Abstract
Gingipains are the major virulence factors of Porphyromonas gingivalis, the main periodontopathogen. It is expected that inhibition of gingipain activity in vivo could prevent or slow down the progression of adult periodontitis. To date, several classes of gingipain inhibitors have been recognized. These include gingipain N-terminal prodomains, synthetic compounds, inhibitors from natural sources, antibiotics, antiseptics, antibodies, and bacteria. Several synthetic compounds are potent gingipain inhibitors but inhibit a broad spectrum of host proteases and have undesirable side effects. Synthetic compounds with high specificity for gingipains have unknown toxicity effects, making natural inhibitors more promising as therapeutic gingipain blockers. Cranberry and rice extracts interfere with gingipain activity and prevent the growth and biofilm formation of periodontopathogens. Although the ideal gingipain inhibitor has yet to be discovered, gingipain inhibition represents a novel approach to treat and prevent periodontitis. Gingipain inhibitors may also help treat systemic disorders that are associated with periodontitis, including cardiovascular disease, rheumatoid arthritis, aspiration pneumonia, pre-term birth, and low birth weight.
Periodontitis is a set of inflammatory diseases affecting the periodontium, that is, the tissues surrounding and supporting the teeth. It causes loss of the alveolar bone around the teeth, and if left untreated, can cause loosening and subsequent loss of teeth. Microorganisms that adhere to and grow on the tooth's surfaces (dental plaque), together with an over-aggressive immune response against them, are generally believed to be the causes of periodontitis. There are seven major categories of periodontitis (Citation1), of which chronic periodontitis and aggressive periodontitis are of particular importance for human health. Periodontitis is a mixed infection where particularly the red complex of bacteria, consisting of Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, is clinically important (Citation2).
Gingipains are the primary virulence factors of P. gingivalis. The direct and indirect activities of gingipains are important in every stage of infection, including attachment and colonization, acquisition of nutrients, evasion of host defense, and tissue invasion and dissemination (Citation3). Gingipains are bacterial house-keeping enzymes; they play a key role in the pathogenic functions of P. gingivalis, such as fimbriae assembly and processing of outer membrane proteins. Gingipains also digest a broad spectrum of host proteins. Some of these are completely degraded, which furnishes peptides for P. gingivalis growth and metabolism, while others are subjected to a limited or selective proteolysis, which leads to the dysregulation of host defensive inflammatory reactions and failure to eliminate P. gingivalis.
The gingipain family comprises three related cysteine proteases that hydrolyze peptide bonds at the carbonyl groups of arginine (Arg-Xaa) and lysine residues (Lys-Xaa). The homologous arginine-specific gingipains, RgpA and RgpB, are products of two related genes rgpA and rgpB, whereas the Lys-specific gingipain, Lys-gingipain (Kgp), is encoded by the kgp gene (Citation4). RgpB is exported into the periplasm as a proprotein composed of an N-terminal prodomain (NPD), a protease domain, and a C-terminal domain (CTD). By contrast to RgpB, RgpA and Kgp have a large hemagglutinin/adhesion domain (HA domain) inserted between the protease and CTD domains. In the periplasm, or during translocation across the outer membrane, progingipains undergo extensive proteolytic processing. NPD and CTD are cleaved off while the HA domain in RgpA and Kgp is fragmented into subdomains. These subdomains are bound to the protease domain via non-covalent interactions (Citation5). Mature gingipains are either secreted in the soluble form, or additionally glycosylated with anionic LPS, which allows them to remain associated with the outer membrane. On the bacterial membrane surface RgpA and Kgp form large multidomain, multifunctional complexes that engage in proteolysis, hem acquisition, platelet activation, red blood cell agglutination, hemolysis, and adhesion to the extracellular matrix. This multi-functionality of gingipains accounts for the severely decreased virulence of gingipain knock-out strains in animal models of bacterial infection, including periodontitis, and immunization with gingipains provides protection from P. gingivalis inoculation-induced pathological changes in vivo. These studies indicate that gingipains are promising targets for the development of inhibitors that could be used for the treatment of periodontitis.
To develop successful therapeutic gingipain inhibitors, the gingipain chiefly responsible for the virulence of P. gingivalis must be clearly identified. Reynolds et al. initially implicated Kgp, and then RgpB, as the primary virulence factor of P. gingivalis in a murine model of alveolar bone loss (Citation6). However, recent findings have assigned this role to RgpA (Citation7). Regardless of this discrepancy, it is clear that the gingipains are indispensable for P. gingivalis virulence and optimally both Kgp and Rgp activity should be targeted for the treatment and/or prevention of periodontitis. An ideal therapeutic compound should also block the proteolytic activity-independent functions of RgpA and Kgp, which have also been implicated in P. gingivalis pathogenicity, although blocking all of the virulence-supporting functions is a challenging and difficult task. Recent developments in the understanding of the mechanism of gingipain processing and secretion have identified these processes as therapeutic targets. Targeting processing and secretion would remove all of the virulence-associated activities of gingipains. Yongqing at al. recently reviewed potential strategies for the inhibition of P. gingivalis Kgp (Citation8) and Grenier and La (Citation9) published a review on proteases in P. gingivalis as potential targets for plant-derived compounds. The aim of this current review is to provide an up-to-date account of research into the different approaches that have been used to inhibit gingipain activity ().
