Abstract
The objective of the present review is to discuss if the yeast vacuole can be used as a target for attenuation of Candida albicans virulence. Literature searches were made electronically using predetermined inclusion criteria. The main searches were made through a systematic strategy in PubMed and authoritative journals in microbiology. It appeared that C. albicans virulence may be reduced by inhibiting vacuolar proton-translocating ATPase (V-ATPase) functions and acidification of the yeast vacuole by V-ATPase inhibitors, by seeking the synergistic effect of antifungals and non-antifungals affecting yeast vacuolar functions, and by inhibiting filament production – also regulated by the vacuole. Accordingly, we may impair C. albicans virulence by inhibiting functions of its vacuole, which plays essential roles during colonization and invasion of the host. Except for drugs where indications for clinical use can be redefined, such interventions may be closer to theory than to reality at the moment. But since the yeast is so difficult to eradicate by antifungal treatment, it could be rewarding to seek new strategies for reducing its virulence rather than trying to eradicate it completely from the microbiota, which often turns out to be impossible.
Candida albicans is recognized as an opportunistic yeast that becomes pathogenic when the host defense deteriorates. Many of us have this yeast in the oral cavity, gut, and genitalia as a commensal. As people are living longer through improvements in standards of life, the risk of acquiring infection by Candida increases due to predisposing factors associated with aged individuals. We might be able to compete with its pathogenicity if we could make the yeast less virulent. Since Candida infections are often considered diseases of the diseased, it may not be so realistic to remove the yeast entirely from the microbiota. Actually, antifungal treatment is very often followed by a relapse unless local and general predisposing factors to infection have been eliminated. Improvement of our host defense is another alternative but not so easy to achieve. It should also be realized that there are limited possibilities for treatment of Candida infections. Available drugs are few and resistance toward antimycotics is increasing. Antifungal therapy is often hampered by toxicity, moderate response rates, and substantial interpatient variation in serum drug levels. This is worrisome because systemic Candida infections, which are increasing, are often associated with high morbidity and mortality. Actually, Candida species are now one of the four most common causes of bloodstream and cardiovascular infections in US hospitals (Citation1, Citation2), and bloodstream infections caused by Candida have a mortality rate as high as 50% (Citation1, Citation3). So the question remains, can we impair the virulence of C. albicans, and if that is the case, how can we do it? C. albicans has a number of virulence factors (Citation4) . The present review will focus on its vacuolar functions as possible targets for attenuation of its virulence.
The yeast vacuole and its ATPase
The vacuole is an organelle in yeasts and plants that is analogous to the mammalian lysosome (). It plays an important role in a number of cellular functions such as in response to stress, adaptation to new environments, and cell differentiation (Citation5). For the vacuole to function optimally, maintenance of an intravacuolar acidic pH is required. This is maintained by the vacuolar proton-translocating ATPase (V-ATPase). This enzyme hydrolyzes ATP for transport of protons from the cytosol into organelles. Important vacuolar functions such as calcium and metal homeostasis (Citation6), cargo sorting and membrane trafficking in endocytic and secretory pathways (Citation7), and drug resistance (Citation8) require V-ATPase-mediated acidification and membrane energization.
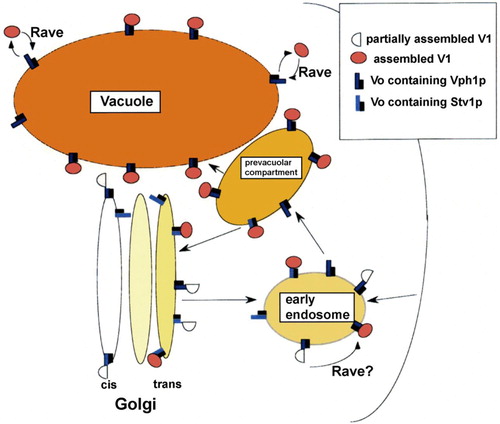
Fig 1. Assembly and trafficking of the yeast V-ATPase. Possible steps in assembly and transport of Vph1p- and Stv1p-containing V-ATPases are shown. Shading of different organelles indicates extent of acidification; the vacuole is most intensely colored as the most acidic compartment in the yeast cell. The first compartment in the secretory pathway showing evidence of acidification is the Golgi apparatus. Vph1p-containing V-ATPases are known to travel to the vacuole via the prevacuolar compartment and are believed to reach this compartment via the early endosome, which is also likely to be somewhat acidic. Stv1p-containing V-ATPases appear to cycle between the prevacuolar compartment and the vacuole, and may also travel through the early endosome. The RAVE complex assists in reassembly of the V1 and V0 complexes at the vacuole, and possibly in assembly at the early endosome. Modified fromCitation9.
