Abstract
Candida albicans is an opportunistic, fungal pathogen of humans that frequently causes superficial infections of oral and vaginal mucosal surfaces of debilitated and susceptible individuals. The organism is however, commonly encountered as a commensal in healthy individuals where it is a component of the normal microflora. The key determinant in the type of relationship that Candida has with its host is how it interacts with the epithelial surface it colonises. A delicate balance clearly exists between the potentially damaging effects of Candida virulence factors and the nature of the immune response elicited by the host. Frequently, it is changes in host factors that lead to Candida seemingly changing from a commensal to pathogenic existence. However, given the often reported heterogeneity in morphological and biochemical factors that exist between Candida species and indeed strains of C. albicans, it may also be the fact that colonising strains differ in the way they exploit resources to allow persistence at mucosal surfaces and as a consequence this too may affect the way Candida interacts with epithelial cells. The aim of this review is to provide an overview of some of the possible interactions that may occur between C. albicans and host epithelial surfaces that may in turn dictate whether Candida removal, its commensal persistence or infection follows.
The Candida genus consists of approximately 200 species of ‘yeast-like’ fungi and collectively represents a highly heterogenic group (Citation1). Taxonomically, the Candida genus is in the class Deuteromycetes, and a feature of Candida species is their ability to grow polymorphically, either in the form of budding yeasts (blastoconidia) or filaments (true hyphae and pseudohyphae). The reason for this heterogeneity in the Candida genus largely stems from the fact that historically, designation of organisms to the genus was based on the absence of a known sexual reproduction stage. In many instances, Candida species have since been shown to reproduce sexually, but have retained their taxonomic status within Candida. As a consequence, Candida species can differ greatly in terms of their biochemistry, morphology, genetic composition and, importantly, their ability to instigate human infection.
In the case of human infections caused by Candida, the terms candidosis (sing.) or candidiasis are used, and candidoses (pl.) can broadly be categorised as being systemic or superficial. Systemic infections generally develop in severely immunocompromised individuals and whilst these infections are relatively rare, they are associated with high mortality. In contrast, superficial infections on moist mucosal surfaces, such as those of the mouth and vagina are more prevalent, but have less damaging effects to the host.
Approximately, 20 Candida species have, at some point, been associated with causing candidosis in humans. The species most frequently isolated from humans and the causative agent of the majority of infections is, however, Candida albicans and it is this species that is the focus of this review.
Candida albicans is an opportunistic pathogen and generally exists as a harmless commensal of humans, primarily on moist mucosal surfaces, particularly of the gut, vagina, and oral cavity. Depending on the population studied, commensal carriage in the oral cavity can range between 40 and 60% (Citation2). In the case of the vagina, Candida colonisation rates again vary with studied groups, with carriage rates of 41 and 21% reportedly occurring in type 1 and type 2 diabetic patients, respectively (Citation3). Women who are pregnant also reportedly have a high incidence of vaginal carriage (Citation4), and vaginal candidosis is one of the most common superficial infections in reproductive-age women (Citation5).
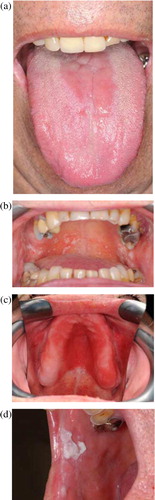
Given that C. albicans colonises host surfaces at such a high prevalence, infections are unsurprisingly often endogenous (Citation6), occurring when there is an ecological shift in the microbiological community, frequently due to debilitation in the host's immune system. Receipt of a broad-spectrum antibiotic, a high frequency intake of carbohydrates, hormonal imbalances, and poor nutrition may also be contributory factors. Interestingly, in the case of oral candidosis four clinically distinct forms of infection are recognised () and these could reflect different forms of interaction between the colonising Candida and host epithelium. The four clinically distinct primary forms of oral candidosis are acute erythematous candidosis, pseudomembranous candidosis, chronic erythematous candidosis, and chronic hyperplastic candidosis. Clinical symptoms of acute erythematous candidosis include redness and soreness of the oral mucosa with the tongue most often affected. Pseudomembranous candidosis is most common in infants and immunocompromised people and typically manifests as creamy white plaques or patches on oral tissues that can usually be scraped off. Chronic erythematous candidosis presents as localised erythema in regions of ill-fitting or inadequately cleaned dentures. Chronic hyperplastic candidosis is seen as firmly adhered white patches on the oral mucosa.