Possible biological effects of administration of gingipain inhibitors
The possible effects of administration of gingipains inhibitors need be studied in vitro and in vivo. A likely effect of inhibitors administration in vivo may result in a reduction in the level of colonization by P. gingivalis. The proteolytic activity of this bacterium is not only disease-related but is there to provide nutrients. Therefore, suppression of proteolytic activity may also reduce concentrations of peptide substrates and micro-nutrients for P. gingivalis. This can be shown in vitro by analysis of gingipain mutants. Furthermore, the loss of proteolytic activity is likely to render the bacterium more susceptible to the normal bacterial clearance operating in the periodontal tissues. Finally, it is possible that inhibition of the hemagglutinin domains may influence the ability of P. gingivalis to adhere to and colonize the tooth surface/periodontal pocket.
Gingipain inhibition via targeting of the NPD
The most common way to spatially and/or temporally control protease activity in vivo is through synthesis of proteases in zymogenic forms. Zymogenicity is often exerted by an NPD. This strategy is employed by P. gingivalis to maintain gingipains enzymatically inert until they are secreted outside the cell. The NPDs of gingipains are composed of about 200 amino acid residues folded in a well-structured domain. NPDs from Rgps expressed in Escherichia coli efficiently inhibit the mature enzyme in trans with a K i in the low nanomolar range (Citation10, Citation11). In the inhibitory complex, the NPD is attached laterally to the catalytic domain through a large concave surface (). Inhibition is maintained by a surface ‘inhibitory loop’, which approaches the active-site cleft of the enzyme on its non-primed side in a substrate-like manner, resulting in the insertion of Arg-126 into the S1 pocket (Citation12). The NPD inhibitory loop matches the enzyme-substrate specificity (Citation13). Downstream of Arg-126, the polypeptide chain of the NPD leaves the cleft, thus avoiding proteolysis. The proteolytic activity of RgpB is also compromised by a strong hydrogen bond between the carbonyl group of Arg-126 in the NPD and the co-catalytic histidine. The hydrogen bond pulls the catalytic histidine residue away from the catalytic cysteine, Cys-473 (Citation12). This structural knowledge should facilitate the development of novel RgpB inhibitors and zymogenic inhibitors for other peptidases.
Fig. 1. The structure of the zymogenic complex of the N-terminal prodomain (NPD) with mature RgpB. (a) Ribbon-type plot in wall-eyed stereo image of the complex between the RgpB PD (in blue/magenta) and the mature RgpB moiety in front view. The latter consists of domains CD (in yellow/orange) and IgSF (in green/brown). The three calcium ions and the barium ions are depicted as red and magenta spheres, respectively. The inhibitory loop and the respective N and C termini are labeled, in turquoise for PD and in brown for CD +IgSF. Arg126 from the PD inhibitory loop and the active-site residues of CD (Cys473, His440, and Glu381) are further shown as sticks for reference of the active site. (b) Orthogonal view of a showing the CD in standard orientation, that is, with the view into the active-site cleft, which runs horizontally from left (non-primed side) to right (primed side). (c) View of the complex in the orientations of a (left) and b (right) showing the regions of the PD and CD engaged in binding in dark blue and orange, respectively. The rest of each molecule is shown in turquoise and yellow, respectively. (d) Close-up view in wall-eyed stereo image of the area around the inhibitory loop delimited by a black rectangle in c. The CD moiety is shown as a tan ribbon, and selected residues are labeled and shown for their side chains as sticks with tan carbons. The inhibitory loop (Lys121–Tyr135) is shown as a stick model with carbons in turquoise. Selected residues are also labeled. The barium ion of the CD is depicted as a magenta sphere. Note that the catalytic cysteine, Cys473, is oxidized to 3-sulfino-L-alanine (residue name CSD). Obtained from de Diego et al. (Citation12).
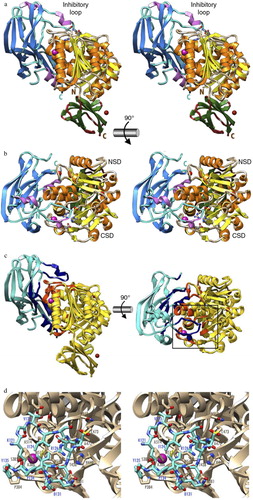
The high-affinity inhibitory interaction between the NPD and the catalytic domain of Rgp suggests that the prodomain must be degraded to release the active, proteolytic form of Rgp during enzyme maturation and secretion The NPD of Kgp has negligible inhibitory activity in trans. Nevertheless, it is likely that it still maintains proKgp inactive when in cis. It is important to emphasize that recombinant NPDs efficiently inhibit the activity of Rgps in whole P. gingivalis cells for prolonged periods of time. Therefore, the therapeutic application of NPDs, formulated to penetrate periodontal pockets, may attenuate P. gingivalis virulence and prevent the development of periodontitis.