There are two subcomplexes in the V-ATPase enzyme: the water soluble V1 and the membrane-embedded V0 (). While the V1 subcomplex forms the site of ATP binding and hydrolysis, the integral membrane V0 subcomplex arranges for transport of protons from the cytosol to the vacuole lumen. There are 14 different subunits in the yeast ATPase with relative molecular masses from 9,000 to 100,000. Eight different subunits A, B, (C), D, E, F, G, and H make up the V1 domain while the V0 domain contains the six remaining subunits called a, c, c ’ , c n , d, and e (Citation10) . The V0a subunit is the only fungal V-ATPase subunit that is encoded by two isoforms, that is, VPH1 and STV1. The driving of protons into the lumen causes an acidic intraluminal pH~6.25. This pH is necessary for optimal function of different degrading enzymes and a membrane potential necessary for secondary transport systems. Interference with the V-ATPase function interrupts a variety of important cellular processes and stress responses important for fungal pathogenicity (Citation5).
V-ATPase activity and ergosterol
Ergosterol is an important constituent of fungal membranes. There seems to be a critical requirement for ergosterol in V-ATPase function, and this requirement may actually underlie the antifungal effect of azoles (Citation11). Thus, Saccharomyces cerevisiae mutants that do not produce ergosterol have alkaline vacuoles and produce the so-called vma phenotype (see later) (Citation5, Citation11). The mutant phenotypes are due to reduced ATP hydrolysis and proton pumping inside the ATPase and not to improper assembly of the ATPase at the vacuolar membrane because of lack of ergosterol. In C. albicans, fluconazole-impaired vacuolar acidification resulted in alkaline vacuoles, and noteworthy, ergosterol feeding restored ATPase function and growth of yeast cells (Citation11). Accordingly, a new regulatory pathway in ATPase function and also a new mechanism of azole toxicity have been identified in C. albicans (Citation11) . This supplements the previous view that azoles cause fungal toxicity through damage of the cell membrane followed by increased cell permeability and lysis (Citation12).
Loss of V-ATPase function
Experiments with S. cerevisiae have demonstrated that loss of ATPase function causes a number of growth defects referred to as the vma phenotype (Citation5, Citation11). This phenotype does not grow on media that are alkaline (pH 7.5–8.5) and contains high concentrations of calcium or non-fermentable carbon sources as the sole carbon source (Citation9). However, growth on acid media (pH 4.0–5.0) is similar to that of the wild type and similar findings have been obtained with C. albicans. By creating a conditional mutant controlled by a tetracycline-regulated promoter (tetR-VMA3 strain), repression of VMA3 in the tet-VMA3 strain prevented V-ATPase assembly at the vacuolar membrane. It also reduced concanamycin A (ATPase inhibitor)-sensitive ATPase-specific activity and proton transport by >90% (Citation5). Later, several other examples will be given on how interference with ATPase function can have serious consequences for the virulence of C. albicans.
Interference with extracellular pH
C. albicans has a broad tolerance spectrum for pH since it survives in ecological niches from highly acidic pH (<2) to alkaline pH (>10) (Citation13, Citation14). The extracellular pH affects dimorphism of C. albicans with the yeast form promoted under acidic conditions and the filament form (pathogenic) under alkaline conditions. This means that increased extracellular pH can increase the virulence through filament production by the yeast (Citation15). C. albicans can actively change the pH of the environment and induce hyphae formation. Mutants showing reduced ability to sense extracellular pH have reduced virulence (Citation16).