Fig. 1. Clinically distinct forms of primary oral candidosis. (a) Acute erythematous candidosis; (b) pseudomembranous candidosis; (c) chronic erythematous candidosis; (d) chronic hyperplastic candidosis.
To successfully persist within the host environment, either as a commensal or as a pathogen, Candida first has to adhere and then colonise host surfaces. These surfaces may take the form of the biomaterials of medical devices, for example, the acrylic of a denture, or the host's mucosal surfaces.
Adherence of Candida to mucosal surfaces
The process of initial adherence of Candida to human epithelial surfaces is complex and multifactorial. Cell surfaces (both Candida and epithelial cells) are generally negatively charged, and establishing successful adherence is, in part, dependent on the sum of non-specific factors contributing to the total free energy of interaction. These include attractive Lifshitz–van der Waals forces, hydrophobic interaction, and Brownian movement forces, as well as the repulsive effects of the electrical double layer of cells. Such interactions form the basis of the extended Derjaguin–Landau–Verwey–Overbeek (DLVO) theory (Citation7).
Once the ‘long-distance’ repulsive forces have been overcome, adherence of Candida is then mediated by specific molecules, referred to as adhesins, on the fungal cell surface and these interact with specific ligands on the host cell surface (). Adhesins on the cell surface of C. albicans can interact with serum proteins, components of the extracellular matrix (ECM), immobilised ligands such as cadherins or integrins, or indirectly via other microorganisms (Citation28). An important serum component that C. albicans can bind to is Factor H (FH; (Citation29)) which is a key regulator of the alternative pathway (AP) of complement and incorporation of FH on the surface of C. albicans prevents AP activation (Citation30). Laminin, fibronectin, collagen, entactin, vitronectin, and tenascin are all ECM proteins that C. albicans can interact with (Citation31).
Table 1 Examples of Candida albicans adhesins and associated host cell ligands
Members of the agglutinin-like sequence (ALS) gene family of C. albicans encode for large cell-wall glycoproteins, some of which are implicated in the adhesion of the organism to host surfaces (Citation25, Citation32). The ALS gene family comprises of eight members (Als1–Als7, and Als9) and all have a similar three-domain structure and are associated with the β-1,6-glucan of the cell wall of C. albicans (Citation23). In the case of C. albicans, Als3 appears to play a key role in adhesion to oral epithelial cells, and it is also related to the extent of subsequent epithelial damage and induction of epithelial cytokines (Citation33).
Hyphal wall protein 1 (Hwp1; encoded for by the HWP1 gene) is another protein involved in C. albicans adhesion to epithelial cells and this protein is perhaps the most widely studied adhesin of C. albicans (Citation34). Glutamine residues in the N-terminal domain of Hwp1 can be cross-linked to unidentified host proteins by host transglutaminase activity and this leads to covalent attachment of the yeast to host epithelial cells. This interaction has been shown to be important for C. albicans colonisation within the oral cavity (Citation35).
The β-1,3-glucan motif of the cell wall of C. albicans and indeed other pathogenic fungi (Citation36), has been shown to interact with Dectin-1 on the surfaces of host cells, primarily on phagocytotic cells including dendritic cells within the oral epithelium. As such, several studies have shown that Dectin-1 belongs to the armoury of pathogen recognition molecules participating in host defence against fungal pathogens, including Candida species and Aspergillus species (Citation37, Citation38). Dectin-1 can synergise with toll-like receptor (TLR) 2 and TLR4 signals and promote Th1 and Th17 responses to activate antifungal host defences (Citation39–Citation41). Further detail concerning Dectin-1 and fungal interactions in respect to immune responses is provided later in this review.