Table 1 List of gingipain inhibitors with references
Gingipain inhibition by proteinaceous protease inhibitors
Mammalian inhibitors of gingipains
Proteinase inhibitors constitute 10% of the protein content of human plasma and are also found in tissue fluids. However, gingipain activity is affected only by a handful of these natural proteolysis regulators. The trypsin-like activity of P. gingivalis, attributed to its Rgp proteases, is inhibited by antithrombin III (ATIII) (K ass =5.65×104 M−1 s−1) in a reaction enhanced by heparin (Citation14). RgpA and RgpB, but not Kgp, are inhibited by human alpha-2-macroglobulin (α2M). A homologous macroglobulin from rat plasma, alpha-1-inhibitor 3, blocked the activity of all three gingipains (Citation15). Conversely, the major cysteine protease inhibitors, the cystatins, have little effect on gingipain activity (Citation16). Nevertheless, chicken cystatin, cystatin C, and cystatin S inhibited the growth of P. gingivalis in culture (Citation17, Citation18). Low molecular mass inhibitors of Rgps had no effect on P. gingivalis growth, indicating that the antibacterial activity of cystatins is not dependent on Rgp inhibition.
Pancreatic secretory Kazal-type trypsin inhibitors are another type of mammalian proteinase inhibitors that block gingipain activity. The porcine Kazal-type trypsin inhibitor, which possesses a Lys residue at the P1 position, exclusively inhibited Kgp, whereas a bovine inhibitor, which possesses an Arg residue at the P1 position, specifically blocked the activity of the Rgps (Citation19). Kazal-type trypsin inhibitors are not expressed in human gingival and periodontal tissues, but human homologues of these inhibitors could be used as a therapy to treat periodontitis.
Virus gingipain inhibitors
Among virally encoded caspase inhibitors, the cowpox virus cytokine-response modifier A (CrmA) serpin and a baculovirus p35 inhibitor of apoptosis inhibit Kgp but not Rgp (Citation20). To achieve Kgp inhibition, CrmA was modified by replacing the P1 Asp residue with a Lys residue at the reactive site. By contrast to CrmA, Kgp was efficiently inhibited by wild-type p35. Gingipains and caspases are members of the CD clan of proteases and share a similar 3D fold (Citation21). Therefore, gingipain inhibition indicates that virally encoded caspases have adopted a mechanism that allows them to regulate disparate members of the clan CD protease family.
Rice-derived inhibitors
Plant fruits and seeds are rich in proteinaceous protease inhibitors. To date, plant-derived proteins capable of inhibiting gingipains have only been characterized in rice (Oryza sativa). The rice grain extract exhibited high levels of gingipain inhibitory activity towards both Kgp and Rgps. The inhibitory activity was highly diverse with respect to molecular mass and isoelectric point. Four proteins, namely the 26-kDa Globulin (NCBI database accession number: 115464709), a plant lipid transfer/trypsin and α-amylase inhibitor (accession # 115471167), an RA17 seed allergen (accession # 115471171), and an α-amylase inhibitor/trypsin inhibitor (accession # 115471201), are the main components responsible for Rgp inhibitory activity in rice extract (Citation22), and are largely responsible for the suppression of gingipain-dependent P. gingivalis virulence and bacterial growth in media with transferrin as an iron source (Citation23). The presence of an Arg residue in the loop of two of the rice gingipain inhibitors, α-amylase inhibitor/trypsin inhibitor and an RA17 seed allergen, correlates with their ability to inhibit Rgps.
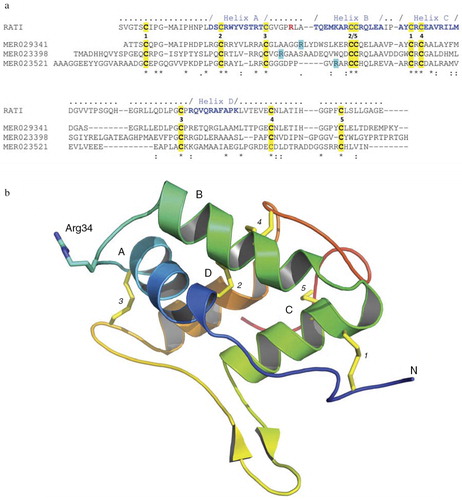
Other rice proteins that interact with Rgps are annotated in the MEROPS peptidase database (http://merops.sanger.ac.uk/cgi-bin/famsum?family=I6) and are denoted as a family of 16 unassigned peptidase inhibitor homologues. They share significant primary structure, including 10 conservative cysteine residues, with an archetypical inhibitor of the I6 family, the ragi seed trypsin/alpha-amylase inhibitor from Eleusine coracana (MEROPS Accession MER018247) (a). This implies conservation of the tertiary structure, including an exposed reactive site loop, located between helixes A and B (a and 2b). Inhibitors belonging to the I6 family exclusively inhibit serine proteases. Therefore, gingipain inhibition by rice I6 family members represents a novel type of inhibitory interaction. I6 family proteins are abundant in many cereal plants, including Sorghum bicolor, Zea mays (maize), Triticum aestivum (wheat), Brachypodium distachyon, Hordeum vulgare (barley), Secale cerede (rye), and Eleusine coracana (millet), and they may be capable of gingipain inhibition. Systematic biochemical studies are needed to isolate such proteins and investigate their enzyme kinetics and structural interactions with gingipains. This could lead to the development of food formula (nutraceuticals) that prevent the initiation and/or progression of periodontitis.