Interference with vacuolar pH
The yeast vacuole is a major target for antifungal drugs preventing it from becoming acidic. Thus, fluconazole is able to block the yeast-to-hyphal transition (Citation17) and inhibits V-ATPase by emptying yeast membranes of ergosterol. This causes alkalization of the vacuole (Citation10, Citation18). Also, amphotericin B, binding to ergosterol, can decrease V-ATPase activity (Citation11). As aforementioned, the azole class of antifungals functions partly by disruption of vacuolar acidification. Loss of C. albicans V-ATPase activity causing alkalization of the vacuolar lumen has several pleiotropic effects affecting pH-dependent growth, and calcium and cold sensitivity (Citation5). Thus, vacuolar acidification is clearly linked to the pathogenicity of the yeast. Another new and interesting target for antifungal drugs is the putative vacuolar transport protein Vac1p that is needed for several cellular functions, particularly those controlling virulence and pathogenicity of C. albicans (Citation19).
It was shown by Poltermann et al. (Citation20) that the vacuolar ATPase subunit Vma7p is involved in vacuole acidification and is needed for hyphal growth and virulence of C. albicans. Null mutants of C. albicans, vma7, had defective proton transport into the vacuole indicated by an alkaline pH. The alkaline vesicular lumen may disturb degradation of intravacuolar structures by its hydrolases and lipases. No hyphal growth was seen by the vma7 null mutant in liquid medium. Vma7p interacts physically with the PI 3-kinase Vps34p, which is a key enzyme in vacuolar protein transport. Poltermann et al. (Citation20) also showed that their respective null mutants, vma7 and vps34, had the same defects in vacuolar acidification and detoxification of metal ions, linking vacuolar proton transport and vacuole acidification with vacuolar protein transport.
The story of the yeast vacuole is additionally intriguing by the fact that fungal V-ATPase, unlike mammalian orthologs, has little isoform diversity in its structural subunits. An exception is the Vo subunit α, which is encoded by the genes VPH1 and STV1 in C. albicans (and S. cerevisiae). These genes, through their functional properties and organelle distribution of V-ATPase complexes, harboring either Vph1p or Stv1p, enabled Patenaude et al. (Citation21) to determine the relative contribution of vacuolar and secretory pathway acidification to pathogenicity and related traits such as the change in morphology from blastoconidiae to hyphae. They showed that the vacuole-specific subunit α isoform, Vph1p, was needed for vacuolar acidification, and that this could not be performed adequately by the Stv1p isoform. This supported previous observations by Tarsio et al. (Citation22) who found that loss of STV1 had little effect on vacuolar pH, while loss of VPH1 caused vacuolar alkalization. The observations were consistent with previous findings in the non-pathogenic yeast S. cerevisiae (Citation21). When injected into a murine model of systemic candidosis, the vph1-null strain did not kill any of the animals during the observation period whereas a reintegrant of VPH1 in the null strain maintained its virulence (Citation21). In other words, the yeast that was unable to achieve vacuolar acidity did not kill the mice.
Raines et al. (Citation23) found that the Vph1p isoform of V-ATPase controlled V-ATPase activity and acidification of the vacuole in C. albicans. However, vacuolar acidification was not essential for filamentation in vitro. They suggested for the first time a role also for Stv1p-containing V-ATPase complexes in C. albicans virulence, particularly if Vph1 is not functional. As a determinant for C. albicans filamentation, they proposed that vacuolar pH alone may be less important than previously thought, at least in vitro, and that both Vph1p- and Stv1p-containing V-ATPase must be inhibited to prevent C. albicans infection. The disparate results of Patenaude et al. (Citation21) and Raines et al. (Citation23) may be related to differences in test strains and methodology. However, they both suggested that ATPase activity could be used to control C. albicans virulence.
Drug synergy in antifungal treatment
It has been mentioned that there is a critical requirement for ergosterol in V-ATPase function, and that this requirement may actually underlie the antifungal effect of azoles (Citation11). Interestingly, combinations of azole drugs with non-antifungals can trigger harmful ion fluxes that can have synergistic antifungal effects. Amiodarone is a drug used therapeutically and prophylactically against serious heart arrhythmia. It causes a surge of cytosolic H+ and Ca2 +, and elicits stress, cell-cycle arrest and fungal death (Citation18, Citation24) (Citation25). Combining amiodarone with fluconazole significantly dampened the transcriptional response to either drug which indicated that the synergism was caused by inhibition of compensatory response pathways (Citation11, Citation26) (Citation27). Dampening was associated with a decrease in total ergosterol levels and reduced the formation of pseudohyphae. In a mouse model of systemic candidosis, the combination of amiodarone with azole was effective in the treatment of the fungal infection (Citation11). Synergy has also been demonstrated between amphotericin B and allicin, the main active component in freshly crushed garlic extract, enhancing the effects of vacuole-targeting fungicidal activity of amphotericin B (Citation28). Interestingly, there is also a considerable potential for azoles used together with amiodarone against resistant C. albicans (Citation27). These findings point to the possibility of allotting new indications to already existing drugs, which may be far less expensive than designing entirely new antifungal drugs. Also, targeting components of the V-ATPase pathway via a downstream element such as Pma1p (fungal-specific protein with no known mammalian homologs) can circumvent the development of drug resistance against current antifungal therapies (Citation29). Anti-Pma1p drugs may also act against yeasts without required drug accumulation within the fungal cell since Pma1p, being a plasma membrane protein, has access to the extracellular space (Citation29).