Recently, the gene encoding the C. albicans protein, EAP1 (Enhanced Adherence to Polystyrene) was identified. This gene was originally investigated because of its ability to encode for a protein mediating adhesion to polystyrene of a Saccharomyces cerevisiae flocculin-deficient strain. EAP1 encodes for a glycosylphosphatidylinositol-anchored, glucan-cross-linked cell-wall protein that has since been shown to facilitate adhesion of C. albicans to epithelial cells as well as polystyrene (Citation28).
Once adherence to mucosal surfaces has been established, colonisation and growth of C. albicans is required to maintain the presence of the organism at the host site. The extent of this colonisation is key to determining whether eradication, commensal carriage, or infection subsequently follows. The ability of C. albicans to generate a biofilm on host surfaces, including mucosa, is also an important attribute toward such persistence.
Biofilm formation by C. albicans on mucosal surfaces
Biofilms are defined as microbial communities that are often attached to solid substrata with the biofilm cells themselves embedded within extracellular polymeric substances (EPS) that they have generated. C. albicans is particularly adept at forming biofilms on the acrylic of dentures and also on mucosal surfaces (). In the case of pseudomembranous candidosis, the pseudomembranes that develop on the oral mucosa have been shown to be typical biofilms and linked to the recalcitrant nature of the condition (Citation42). Once the biofilm has formed, the EPS encasing the cells may contribute to persistence of the organism by several possible mechanisms. First, the EPS may serve to sequester antimicrobial substances that are present in oral secretions or within administered agents thereby limiting diffusion into the biofilm and access to cells. Similarly, restricted access of phagocytotic cells to C. albicans within the biofilm will also occur. It has also been suggested that altered cell phenotypes, potentially with reduced growth rates of biofilm cells provides an additional means of protection against host defence molecules. An important regulator of C. albicans biofilm formation is the transcription factor Bcr1, which is a positive regulator of several candidal adhesin genes including HYR1, HWP1, CHT2, ECE1, RBT5, ALS1, and ALS3 (Citation43, Citation44). The importance of Bcr1 in C. albicans biofilm formation within in a mouse model of oral infection has recently been demonstrated (Citation45).
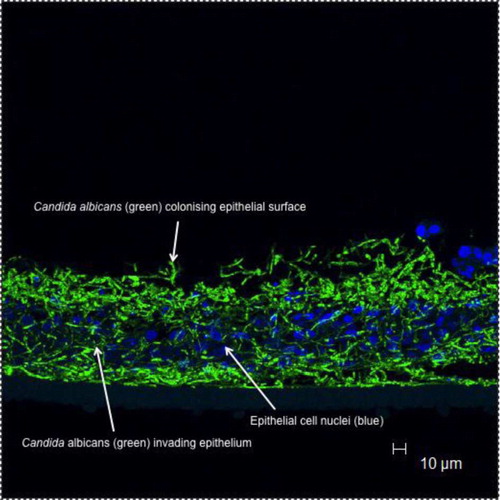
Fig. 2. Candida albicans biofilm formation on oral mucosal surfaces.
Arrows indicate green fluorescing C. albicans (stained with a labelled peptide nucleic acid probe) infecting a reconstituted oral epithelium generated commercially from transformed human keratinocytes of the cell line TR146 (from a squamous cell carcinoma of the buccal mucosa; SkinEthic Laboratories, Nice, France); nuclei of epithelial keratinocytes are shown as blue (Hoescht staining).