Fig. 2. Rice-derived proteins inhibiting gingipains. (a) Crystal structure-guided alignment of a bifunctional inhibitor of alpha-amylase and trypsin (RATI) from ragi seeds (Indian finger millet, Eleusine coracana Gaertneri) (MER018247) and rice-derived proteins inhibiting gingipains: alpha-amylase/trypsin inhibitor (MER029341), seed allergic protein RA17 precursor (MER023398) and plant lipid transfer/seed storage/trypsin-alpha amylase inhibitor domain containing protein (MER023521). Conserved cysteine residues and arrangement of disulfide bridges are highlighted yellow and induced by the identical digit between the RATI and MER029341 sequences, respectively. Amino acid residues forming alpha-helixes (A, B, C, and D) are in bold blue font and the reactive site loop is located between helixes A and B. The reactive site Arg residue in the RATI structure is in the bold red font. Arg residues in rice proteins, which may be recognized by Rgps as the P1 residues in the inhibitory reaction are highlighted turquoise. ‘-’ gaps introduced into sequences to facilitate the alignment using the ClustalW program (http://embnet.vital-it.ch/software/ClustalW.html). (b) The crystal structure of RATI (PDB database: 1B1U). The reactive site residue Arg34 is shown in ball-and-stick representation in purple.
Gingipain inhibition by proteins, peptides, and protein-derived peptides
Lactoferrin
Lactoferrin, an 80-kDa-iron-binding glycoprotein present in saliva and gingival crevicular fluid, inhibits P. gingivalis proteolytic activity and exhibits sustained biofilm inhibitory activity even when incubated with P. gingivalis (Citation24). This activity is probably related, at least in part, to its anti-protease effect. Lactoferrin is relatively resistant to hydrolysis by P. gingivalis proteases and may play an important role in preventing P. gingivalis-associated diseases via innate immunity.
Histatin
Histatin 5 is a salivary peptide rich in His residues with antifungal and antibacterial activity. It is not a typical protease inhibitor; however, it inhibits both the host matrix metalloproteases, MMP-2 and MMP-9, with IC50 values of 0.57 and 0.25 µM, respectively, and gingipains. Kinetic analysis has revealed that histatin 5 is a competitive inhibitor of Rgps, with a K i of 15 µM. Histatin 5 also inhibits Kgp but the mechanism of inhibition was not determined (Citation25). Since inhibition of both bacterial and host proteases occurs at physiological concentrations, histatin 5 could be an interesting candidate in clinical trials directed toward finding a therapy for the treatment and/or prevention of periodontitis.
κ-casein
Bovine milk caseins are a rich natural source of peptides with biological activity. Toh et al. (Citation26) identified a κ-casein (residues 109–137) from a chymosin digest of casein, as an inhibitory peptide of all P. gingivalis gingipains. The synthesized peptide inhibited the proteolytic activity associated with whole cells of P. gingivalis, purified RgpA-Kgp adhesion complexes, and purified RgpB. The κ-casein acted synergistically with Zn (II) against both Rgps and Kgp. Preincubation of P. gingivalis with κ-casein significantly reduced lesion development in a murine infection model.
Cyanate hydratase-derived peptide
Another peptide with inhibitory activity against gingipains is derived from cyanate hydratase, a rice extract protein. A synthetic dodecapeptide (RRLMAAKAESRK) corresponding to residues 33–48 of cyanate hydratase interfered with both Rgp and Kgp and inhibited bacterial growth in a dose-dependent manner (Citation22).
Gingipain inhibition by peptide analogs
Aza-peptide Michael acceptors
Ekici et al. (Citation27) found that aza-peptide Michael acceptors are highly potent and specific for the clan CD cysteine protease family to which gingipains belong. Aza-Lys and aza-Orn derivatives potently inhibited Kgp and clostripain, and showed no cross-reactivity with clan CA cysteine proteases, such as papain, cathepsin B, and calpain. Unfortunately, none of these inhibitors were tested for their effect on P. gingivalis virulence.
A71561
A compound, 1-(3-phenylpropionyl)piperidine-3-(R,S)-carboxylic acid-[4-amino-1(S)-(benzothiazole-2-carbonyl)butyl] amide (A71561), is a specific, slowly reversible inhibitor of Kgp with a K i in the nanomolar range. A71561 reduced the virulence of P. gingivalis in a murine in vivo infection model if bacteria were pre-incubated with the compound before inoculation (Citation28). However, to exert this beneficial effect, wild-type P. gingivalis cells had to be exposed to a high concentration (2 mM) of A71561, despite complete Kgp inhibition in the inoculum at concentrations<0.1 mM.
KYT inhibitors
Small peptide analogs, such as carbobenzoxy-Lys-Arg-CO-Lys-N-(CH3)2 (KYT-1) and carbobenzoxy-Glu(NHN(CH3)Ph)-Lys-CO-NHCH2Ph (KYT-36), strongly inhibit the degradation of human type 1 collagen, immunoglobulins, fibronectin, fibrinogen, the disruption of the bactericidal activity of polymorphonuclear leukocytes, and increases in vascular permeability (Citation29). KYT-1 and KYT-36 have low toxicity and are potent, subnanomolar and selective inhibitors for Kgp and Rgp, respectively. Recently KYT-41, a dual inhibitor of both types of gingipains was developed (Citation30). Evaluation of its biological potency in vitro and in vivo revealed very good selectivity for gingipains over host proteases, high inhibitory (Ki=40 nM and 0.27 nM for Rgp and Kgp, respectively) and ability to abrogate manifold pathological functions of P. gingivalis. Importantly, the therapeutic potential of KYT-41 was revealed by ability to suppress the vascular permeability that was enhanced in guinea pigs by P. gingivalis and the gingival inflammation in a beagle dog periodontitis model. Therefore among all the inhibitors already mentioned in this review, this makes them the most suitable candidates for the treatment of periodontitis.