Anti-V-ATPase drugs
Anti-V-ATPase drugs may be effective in the treatment of candidosis (Citation29). By 2009, eight types of V-ATPase inhibitors had been described (Citation30). Among them were naturally occurring compounds that inhibit V-ATPase such as specific and potent inhibitors produced by Streptomyces species: bafilomycin A1 and concanamycin A (Citation30, Citation31). Unfortunately, these compounds are unable to discriminate between fungal and mammalian V-ATPase (Citation32, Citation33). In a screening of two compound libraries, the ENZO and the FIMM oncology collection library for anti-Candida activity, Stylianou et al. (Citation34) found seven off-target drugs previously reported to be active against Candida species and seven new agents (amonafide, tosedostat, megestrol acetate, melengestrol acetate, stanozolol, trifluperidol, and haloperidol) with anti-Candida activity. The aminopeptidase inhibitor tosedostat, which is being clinically trialled for anti-cancer therapy, had a broad antifungal activity against different Candida species, including C. glabrata.
Chan et al. (Citation35) used the pHluorin screening method on the Prestwick Chemical Library containing 1,120 off-patent drugs and identified alexidine dihydrochloride and thonzonium bromide as new and specific ATPase inhibitors. They also confirmed disulfiram as an inhibitor of V-ATPase in C. albicans. Noteworthy, most drugs in the Prestwick Chemical Library had known safety and toxicity profiles. In addition, alexidine dihydrochloride and thonzonium bromide showed general toxicity to C. albicans cells. Therefore, anti-V-ATPase drugs may be used clinically. Most importantly, they may evade the problem of multiresistance experienced with azoles.
Interference with filament production
Interference with the V-ATPase function can also disable the capacity of the yeast to produce filaments. Many authors consider filaments as the pathogenic form of the organism. This was demonstrated with the vma7 -1- strain of C. albicans in serum or liquid Spider medium with mannitol (Citation20). Normally, hyphal growth is induced under these growth conditions. This was not the case with the vma7 null mutant, which was also avirulent in a mouse model of systemic candidosis (Citation20). Treatment with fluconazole and ERG3 deletion also inhibited filamentation in C. albicans (Citation17, Citation36). When a wild-type ERG3 allele was reintroduced into a homozygous deletion mutant of C. albicans, virulence, ergosterol synthesis, and susceptibility to azoles were restored. It was concluded that the loss of virulence was most likely due to the erg3 mutant's failing ability to form hyphae. Recently, Rane et al. (Citation5) found that repression of VMA3, which is a gene that encodes the c subunit of the V0 subcomplex, suppressed filamentation in C. albicans. Specific chemical inhibition of Vma3p function also reduced filament production. There were also fission defects causing reduced protease and lipase secretion and ineffective macrophage killing (Citation5). Furthermore, damage to the host and lethality were shown to be associated with active invasive filamentous growth in mice injected with C. albicans into the tail vein (Citation37). Therefore, inhibition of filamentation was suggested as a method for treatment of disseminated candidosis.
Host cell toxicity and drug resistance
As mentioned, there are naturally occurring compounds that inhibit V-ATPase such as specific and potent inhibitors produced by Streptomyces species: bafilomycin A1 and concanamycin A (Citation31, Citation32). Unfortunately, these compounds are unable to discriminate between fungal and mammalian V-ATPase (Citation30, Citation33).