Damage induced by C. albicans to epithelial cell surfaces
To propagate at mucosal sites, nutrients may be acquired from the surrounding milieu or through degradation of host tissue structures. The latter may also allow penetration of C. albicans hyphae into deeper layers of the epithelium (), which would further enhance persistence of the organism on oral surfaces, which, in the oral cavity has a high cellular turnover partly serving as a defence mechanism to remove colonised cells.
As previously mentioned, C. albicans is an opportunistic pathogen and as such it can be argued that it does not possess potent virulence factors, certainly when compared with other strict pathogens. However, C. albicans can generate a number of hydrolytic enzymes with broad substrate activity that can damage host cell structures. Perhaps, the most extensively studied extracellular hydrolytic enzymes of C. albicans are the secreted aspartyl proteinases (SAPs).
The SAP family of C. albicans is currently known to comprise 10 genes encoding for proteinases with masses of 35–50 kDa. SAPs 1–3 and SAPs 4–6 are thought to represent two subfamilies (Citation46). The SAP genes are differentially regulated depending on the surrounding environment and are thought to be involved in the pathogenesis of C. albicans. SAP1–6 gene expression appears to be related to adherence, tissue damage, and changes in the immune response (Citation46–Citation49). SAPs 4–6 are expressed by C. albicans during hyphal invasion of a reconstituted human oral epithelium (RHE; (Citation50)) and oral infection (Citation51). SAPs 4–6 are also linked with hyphal formation, invasion of the epithelium (Citation52), and apoptosis of epithelial cells (Citation53). SAPs 2 and 6 are also potent inducers of IL-1β, TNF-α, and IL-6 production by monocytes (Citation54). The SAP gene products are suggested to contribute to various virulence processes in vitro including adherence to and invasion of the epithelial cells (Citation55, Citation56). However, all the SAPs have distinct pH optima and the extent of their functional activity at the generally neutral pH of oral mucosa remains to be ascertained.
E-cadherin is a protein associated with epithelial cell junctions that serves to maintain a functional barrier to invasion. In vitro breakdown of E-cadherin, produced by oral epithelial cells by SAP5, has been demonstrated and this could represent a mechanism by which C. albicans mediates invasion of oral mucosa (Citation56).
In addition to SAPs, C. albicans also has two other gene families, namely the lipases (LIP) and phospholipases (PL) that produce extracellular hydrolytic enzymes that could play roles in candidal adhesion, nutrient acquisition and invasion of epithelial surfaces (Citation57, Citation58). The LIP gene family of C. albicans comprises at least 10 genes (LIP1–10) (Citation59), whilst seven phospholipase genes of C. albicans have been identified (PLA, PLB1, PLB2, PLC1, PLC2, PLC3, and PLD1) (Citation60). Constitutive expression of the LIP genes and PLB has been demonstrated in C. albicans biofilms generated on an RHE (Citation61).
Epithelial cell responses to C. albicans
Epithelial cells of host mucosal surfaces represent the first line of defence against Candida infection. As key cells in the innate immunity of the host, epithelial cells express pattern recognition receptors (PRRs), which recognise C. albicans. PRRs interact with pathogen-associated molecular patterns (PAMPs) on microbial cells and examples of these in C. albicans include cell-wall components and nucleic acids. PRRs are divided into three major groups, TLRs, C-type lectin receptors (CLRs) and nod-like receptors (NLRs). Within these receptor groups, only certain TLRs and CLRs on epithelial surfaces recognise Candida. In addition to PRRs, other cell-surface proteins, such as E-cadherin and Epidermal Growth Factor Receptor (EGFR), can also recognise Candida and as discussed previously, these are unsurprisingly implicated in Candida adherence and endocytosis (Citation62, Citation63).
Cell-surface recognition of Candida produces a cascade cell signaling reaction, which leads to gene expression in epithelial cells for a number of growth factors, chemokines/cytokines, antimicrobial peptides, and cell matrix proteins (Citation64, Citation65). Epithelial responses to Candida may not however, result in a strong host immune response and inflammation. Indeed, certain candidal factors as well as proteins produced by epithelial cells may actually result in anti-inflammatory effects and subsequent immune tolerance (Citation66). The precise features that determine whether epithelial cells induce inflammation or are acquiescent toward C. albicans remain unclear. In the following section, we will summarise the general mechanisms involved in epithelial cell responses to Candida, including cell-surface receptor binding, cell signaling triggering, and the factors produced by the epithelial cells.
