely discontinued for 11 of the 13 subjects with diabetes and dosages were reduced for the remaining 2. All post-RYGB subjects with T2DM noted that their blood glucose levels were maintained below 200 mg/dL and the improvement in glycemic control was confirmed by the subsequent reduction in HbA1c levels (B). At 14 days postsurgery, dietary intake of carbohydrates, protein, and fat were all significantly reduced (p<0.0001). Post-RYGB weight loss during the first 2 weeks after surgery was not significant (p>0.05) and all subjects remained morbidly obese. However, by 12 weeks post-RYGB, the weight loss was significant (p<0.001, A).
Markers of inflammation
Prior to surgery, there was a higher baseline serum endotoxin level in subjects with T2DM (4.15±0.77 EU/ml) than in those without diabetes (2.51±0.43 EU/ml). Two weeks after surgery, endotoxin levels in subjects with T2DM had fallen to 2.91 + 0.54 EU/ml and remained at that low level up to week 12 (A).
Fig. 2. Inflammatory markers. Serum levels of endotoxin (A), TNF-α (B), and CRP (C) in subjects with or without T2DM before RYGB and levels in subjects with T2DM at 2 weeks and 12 weeks after surgery.
**p<0.01.
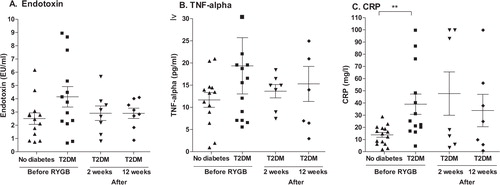
Prior to surgery, the serum levels of TNF-α were also higher in the participants with T2DM (19.36±6.34 pg/ml) than in those without diabetes (11.68±1.67 pg/ml). At 2 weeks after surgery, levels in the subjects with T2DM had fallen to 13.64±1.50 pg/ml and remained at that level at week 12 (B).
Serum levels of CRP, prior to surgery, were significantly higher in the patients with T2DM (39.0±8.3 mg/l) than in those without diabetes (13.8±2.0 mg/l, p<0.01). The mean level in the subjects with T2DM was slightly increased after surgery (C).
Microflora
The RTq-PCR was found to be highly specific. Each primer pair induced amplification of the appropriate reference DNA only, except that the bacteroidites primer amplified DNA from P. gingivalis and B. thetaiotaomicron with almost equal efficiency, consistent with their known relatedness Citation28. The ‘All Bacteria’ primers did amplify DNA of M. smithii, which is consistent with its original classification as a bacterium.
Prior to surgery, subjects with T2DM had a 10-fold greater concentration of ‘All Bacteria’ in the oral rinses than the subjects without diabetes (2.2×109±8.7×108 genomes/ml compared to 3.6×108±1.17×108) (A). However, when the T2DM subjects were categorized by level of glycemic control, the oral levels of Bifidobacteria were lower in the subjects with HbA1c > 6.5% than in those with levels of HbA1c < 6.5% (2.6×104±9.4×103 genomes/ml compared to 6.8×104±2.85×104) (B).
Fig. 3. Pre-surgical microflora. Concentrations of microbes in oral rinses (A, B) and stool (C, D) before RYGB, with subjects stratified as no diabetes/T2DM (A, C) and as HbA1c < 6.5%/HbA1c > 6.5% (B, D).
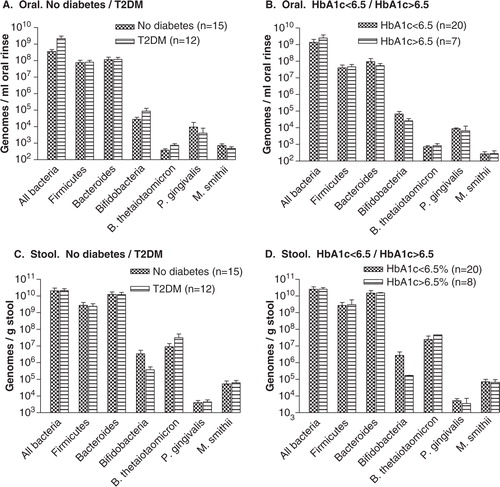
Prior to surgery, there were no differences in stool samples between levels of ‘All Bacteria’ between subjects with or without T2DM (C). However, levels of Bifidobacteria were approximately 10-fold lower in subjects with T2DM compared with those without diabetes (3.75×105±1.8×105/genomes/g compared to 3.4×106±2.1×106 genomes/g). This difference was greater when subjects were stratified by HbA1c levels > 6.5% or < 6.5% (1.67×105±4.7×103 genomes/g compared to 2.71×106±1.6×106 genomes/g, D).