DX-9065a
DX-9065a is a selective factor Xa (FXa) inhibitor that blocks the protease activity of FXa in an anti-thrombin III-independent manner (Citation31). This compound was shown to efficiently inhibit gingipains activity and suppress the growth of P. gingivalis and Prevotella intermedia (Citation32).
Chloromethane and chloromethyl ketones
Potempa et al. (Citation33) found that chloromethane compounds inhibit all three forms of gingipains to varying degrees depending on the peptidyl component of the inhibitor. Compounds containing a basic residue at P1 rapidly inactivated gingipains with some specificity being conferred by the P2 site. Kgp was rapidly inhibited by the (acyclo)methane inhibitor Cbz-Phe-Lys-CH2OCO-2,4,6-Me3-Ph. This inhibitor and the peptidyl chloromethanes D-Phe-Pro-Arg-CH2Cl and D-Phe-Phe-Arg-CH2Cl were the most specific among those tested for Rgp inhibition.
Chloromethyl ketones have been used as model compounds to study the specificity of the Sn binding region of RgpB and Kgp and to reveal the structural preferences of gingipains in this region (Citation34). Among three series of inhibitors with Arg or Lys at the P1 position and various substitutions at P2 and P3, small ligands, such as dipeptide analogs, were found to display high potency (k obs/[1]~107 M−1 s−1) for gingipains. Unfortunately, chloromethyl ketones are not suitable for in vivo studies because they react with primary amino groups.
FA-70C1
FA-70C1 is an antipain analog composed of phenylalanyl-ureido-citrullinyl-valinyl-cycloarginal isolated from the culture supernatant of the Streptomyces species strain FA-70 (Citation35). FA-70C1 inhibits Rgps with a K i of 4.5 nM and human cathepsins B, L, and H with K i values much higher than those of Rgps. FA-70C1 dose-dependently prevented the adverse effects of the P. gingivalis culture supernatant on the bactericidal activity of neutrophils and the viability of human fibroblasts and umbilical vein endothelial cells. FA-70Cl blocked the enhancement of vascular permeability induced by in vivo administration of the P. gingivalis culture supernatant. Additionally, FA-70C1 dose-dependently suppressed the growth of P. gingivalis. All these beneficial effects were exerted despite the fact that FA-70C1 does not inhibit Kgp. This suggests that Rgps should be the primary targets for the development of inhibitor-based therapies, and FA-70C1 could be a useful tool for the prevention of P. gingivalis virulence.
Gingipain inhibition by antibiotics and antiseptics
Antibiotics can target bacterial proteases (Citation36). Benzamidine derivatives inhibit the activity of RgpA and RgpB (Citation37, Citation38). Among these derivatives, bis-benzamidine, with a urea linker, was the most potent for Rgp inhibition. Application of low concentrations of zinc increased the benzamide derivative inhibition of Rgps by two- to three-fold. Benzamidine derivatives also bind to the P. gingivalis chaperone, GroEL, as well as to Rgps, and it is unclear which interaction is responsible for reduction of the mortality rate in a fertilized egg model of P. gingivalis pathogenicity (Citation39).
Chlorhexidine, tetracyclines, and non-antimicrobial chemically modified tetracycline derivatives have all been reported to inhibit gingipains and inhibition was increased by the addition of zinc (Citation40–(Citation42)). Chlorhexidine, cetylpyridium chloride, minocycline, and doxycycline all partially inhibited the Rgp-induced degradation of collagen-guided tissue regeneration membranes in P. gingivalis vesicles in solution, whereas metronidazole had no such effect (Citation43). Tetracyclines at 100 µM totally inhibited the amidolytic activity of RgpA and RgpB. These effects on gingipains may partially explain the therapeutic effect of tetracyclines in periodontitis treatment.
Gingipain inhibition by sword bean extract and canavanine
The fruit of the domesticated legume, Canavalia gladiate (commonly referred to as sword bean), is used in Chinese and Japanese herbal medicine for treating pus discharge. The fruit contains L-canavanine, in which the N-methylene of L-arginine is replaced by N-O. L-Canavanine inhibits microbial arginine deiminases (ADIs); thus, it may be the compound responsible for the pharmacological effect of sword bean extract (SBE) (Citation44). Recently, Nakatsuka et al. (Citation45) showed that SBE and canavanine inhibited the growth of P. gingivalis and Fusobacterium nucleatem. SBE also inhibited the activity of P. gingivalis cell-associated Rgp with an efficacy comparable to that of leupeptin. SBE inhibition of Kgp was weaker than that of Rgp. Although canavanine has been implicated as the compound responsible for gingipain inhibition, the direct effect of pure canavanine on gingipain activity has not been tested. However, oral administration of SBE suspended in carboxyl methylcellulose completely suppressed P. gingivalis-induced alveolar bone resorption in a rat model of periodontitis. This therapeutic effect of SBE is likely to be due to a combination of the direct antimicrobial action of canavanine and canavanine inhibition of gingipains. It is important to note that canavanine can also inhibit two other P. gingivalis enzymes; namely, extracellular peptidyl arginine deiminase (PPAD) and cytoplasmic ADI (Citation46).