TLRs are a family of PRRs whose involvement in host innate immune responses to various pathogens has been well studied. Up to 13 TLRs have been identified in both humans and mice. Expression of TLR1, 2, 4, 5, and 6 has been demonstrated in human mucosal epithelial cells (Citation64–Citation68), and their expression in response to C. albicans infecting both oral and vaginal epithelial cell lines has been shown to be similar (Citation69). The exact composition of the PRRs used by epithelial cells in response to infections with C. albicans is, however, unknown (Citation69). TLR2 and TLR5 are both expressed at high levels by oral cells and are frequently associated with epithelial repair, growth, and survival (Citation70, Citation71).
Candida stimulates human epithelial cells to express (granulocyte-macrophage colony-stimulating factor) GM-CSF, which is a highly potent cytokine that stimulates dendritic cell maturation to mediate mucosal inflammation. Interestingly, TLR4 is not involved in GM-CSF stimulation and it has also been shown that candidal viability is also required in GM-CSF induction (Citation72).
Aside from TLRs, perhaps the most important PRRs for Candida recognition are CLRs. CLRs comprise a family of six cell-surface proteins. Dectin-1 and Dectin-2 belong to this family, and are confirmed receptors for Candida recognition (Citation73–Citation75). Whilst the role of Dectin-1 and 2 in host immunity against Candida infection has been extensively studied in animal models and human macrophages/dendritic cells, only Dectin-1 expression in human oral gingival epithelial cells has been reported, and its expression is at best weak (Citation76). Therefore, the exact role of Dectin-1 expression in oral mucosal immunity remains unclear.
Epithelial cells can also recognise the morphology of the colonising Candida. C. albicans yeast and hyphae both trigger Nuclear Factor Kappa β (NFkβ) activation in epithelial cells, but NFkβ activation alone does not lead to cytokine release. Only C. albicans hyphae appear to be able to also induce mitogen-activated protein kinases (MAPK) phosphorylation, which combined with NFkβ activation results in production of IL-6 and GM-CSF by epithelial cells (Citation34, Citation77) (Citation78). Despite the identification of such downstream inflammatory signaling cascades, the oral epithelial receptor(s) that induce(s) cytokine responses to Candida have yet to be identified. The receptors described above might be involved in differential detection of Candida hyphae, thus representing a possible mechanism by which the host distinguishes between commensal Candida yeast carriage (resulting in immune tolerance) and invasive Candida hyphal infection (resulting in inflammatory immune responses).
Many downstream mechanisms have been identified as influencing immune tolerance and activation following Candida colonisation. Examples include involvement of the resident macrophages in the mucosa that produce anti-inflammatory cytokines to regulate host immune responses (Citation66). Nevertheless, details of the mechanisms of Candida recognition and host tolerance by mucosal epithelial cells still need to be clarified.
Candida also induces in vitro upregulation of various antimicrobial peptides such as β-defensins and LL-37 (Citation79, Citation80), which are known to have candidacidal activity and could play significant roles in combating infections and invasion, as well as initiating other immune responses (Citation81, Citation82).
Summary
It is clear that a delicate balance exists between C. albicans and host epithelial surfaces. The type of response elicited by the epithelial surface to colonising Candida is extremely important given that such surfaces are the first line of defence of the host to infection. The nature of mucosal responses is affected by many variables including host factors such as immune dysfunction, underlying disease, other forms of host debilitation, and the composition of the existing microflora community. In addition, there are factors associated with the strain of C. albicans involved that are also important in determining responses of the epithelium. These include the level of expression of putative virulence factors including cell-surface adhesins, extracellular hydrolytic enzymes, and the type of morphology exhibited by the colonising C. albicans. Given the heterogeneity associated with such factors in both the Candida genus and amongst strains of C. albicans, it could readily be postulated that those strains able to adapt to the conditions at the mucosal surface without inducing host responses represent those most likely to successfully persist as commensals. Strains that rely on virulence factors to persist are those that lead either to pathology or become eradicated through the activity of host defences.