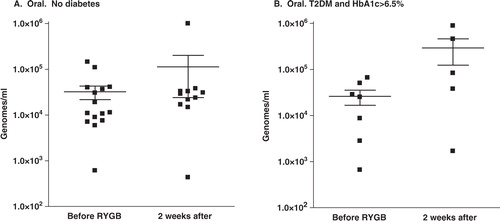
At 2 weeks after RYGB, subjects were not able to provide stool samples reliably because intestinal mobility was still reduced. However, all subjects were able to provide oral rinses. At this time, no organisms showed a change in the oral levels that exceeded 2-fold except for the Bifidobacteria species. The oral microflora of the participants without diabetes showed a 2.4-fold increase in the levels of Bifidobacteria, from 3.2×104±1.0×104 genomes/ml to 1.1×105±8.9×104 genomes/ml (A). In the participants with T2DM and HbA1c > 6.5%, the level of Bifidobacteria in the oral rinses increased approximately 10-fold, from 2.61×104±9.4×103 genomes/ml to 2.92×105±1.69×105 genomes/ml (B). However, all of these changes were at a significance level of p>0.05.
Statistical analysis
Power analysis was applied to find the sample size that would be necessary to detect the differences in the numbers of Bifidobacteria, given the means and distributions shown in B. It was determined that to test the hypothesis that the concentrations of Bifidobacteria increase after surgery with a power of 80% and α < 0.05, we would require 25 subjects.
Discussion
The role of microbes in the etiology of T2DM and other comorbidities of obesity might be made clear by studies on patients undergoing RYGB, since glycemic control is reported to improve within days Citation17. However, this approach is made difficult by the lack of stool samples immediately after surgery, when intestinal mobility is temporarily impaired. Studies at several months after RYGB Citation29–Citation31 do not reflect immediate (pre-weight loss) postoperative changes. Therefore, in the present study, we examined bacteria from the oral cavity within 2 weeks of surgery when the most important changes are likely to have occurred.
Before RYGB, the subjects with T2DM had slightly higher levels of circulating endotoxin and TNF-α, and significantly higher levels of CRP (), consistent with previous reports Citation15 Citation16 Citation32. At 2 weeks post-RYGB, levels of circulating endotoxin and TNF-α had fallen in subjects with T2DM (). This rapid decline in levels of endotoxin and TNF-α may be related to the changes in the microbiome and thus to the resolution of the hyperglycemia. However, it cannot be excluded that dietary changes were an important factor. The observed changes are not consistent with the notion that an elevated level of TNF-α is due solely to the presence of adipose tissue since the subjects were still morbidly obese. The small increase in the circulating level of CRP after surgery could be a consequence of the surgical procedure itself.
After RYGB, an improvement in glycemic control was seen in subjects with T2DM, with most patients being able to discontinue their hypoglycemic medication. This was expected and is consistent with previous reports Citation17. The levels of Bifidobacteria in stool samples were lower in patients with T2DM prior to surgery, consistent with other reports Citation14.
Since the clinical and laboratory features of the participants conformed to what might be expected from the previously published literature, the study population does make a suitable group for the study of obesity-related conditions, such as T2DM and periodontal disease. On the contrary, it must be noted that patients undergoing RYGB are not a representative cross section of the global population of obese persons. The availability of RYGB is limited to individuals who meet specific criteria of motivation, compliance and resources. All of the subjects were drawn from the same geographical area, and the sample size was small. Possibly as a result of this selection process, no patient had periodontal disease despite the well-established association of periodontal disease with obesity Citation2. Therefore, it might be difficult to study the progress of periodontal disease in this group.