SBE and canavanine have lower cytotoxicity than chlorhexidine gluconate (Citation45), a product already used as an active substance in wound dressings and antiseptic mouthwashes, and as a preservative in eye drops. This reduces safety concerns for SBE and would facilitate the testing of SBE in human clinical trials as an adjunct therapy for the routine mechanical treatment of periodontitis.
Gingipain inhibition by cranberry-derived polyphenols
Proteases of periodontopathogens (P. gingivalis, T. forsythia, and T. denticola) were efficiently inhibited by a high-molecular-weight fraction isolated from cranberry juice concentrate as non-dialyzable material (NDM). NDM containing polyphenols (65% proanthocyanidins) also prevented degradation of type 1 collagen and transferrin by whole P. gingivalis cells (Citation47). Inhibition of Rgp and Kgp activity by the polyphenol fraction of cranberry was confirmed in an independent study, which showed that this fraction also significantly inhibited synergistic biofilm formation by P. gingivalis and F. nucleatum. Significant inhibition of bacterial cell-associated gingipain activity occurred at an NDM concentration of approximately 1 µg/mL (Citation48). Cranberry-derived proanthocyanidins neutralized all the virulence properties of P. gingivalis but did not interfere with bacterial growth. NDM reduced activation of the NF-κB p65 proinflammatory pathway (Citation49), inhibited the adhesion of P. gingivalis and F. nucleatum to epithelial cells, and interfered with the coaggregation of these two species in a dose-dependent manner. Locally, in infected subcutaneous chambers, NDM reduced TNF-α levels induced by inoculation of a P. gingivalis and F. nucleatum mixture. In addition, NDM quenched TNF-α expression by macrophages stimulated with these two bacteria. These anti-inflammatory features of NDM explain why NDM reduced the severity of experimental periodontitis in mice treated with this agent (Citation50).
Gingipain inhibition by green tea-derived polyphenols
Traditional Chinese medicine has used tea as a medicament for many illnesses since ancient times. Recent epidemiological and clinical studies show that consumption of green tea and/or selected tea constituents contributes to a reduction in the risk of cardiovascular disease and cancer, and may provide oral health benefits. Tea polyphenols (catechins) are thought to be responsible for the biological properties of green tea since they exhibit antioxidant, anti-inflammatory, anti-cancer, and antimicrobial activities. In the case of periodontal disease, case-controlled, randomized, split-mouth clinical studies showed significant improvement of clinical parameters (gingival index, probing pocket depth, clinical attachment loss, and plaque index) in sites treated with green tea catechins delivered locally, in comparison to sites that received only a routine treatment of subgingival scaling and root planning (Citation51–(Citation55)). This improvement of periodontal status at sites treated with catechins is due to direct inhibition of host proteases (Citation56, Citation57) and suppression of the release of proinflammatory cytokines and chemokines from oral epithelial cells and fibroblasts (Citation56, Citation58). Catechins can also directly inhibit gingipains and this may contribute to the therapeutic effect of green tea extract. Catechin derivatives, including epigallocatechin gallate, the main constituent of green tea polyphenols, epicatechin gallate, gallocatechin gallate, and catechin gallate, significantly inhibited Rgp activity with an IC50 in the range of 3–5 µM (Citation59). The potency of catechins to inhibit gingipains together with their ability to interfere with P. gingivalis growth and suppress the inflammatory reaction make these compounds perfect candidates for the development of therapeutics against periodontitis and potentially associated systemic illnesses, such as atherosclerosis (Citation60).
Gingipain inhibition by Myrothamnus flabellifolia extract
A polyphenol-enriched extract from Myrothamnus flabellifolia (MF) is a more potent inhibitor for Rgp than Kgp (Citation61). At 50 mg/mL, MF reduced Rgp activity by 70–80% and 100 mg/mL MF reduced Rgp activity by approximately 80%. By contrast to Rgp, Kgp activity was only reduced by approximately 50% with 100 mg/mL MF. MF also had anti-adhesive effects mediated by its interaction with bacterial outer membrane proteins (OMPs). However, it exhibits high toxicity, which may limit its clinical use to multiple low-dose applications.
Gingipain inhibition by antibodies and vaccines
Antibody-binding epitopes in the vicinity of the substrate-binding cleft of certain proteases can be used to limit their protease activity. The best examples of this are the natural antibodies against IgA1 proteases produced by mucosal pathogens (Citation62–(Citation65)). Although no natural antibodies with gingipain inhibitory activity are known, IgY antibodies isolated from the yolks of hens immunized with purified gingipains exerted dose-dependent inhibition of gingipain hydrolysis of synthetic substrates. In addition, the anti-gingipain IgY antibodies strongly interfered with gingipain-induced human epithelial cell detachment (Citation66), and were effective immunotherapeutic agents in the treatment of periodontitis in humans. A small scale clinical trial revealed that periodontal sites treated with anti-gingipain IgY in addition to routine subgingival scaling and root planning, showed significantly reduced numbers of P. gingivalis, decreased pocket probing depth (attachment loss), and attenuated bleeding on probing (BOP) in comparison to sites treated only with subgingival scaling and root planning (Citation67).