Conflict of interest and funding
There is no conflict of interest in the writing of this article for any of the authors.
Acknowledgements
All clinical images used in this manuscript were generated by the authors from consenting patients attending the School of Dentistry, Cardiff University.
References
- Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002; 78: 455–9.
- Samaranayake L. Commensal oral Candida in Asian cohorts. Int J Oral Sci. 2009; 1: 2–5.
- de Leon EM, Jacober SJ, Sobel JD, Foxman B. Prevalence and risk factors for Candida colonization in women with type 1 and type 2 diabetes. BMC Infect Dis. 2002; 2: 1.
- Leli C, Mencacci A, Meucci M, Bietolini C, Vitali M, Farinelli S, etal. Association of pregnancy and Candida vaginal colonization in women with or without symptoms of vulvovaginitis. Minerva Ginecol. 2013; 65: 303–9.
- Foxman B, Marsh JV, Gillespie B, Sobel JD. Frequency and response to vaginal symptoms among white and African American women: results of a random digit dialing survey. J Womens Health. 1998; 7: 1167–74.
- Ruby J, Barbeau J. The buccale puzzle: the symbiotic nature of endogenous infections of the oral cavity. Can J Infect Dis. 2002; 13: 34–41.
- van Oss CJ. Interfacial forces in aqueous media. 1994; New York: Marcel Dekker.
- Bendel CM, Hostetter MK. Distinct mechanisms of epithelial adhesion for Candida albicans and Candida tropicalis. Identification of the participating ligands and development of inhibitory peptides. J Clin Invest. 1993; 92: 1840–9.
- Eigentler A, Schulz TF, Larcher C, Breitwieser EM, Myones BL, Petzer AL, etal. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect Immun. 1989; 57: 616–22.
- Gilmore BJ, Retsinas EM, Lorenz JS, Hostetter MK. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988; 157: 38–46.
- Hostetter MK. Adhesins and ligands involved in the interaction of Candida albicans. Clin Microbiol Rev. 1994; 7: 29–42.
- Klotz SA, Hein RC, Smith RL, Rouse JB. The fibronectin adhesin of Candida albicans. Infect Immun. 1994; 62: 4679–81.
- Skerl KG, Calderone RA, Segal E, Sreevalsan T, Scheld WM. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984; 30: 221–7.
- Yan S, Nègre E, Cashel JA, Guo N, Lyman CA, Walsh TJ, etal. Specific induction of fibronectin binding activity by hemoglobin in Candida albicans grown in defined media. Infect Immun. 1996; 64: 2930–5.
- Critchley IA, Douglas LJ. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987; 133: 629–36.
- Critchley IA, Douglas LJ. Role of glycosides as epithelial cell receptors for Candida albicans. J Gen Microbiol. 1987; 133: 636–43.
- Douglas LJ, Bennett JE, Hay RJ, Peterson PK. Mannoprotein adhesins of Candida albicans. New strategies in fungal disease. 1992; Edinburgh: Churchill Livingstone. 34–53.
- Vardar-Ünlü G, McSharry C, Douglas L. Fucose-specific adhesins on germ tubes of Candida albicans. FEMS Immunol Med Microbiol. 1998; 20: 55–67.
- Yu L, Lee KK, Sheth HB, Lane-Bell P, Srivastava G, Hindsgaul O, etal. Fimbria mediated adherence of Candida albicans to glycosphingolipid receptors on human buccal epithelial cells. Infect Immun. 1994; 62: 2843–8.