More promising is the fungus-specific V-ATPase subunit c′, which is encoded by VMA11 and has no mammalian homolog (Citation5). The combination of antifungals with non-antifungal drugs is also promising in terms of toxicity. By achieving synergy, the doses of each drug may be reduced so that they can be tolerated by the host. As previously mentioned, drug synergy is a principle that also can be useful against antifungal drug resistance (Citation27).
A prerequisite for all the approaches to reduce C. albicans virulence in man is that they have been tested for host cell toxicity and have been shown to be without major side effects. Until this has been done, they cannot be used for reducing morbidity and mortality even in seriously ill patients. Fortunately, targeting pathogen-specific factors may allow a very specific action on Candida and enable reduced incidence of resistance (Citation38). Interestingly, low levels of azoles did not affect C. albicans growth in vitro but inhibited extensive hyphal elongation (Citation39) suggesting another therapeutic option that could be well tolerated by patients.
Conclusions
In conclusion, we may impair C. albicans virulence through affecting functions of its vacuole. The vacuole has an essential role in supporting yeast pathogenicity by performing a variety of important functions during colonization and invasion of the host. Impairment can theoretically be done by inhibiting V-ATPase functions and acidification of the vacuole, by seeking the synergistic effect of antifungals together with non-antifungals, by applying V-ATPase inhibitors, and by inhibiting filament production, also guided by the vacuole.
Except for drugs where indications for clinical use can be redefined, some of these approaches may be closer to theory than to reality. However, since the yeast is so difficult to eradicate by antifungal treatment, it may be more rewarding to seek new strategies for reducing its virulence than trying to wipe it out of the entire microbial population. This often turns out to be impossible.
Conflict of interest and funding
The author has no conflict of interest and has single-handedly written the review.
Acknowledgements
The author wants to acknowledge funding through a grant from the European Commission (FP7-HEALTH-306029 ‘TRIGGER’).
References
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, etal. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003; 37: 1172–7.
- Calderone RA. Candida and candidiasis. 2002; Washington, DC: ASM Press. Volume 8, Number 8.
- Eggimann P, Garbino J, Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect Dis. 2003; 3: 772–85.
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013; 4: 119–28.
- Rane HS, Bernardo SM, Raines SM, Binder JL, Parra KJ, Lee SA. Candida albicans VMA3 is necessary for V-ATPase assembly and function and contributes to secretion and filamentation. Eukaryot Cell. 2013; 12: 1369–82.
- Forster C, Kane PM. Cytosolic Ca2 + homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J Biol Chem. 2000; 275: 38245–53.
- Huang C, Chang A. pH-dependent cargo sorting from the Golgi. J Biol Chem. 2011; 286: 10058–65.
- Zhang Y, Rao R. The V-ATPase as a target for antifungal drugs. Curr Protein Pept Sci. 2012; 13: 134–40.
- Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar ATPase. Microbiol Mol Biol Rev. 2006; 70: 28872–81.
- Zhang Z, Zheng Y, Mazon H, Milgrom E, Kitagawa N, Kish-Trier E, etal. Structure of the yeast vacuolar ATPase. J Biol Chem. 2008; 283: 35983–95.
- Zhang Y-Q, Gamarra S, Garcia-Effron G, Park S, Perlin DG, Rao R. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLOS Pathog. 2010; 6: e1000939.
- Chapman SW, Sullivan DC, Cleary JD. In search of the holy grail of antifungal therapy. Trans Am Clin Climatol Assoc. 2008; 119: 197–216. [PubMed Abstract] [PubMed CentralFull Text].
- De Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998; 66: 3317–25. [PubMed Abstract] [PubMed CentralFull Text].
- Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011; 2: e00055–11.
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011; 9: 737–48.
- Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol. 2009; 12: 365–70.
- Ha KC, White TC. Effects of azole antifungal drugs on the transition from yeast cells to hyphae in susceptible and resistant isolates of the pathogenic yeast Candida albicans. Antimicrob Agents Chemother. 1999; 43: 763–8. [PubMed Abstract] [PubMed CentralFull Text].
- Zhang Y-Q, Rao R. Beyond ergosterol: linking pH to antifungal mechanisms. Virulence. 2010; 1: 551–4.
- Franke K, Nguyen M, Härtl A, Dahse HM, Vogl G, Würzner R, etal. The vesicle transport protein Vac1p is required for virulence of Candida albicans. Microbiology. 2006; 152: 3111–21.