Vaccination with gingipains has been reported to yield a protective effect against P. gingivalis infection (Citation68–(Citation70)). The vaccines tested were mainly peptide and DNA vaccines. While DNA vaccines induce both cellular and humoral immunity, peptide vaccines induce only humoral immunity. In a review on P. gingivalis vaccines, RgpA and Kgp were proposed as potential vaccines for the prevention of P. gingivalis-induced periodontitis (Citation71). O'Brien-Simpson et al. (Citation72) reported that RgpA-Kgp peptide-based immunogens together with incomplete Freund's adjuvant gave protection against P. gingivalis in a murine lesion model. The protease domain of Kgp serves as a hemoglobin-binding domain and may also constitute an immunogen for the induction of immunity from P. gingivalis infection (Citation73). Immunization in a rat periodontitis model with the RgpA-Kgp protease-adhesion complex protected against colonization of the subgingival sulcus with P. gingivalis and reduced periodontal bone loss by inducing a high titer of serum IgG2 (Citation74). Immunization with RgpA stimulated the production of HA domain-specific antibodies, which contributed to the prevention of periodontal disease in a murine model (Citation69). However, only certain parts of HA domain-specific antibodies have protective functions (Citation75); therefore, antibodies produced against the protease domain may be more promising. Genco et al. (42) found that antibodies directed against the amino-terminal region of the protease domain of Rgps induced a protective immune response against P. gingivalis infection in a mouse chamber model. A study in humans suggested that inability to produce sufficient antibodies to the protease domain of gingipains may be an etiologic factor for chronic periodontitis and antibodies against the protease domain are important in the prevention of periodontitis (Citation76).
RgpA contains a proprotein, a protease domain, and a hemagglutinin-adhesion (HA) domain consisting of consecutive hemagglutinin polypeptides of 44 kDa, 15 kD, 17 kDa, and 27 kDa (HGP44, HGP15, HGP17, and HGP27, respectively). The HA domain is similar to that encoded by the hemagglutinin A (HagA) gene and the HA domains of Kgp (Citation77). Of note, recolonization of patients subjected to periodontal treatment with P. gingivalis was prevented by local passive immunization with monocolonal antibodies (Citation78) recognizing heamagglutinating epitope in HGP44 (Citation79). This important observation constitutes a proof of concept that gingipains constitute perfect target for vaccine development. Indeed, immunization of mice with RgpA stimulated production of P. gingivalis- and RgpA-specific IgG antibodies directed primarily against the HA domain of this protein (Citation68). O'Brien-Simpson et al. (Citation80) found that when the RgpA-Kgp complex, functional binding motif, or active-site peptides thereof were used as a vaccine, a Th2 response was induced that blocked the function of the RgpA-Kgp complex and protected against periodontal bone loss in the murine periodontitis model. Recently, Muramatsu et al. (Citation81) examined the protective effect of immunization with RgpA domain vaccines and reported that HGP44 induced protection against P. gingivalis-induced alveolar bone loss in mice. The HGP44 domain-coding DNA vaccine was responsible for this protection. The HPG44-coding region could thus be a candidate antigen for a DNA vaccine. Previously, antibodies induced by the HPG44 domain inhibited binding of the RgpA-Kgp complex to fibrinogen, fibrinonectin, and collagen type IV (Citation80) and enhanced opsonization and killing of invasive and non-invasive strains of P. gingivalis (Citation82). Yonezawa et al. (Citation83) found that immunization by an RgpA DNA vaccine protected mice against invasive P. gingivalis infection through regulation of interferon-γ. The antibodies produced inhibited several P. gingivalis virulence activities including Rgp-mediated hemagglutination and binding to collagen. This could be an important consideration for the immunization of animals against periodontitis.
Gingipain inhibition by other bacteria
Other bacteria present in dental plaque may modulate the proteolytic activity of P. gingivalis. Tenorio et al. (Citation84) found that a number of oral bacterial strains in subgingival plaque were capable of reducing the cytotoxic effects of P. gingivalis in vitro and interfering with its growth and proteolytic activity. Further research is required to determine whether this observation could lead to new probiotic agents and novel strategies for treatment of periodontal disease.