The use of oral rinses to evaluate the GI tract microflora was found to be feasible. The major organisms of interest were readily detected in the oral rinses, and changes and differences were consistent with expectations. Bifidobacteria in the mouth were somewhat less in the subjects with T2DM who had HbA1c > 6.5% () and the concentration increased 10-fold within 2 weeks of surgery (). Such large differences were not observed for the other organisms examined. The levels of Bifidobacteria in the mouth appeared to reflect those in the lower GI tract, suggesting that a study of oral organisms could provide data with systemic implications. Although the magnitude of the postsurgical change did not achieve the 0.05 level of significance, a power analysis showed that this could feasibly be tested with a larger sample.
Simultaneous changes in oral and lower GI microbes could be due to the surgical procedure correcting a systemic mucosal immune defect as we previously proposed Citation19 Citation33. This could allow selected bacterial species to return to normal levels. Alternatively, the oral microflora could directly influence the postgastric microbiota, given that approximately 1 g of bacteria are swallowed per day Citation34.
Two mechanisms have been proposed to explain the beneficial effects of Bifidobacteria and related bacilli in T2DM Citation8 Citation9. The first is that they compete with pathogenic organisms, displacing them from the normal microflora Citation35. If this is the case, then a microbiome-wide study of the oral organisms should identify organisms that are displaced. The second mechanism is that Bifidobacteria binds endotoxin to their surface, preventing it from being transported across the mucosal barrier and into the circulation. Although some strains of Bifidobacteria are extremely efficient at this, others are not Citation36. Future studies of the oral microbiome should be aimed at examining variants of Bifidobacteria or related organisms and explore possible associations with improvements in glycemic control. Any role for Bifidobacteria or related organisms in protection from T2DM or other comorbidities of obesity has obvious implications for contributing to the prevention and treatment of the conditions.
Conclusion
This study investigated the feasibility of studying the oral microflora in patients undergoing gastric bypass surgery as a novel approach to increasing our understanding of obesity and its comorbidities – T2DM and periodontal disease. The data are consistent with the hypotheses that RYGB affects the microbiome of the GI tract and that studies of the oral flora can provide useful information to direct potential new treatments. Future studies will require larger patient populations, microbiome-wide observations, and investigations of the mechanisms of selected organisms.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
Acknowledgements
We thank Drs. A. Tanner and J. Gordon for supplying bacteria and DNA and Drs. R. Genco and A. Tanner for helpful discussions. We thank the staff at the Bariatric Surgery Center and Clinical Research Unit of SUNY Upstate Medical University for their assistance.
References
- Catenacci V, Hill J, Wyatt H. The obesity epidemic. Clin Chest Med. 2009; 30: 415–44. 10.3402/jom.v4i0.19013.
- Pischon N, Heng N, Bernimoulin J, Kleber BM, Willich SN, Pischon T. Obesity, inflammation and periodontal disease. J Dent Res. 2007; 86: 400–9. 10.3402/jom.v4i0.19013.
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock N, Magness S, et al.. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in the mouse. PLoS One. 2010; 5: e12191. 10.3402/jom.v4i0.19013.
- Membrez M, Blancher F, Jaquet M, Biblioni R, Cani PD, Burcelin RG, et al.. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008; 22: 2416–26. 10.3402/jom.v4i0.19013.
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al.. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008; 57: 1470–81. 10.3402/jom.v4i0.19013.
- Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit JS, et al.. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012; 153: 794–9. 10.3402/jom.v4i0.19013.
- Krogh-Madsen R, Plomgaard P, Moller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006; 291: E108–E14. 10.3402/jom.v4i0.19013.
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al.. Selective increases of Bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxemia. Diabetologia. 2007; 50: 2374–83. 10.3402/jom.v4i0.19013.
- Kondo S, Xiao J-Z, Satoh T, Odamaki T, Takahashi S, Sugahara H, et al.. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010; 74: 1656–61. 10.3402/jom.v4i0.19013.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006; 444: 1022–3. 10.3402/jom.v4i0.19013.
- Haffajee AD, Socransky SS. Relation of body mass index, periodontitis and Tannerella forsythia. J Clin Periodontol. 2009; 36: 89–99. 10.3402/jom.v4i0.19013.
- Goodson JM, Groppo D, Halem S. Carpino E. Is obesity an oral bacterial disease?. J Dent Res. 2009; 88: 519–23. 10.3402/jom.v4i0.19013.