- Poltermann S, Nguyen M, Günther J, Wendland J, Härtl A, Künkel W, etal. The putative vacuolar ATPase subunit Vma7p of Candida albicans is involved in vacuole acidification, hyphal development and virulence. Microbiology. 2005; 151: 1645–55.
- Patenaude C, Zhang Y, Cormack B, Köhler J, Rao R. Essential role for vacuolar acidification in Candida albicans virulence. J Biol Chem. 2013; 288: 26256–64.
- Tarsio M, Zheng H, Smardon AM, Martinez-Muñoz GA, Kane PM. Consequences of loss of Vph1 protein-containing vacuolar ATPases (V-ATPases) for overall cellular pH homeostasis. J Biol Chem. 2011; 286: 28089–96.
- Raines SM, Rane HS, Bernardo SM, Binder JL, Lee SA, Parra KJ. Deletion of vacuolar proton-translocating ATPase Voa isoforms clarifies the role of vacuolar pH as a determinant of virulence-associated traits in Candida albicans. J Biol Chem. 2013; 288: 6190–201.
- Maresova L, Muend S, Zhang YQ, Sychrova H, Rao R. Membrane hyperpolarization drives cation influx and fungicidal activity of amiodarone. J Biol Chem. 2009; 284: 2795–802.
- Muend S, Rao R. Fungicidal activity of amiodarone is tightly coupled to calcium influx. FEMS Yeast Res. 2008; 8: 425–31.
- Gamarra S, Rocha EMF, Zhang Y-Q, Park S, Rao R, Perlin DS. Mechanism of the synergistic effect of amiodarone and fluconazole on Candida albicans. Antimicrob Agents Chemother. 2010; 254: 1753–61.
- Guo Q, Sun S, Li Y, Shi C. In vitro interactions between azoles and amiodarone against clinical Candida albicans. Int J Antimicrob Agents. 2008; 31: 88–90.
- Ogita A, Fujita K-I, Tanaka T. Enhancing effects on vacuole-targeting fungicidal activity of amphotericin B. Front Microbiol. 2012; 3: 100.
- Hayek SR, Lee SA, Parra KJ. Advances in targeting the vacuolar protein-translocating ATPase (V-ATPase) for anti-fungal therapy. Front Pharmacol. 2014; 5: 1–8.
- Bowman EJ, Graham LA, Stevens TH, Bowman BJ. The bafilomycin/concanamycin binding site in subunit c of the V-ATPase from Neurospora crassa and Saccharomyces cerevisiae. J Biol Chem. 2004; 279: 33131–8.
- Bowman EJ, Siehers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPase from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988; 85: 7972–6.
- Bowman EJ, Bowman BJ. V-ATPase as drug targets. J Bioenerg Biomembr. 2005; 37: 431–5.
- Huss M, Wieczorek H. Inhibitors of V-ATPases: old and new players. J Exp Biol. 2009; 212: 341–6.
- Stylianou M, Kulesskiy E, Lopes JP, Granlund M, Wennerberg K, Urban CF. Antifungal application of non-antifungal drugs. Antimicrob Agents Chemother. 2014; 58: 1055–62.
- Chan C-Y, Prudom C, Raines SM, Charkhzarrin S, Melman SD, De Haro LP, etal. Inhibitors of V-ATPase proton transport reveal uncoupling functions of tether linking cytosolic and membrane domains of V0 subunit a (Vph1p). J Biol Chem. 2012; 287: 10236–50.
- Chau AS, Gurnani M, Hawkinson R, Laverdiere M, Cacciapouti A, McNicholas PM. Inactivation of sterol Δ5, 6-desaturase attenuates virulence in Candida albicans. Antimicrob Agents Chemother. 2005; 49: 3646–51.
- Saville SP, Lazzell AL, Bryant AP, Fretzen A, Monreal A, Solberg EO, etal. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob Agents Chemother. 2006; 50: 3312–16.
- Jacobsen ID, Wilson D, Wächtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic agent. Expert Rev Anti Infect Ther. 2012; 10: 85–93.
- Wächtler B, Wilson D, Hube B. Candida albicans adhesion to and invasion and damage of vaginal epithelial cells: stage-specific inhibition by clotrimazole and bifonazole. Antimicrob Agents Chemother. 2011; 55: 4436–9.