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999; 283: 1535–8.
- Sundstrom P. Adhesion in Candida spp. Cell Microbiol. 2002; 4: 461–9.
- Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, etal. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002; 44: 61–72.
- Kapteyn JC, Hoyer LL, Hecht JE, Müller WH, Andel A, Verkleij AJ, etal. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. 2000; 35: 601–11.
- Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, etal. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004; 150: 2415–28.
- Zhao X, Pujol C, Soll DR, Hoyer LL. Allelic variation in the contiguous loci encoding Candida albicans ALS5, ALS1 and ALS9. Microbiology. 2003; 149: 2947–60.
- Hamada K, Terashima H, Arisawa M, Kitada K. Amino acid sequence requirement for efficient incorporation of glycosylphosphatidylinositol-associated proteins into the cell wall of Saccharomyces cerevisiae. J Biol Chem. 1998; 273: 26946–53.
- Hamada K, Terashima H, Arisawa M, Yabuki N, Kitada K. Amino acid residues in the omega-minus region participate in cellular localization of yeast glycosylphosphatidylinositol-attached proteins. J Bacteriol. 1999; 181: 3886–9.
- Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003; 149: 353–62.
- Zipfel PF, Skerka C, Kupka D, Luo S. Immune escape of the human facultative pathogenic yeast Candida albicans: the many faces of the Candida Pra1 protein. Int J Med Microbiol. 2011; 301: 423–30.
- Meri T, Amdahl H, Lehtinen MJ, Hyvärinen S, McDowell JV, Bhattacharjee A, etal. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 2013; 9: e1003308.
- Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008; 72: 495–544.
- Hoyer LL, Hecht JE. The ALS5 gene of Candida albicans and analysis of the Als5p N-terminal domain. Yeast. 2001; 18: 49–60.
- Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, etal. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS One. 2012; 7: e33362.
- Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, etal. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010; 8: 225–35.
- Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002; 185: 521–30.
- Yokota K, Takashima A, Bergstresser PR, Ariizumi K. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene. 2001; 272: 51–60.
- Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, etal. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005; 1: e42.
- Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, etal. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007; 196: 1565–71.
- Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003; 197: 1119–24.
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, etal. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009; 361: 1760–7.
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, etal. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007; 8: 630–8.
- Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009; 4: e7967.
- Nobile C, Nett JE, Andes DR, Mitchell AP. Function of Candida albicans adhesion Hwp1 in biofilm formation. Eukaryot Cell. 2006; 5: 1604–10.
- Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005; 15: 1150–5.
- Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, etal. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS One. 2011; 6: e16218.
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003; 67: 400–28.
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001; 9: 327–35.
- Naglik J, Albrecht A, Bader O, Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004; 6: 915–26.
- Schaller M, Januschke E, Schackert C, Woerle B, Korting HC. Different isoforms of secreted aspartyl proteinases (Sap) are expressed by Candida albicans during oral and cutaneous candidosis in vivo. J Med Microbiol. 2001; 50: 743–7.
- Malic S, Hill KE, Ralphs JR, Hayes A, Thomas DW, Potts AJ, etal. Characterization of Candida albicans infection of an in vitro oral epithelial model using confocal laser scanning microscopy. Oral Microbiol Immunol. 2007; 22: 188–94.
- Naglik JR, Newport G, White TC, Fernandes-Naglik LL, Greenspan JS, Greenspan D, etal. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun. 1999; 67: 2482–90.
- Staib P, Kretschmar M, Nichterlein T, Hof H, Morschhäuser J. Differential activation of a Candida albicans virulence gene family during infection. Proc Natl Acad Sci USA. 2000; 97: 6102–7.
- Wu H, Downs D, Ghosh K, Ghosh AK, Staib P, Monod M, etal. Candida albicans secreted aspartic proteases 4–6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism. FASEB J. 2013; 27: 2132–44.
- Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, etal. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol. 2013; 43: 679–92.