- Zeigler C, Persson G, Wondimu B, Marcus C, Sobko T, Modeer T. Microbiota in the oral sub-gingival biofilm is associated with obesity in adolescence. Obesity. 2012; 20: 157–64. 10.3402/jom.v4i0.19013.
- Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, et al.. Molecular characterization of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010; 61: 69–78. 10.3402/jom.v4i0.19013.
- Dandona P, Weinstock R, Thusdu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998; 83: 2907–10. 10.3402/jom.v4i0.19013.
- Engebretson S, Chertog R, Nichols A, Hey-Hadavi J, Celenti R, Grbic J. Plasma levels of tumor necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J Clin Periodontol. 2007; 34: 18–24. 10.3402/jom.v4i0.19013.
- Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obesity Surg. 2005; 15: 474–81. 10.3402/jom.v4i0.19013.
- NIH Conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991; 115: 956–61.
- Culnan DM, Cooney RN, Stanley B, Lynch CJ. Apolipoprotein A-IV, a putative satiety/anti-atherogenic factor, rises after gastric bypass. Obesity. 2009; 17: 46–52. 10.3402/jom.v4i0.19013.
- Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol. 2008; 47: 367–73. 10.3402/jom.v4i0.19013.
- Haakensen M, Dobson CM, Deneer H, Ziola B. Real-time PCR detection of bacteria belonging to the Firmicutes Phylum. Int J Food Microbiol. 2008; 125: 236–41. 10.3402/jom.v4i0.19013.
- Matsuki TK, Watanabe J, Fujimoto Y, Miyamoto T, Takada K, Matsumoto K, et al.. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol. 2002; 68: 5445–51. 10.3402/jom.v4i0.19013.
- Liu C, Song Y, McTeague M, Vu AW, Wexler H, Finegold SM. Rapid identification of the species of the Bacteroides fragilis group by multiplex PCR assays using group- and species-specific primers. FEMS Microbiol Lett. 2003; 222: 9–16. 10.3402/jom.v4i0.19013.
- Yoshida A, Nagashima S, Ansai T, Tachibana M, Kato H, Watari H, et al.. Loop-mediated isothermal amplification method for rapid detection of the periodontopathic bacteria Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola. J Clin Microbiol. 2005; 43: 2418–24. 10.3402/jom.v4i0.19013.
- Dridi B, Henry M, El Khechine A, Raoult D, Drancouort M. High prevalence of Methanobrevibacter smithii and Methanospaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009; 4: 1–6. 10.3402/jom.v4i0.19013.
- Motulsky H. Intuitive Biostatistics. Oxford University Press. New York, 1995
- Page R, Eke P. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007; 78: 1387–99. 10.3402/jom.v4i0.19013.
- Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, et al.. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J Bacteriol. 2003; 185: 5591–601. 10.3402/jom.v4i0.19013.
- Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al.. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009; 106: 2365–70. 10.3402/jom.v4i0.19013.
- Furet JP, Kong LC, Tap J, Poitou C, Basdevamt A, Bouillot JL, et al.. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss. Diabetes. 2010; 59: 3049–57. 10.3402/jom.v4i0.19013.
- Li J, Ashrafian H, Bueter M, Kinross J, Sands C, LeRoux C, et al.. Metabolic surgery profoundly influences gut microbial-host metabolic cross talk. Gut. 2011; 60: 1214–23. 10.3402/jom.v4i0.19013.
- Lee C, Adler A, Sandhu MS, Sharp S, Forouhi N, Ergou S, et al.. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009; 52: 1040–7. 10.3402/jom.v4i0.19013.
- Cottam DR, Schaffer PA, Shaftan GW, Velcu L, Angus LDG. Effect of surgically-induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obesity Surg. 2002; 12: 335–42. 10.3402/jom.v4i0.19013.
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005; 38: 135–87. 10.3402/jom.v4i0.19013.
- Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004; 28: 405–40. 10.3402/jom.v4i0.19013.
- Park M, Kim M, Ji G. Assessment of lipopolysaccharide-binding activity of Bifidobacterium and its relationship with cell surface hydrophobicity, autoaggregation, and inhibition of interleukin-8 production. J Microbiol Biotechnol. 2007; 17: 1120–6.