- Schaller M, Bein M, Korting HC, Baur S, Hamm G, Monod M, etal. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect Immun. 2003; 71: 3227–34.
- Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007; 75: 2126–35.
- Gácser A, Trofa D, Schäfer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007; 117: 3049–58.
- Stehr F, Felk A, Gácser A, Kretschmar M, Mähnss B, Neuber K, etal. Expression analysis of the Candida albicans lipase gene family during experimental infections and in patient samples. FEMS Yeast Res. 2004; 4: 401–8.
- Hube B, Stehr F, Bossenz M, Mazur A, Kretschmar M, Schäfer W. Secreted lipases of Candida albicans: cloning, characterisation and expression analysis of a new gene family with at least ten members. Arch Microbiol. 2000; 174: 362–74.
- Samaranayake YH, Dassanayake RS, Cheung BP, Jayatilake JA, Yeung KW, Yau JY, etal. Differential phospholipase gene expression by Candida albicans in artificial media and cultured human oral epithelium. APMIS. 2006; 114: 857–66.
- Nailis H, Kucharíková S, Ricicová M, Van Dijck P, Deforce D, Nelis H, etal. Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and -independent gene expression. BMC Microbiol. 2010; 10: 114.
- Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, etal. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007; 5: e64.
- Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, etal. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010; 6: e1001181.
- Saegusa S, Totsuka M, Kaminogawa S, Hosoi T. Candida albicans and Saccharomyces cerevisiae induce interleukin-8 production from intestinal epithelial-like Caco-2 cells in the presence of butyric acid. FEMS Immunol Med Microbiol. 2004; 41: 227–35.
- Bahri R, Saidane-Mosbahi D, Rouabhia M. Candida famata modulates toll-like receptor, beta-defensin, and proinflammatory cytokine expression by normal human epithelial cells. J Cell Physiol. 2010; 222: 209–18.
- Zheng XF, Hong YX, Feng GJ, Zhang GF, Rogers H, Lewis MA, etal. Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PLoS One. 2013; 8: e63967.
- Filler SG. Candida-host cell receptor–ligand interactions. Curr Opin Microbiol. 2006; 9: 333–9.
- Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, etal. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005; 7: 1117–27.
- Naglik JR, Moyes D. Epithelial cell innate response to Candida albicans. Adv Dent Res. 2011; 23: 50–5.
- Rhee SH, Im E, Riegler M, Kokkotou E, O'Brien M, Pothoulakis C. Pathophysiological role of toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005; 102: 13610–5.
- Shaykhiev R, Behr J, Bals R. Microbial patterns signaling via toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS One. 2008; 3: e1393.
- Li L, Dongari-Bagtzoglou A. Epithelial GM-CSF induction by Candida glabrata. J Dent Res. 2009; 88: 746–51.
- Goodridge H, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007; 178: 3107–15.
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, etal. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009; 206: 2037–51.
- Rogers H, Williams DW, Feng GJ, Lewis MA, Wei XQ. Role of bacterial lipopolysaccharide in enhancing host immune response to Candida albicans. Clin Dev Immunol. 2013; 2013: 320168.
- Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dent Res. 2010; 89: 666–75.
- Moyes DL, Murciano C, Runglall M, Kohli A, Islam A, Naglik JR. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol. 2012; 201: 93–101.
- Netea MG, Kullberg BJ. Epithelial sensing of fungal invasion. Cell Host Microbe. 2010; 8: 219–20.
- Li M, Chen Q, Tang R, Shen Y, Liu WD. The expression of beta-defensin-2, 3 and LL-37 induced by Candida albicans phospholipomannan in human keratinocytes. J Dermatol Sci. 2011; 61: 72–5.
- Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC. Human defensins. J Mol Med. 2005; 83: 587–95.
- Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005; 125: 108–15.
- Vylkova S, Nayyar N, Li W, Edgerton M. Human beta defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother. 2007; 51: 154–61.