Concluding remarks
A number of gingipain inhibitors have been detected. Broadly speaking they can be classed as NPDs of gingipains, synthetic inhibitors, inhibitors from natural sources, antibiotics, antiseptics, antibodies, and bacteria. A few of the synthetic inhibitors were devised to elucidate the structures, catalytic mechanisms, and reaction intermediates of gingipains. Although there is no doubt that several of these inhibitors are highly potent, they are generally unsuitable for in vivo use because of their chemical reactivity and/or ability to interfere with activity of essential host proteases. Many other synthetic compounds have high or unknown toxicity. Nevertheless, these synthetic compounds are effective in preventing P. gingivalis pathogenicity if bacteria are pre-incubated with the inhibitors prior to inoculation of experimental animals. This represents an interesting strategy to arrest P. gingivalis-induced periodontal disease. The KYT inhibitors are the most promising synthetic compounds since they have low toxicity and high selectivity for gingipains. Taking into account the absolute dependence of P. gingivalis virulence on gingipain activity it is perplexing that none of the synthetic compounds have been developed and tested in pre-clinical trials. Clearly, more work is required to develop synthetic inhibitors and assess them in clinical trials. Inhibitors from natural sources are at a more advanced stage with several undergoing testing on a limited scale in clinical trials and showing promising therapeutic results. Cranberry and rice extracts are especially interesting not only because they interfere with gingipains, but also because they prevent growth and biofilm formation by periodontopathogens. Despite all this research, prophylactic and therapeutically useful inhibitors to treat and/or prevent periodontitis still require further development. In doing this it is important to differentiate between inhibition of proteolytic enzyme activity through some form of active site-directed strategy versus interference with the hemagglutination/adherence properties of RgpA and Kgp. It is not clear how effective a strategy it will be that targets only a single organism in the complex dysbiotic microbiota of periodontal disease. Since additional periodontopathogens such as T. forsythia and T. denticola also produce proteinases, future research should investigate their susceptibility to gingipain inhibitors as well.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
Acknowledgements
The authors want to acknowledge funding through project 2975/7.PR/13/2014/2 from Polish MNiSW and through the European Commission (FP7-HEALTH-306029 ‘TRIGGER’).
References
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999; 4: 1–6. [PubMed Abstract].
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998; 25: 134–44. [PubMed Abstract].
- Guo Y, Nguyen K-A, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaning substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010; 54: 15–44. [PubMed Abstract] [PubMed CentralFull Text].
- Fitzpatrick RE, Wijeyewickrema LC, Pike RN. The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 2009; 4: 471–87. [PubMed Abstract].
- Sztukowska M, Veillard F, Potempa B, Bogyo M, Enghild JJ, Thogersen IB, etal. Disruption of gingipain oligomerization into non-covalent cell-surface attached complexes. Biol Chem. 2012; 393: 971–7. [PubMed Abstract] [PubMed CentralFull Text].
- Pathirana RD, O'Brien-Simpson NM, Brammar GC, Slakeski N, Reynolds EC. Kgp and RgpB, but not RgpA, are important for Porphyromonas gingivalis virulence in the murine periodontitis model. Infect Immun. 2007; 75: 1436–42. [PubMed Abstract] [PubMed CentralFull Text].
- Wilensky A, Polak D, Houri-Haddad Y, Shapira L. The role of RgpA in the pathogenicity of Porphyromonas gingivalis in the murine periodontitis model. J Clin Periodontol. 2013; 40: 924–32. [PubMed Abstract].
- Yongqing T, Potempa J, Pike RN, Wijeyewickrema LC. The lysine-specific gingipain of Porphyromonas gingivalis: importance to pathogenicity and potential strategies for inhibition. Adv Exp Med Biol. 2011; 712: 15–29. [PubMed Abstract].
- Grenier D, La VD. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: a review article. Curr Drug Targets. 2011; 12: 322–31. [PubMed Abstract].
- Veillard F, Sztukowska M, Mizgalska D, Ksiazek M, Houston J, Potempa B, etal. Inhibition of gingipains by their profragments as the mechanism protecting Porphyromonas gingivalis against premature activation of secreted proteases. Biochim Biophys Acta. 2013; 1830: 4218–28. [PubMed Abstract] [PubMed CentralFull Text].
- Huq NL, Seers CA, Toh EC, Dashper SG, Slakeski N, Zhang L, etal. Propeptide-mediated inhibition of cognate gingipain proteinases. PLoS One. 2013; 8: e65447. [PubMed Abstract] [PubMed CentralFull Text].
- de Diego I, Veillard FT, Guevara T, Potempa B, Sztukowska M, Potempa J, etal. Porphyromonas gingivalis virulence factor gingipain RgpB shows a unique zymogenic mechanism for cysteine peptidases. J Biol Chem. 2013; 288: 14287–96. [PubMed Abstract] [PubMed CentralFull Text].
- Veillard F, Potempa B, Poreba M, Drag M, Potempa J. Gingipain aminopeptidase activities in Porphyromonas gingivalis. Biol Chem. 2012; 393: 1471–6. [PubMed Abstract].
- Curtis MA, Slaney JM, Carman RJ, Pemberton PA. Interaction of a trypsin-like enzyme of Porphyromonas gingivalis W83 with antithrombin III. FEMS Microbiol Lett. 1993; 108: 169–74. [PubMed Abstract].
- Grøn H, Pike R, Potempa J, Travis J, Thøgersen IB, Enghild JJ, etal. The potential role of alpha 2-macroglobulin in the control of cysteine proteinases (gingipains) from Porphyromonas gingivalis. J Periodontal Res. 1997; 32: 61–8.
- Blankenvoorde MF, Henskens YM, van't Hof W, Veerman EC, Nieuw Amerongen AV. Inhibition of the growth and cysteine proteinase activity of Porphyromonas gingivalis by human salivary cystatin S and chicken cystatin. Biol Chem. 1996; 377: 847–50. [PubMed Abstract].
- Blankenvoorde MF, van't Hof W, Walgreen-Weterings E, van Steenbergen TJ, Brand HS, Veerman EC, etal. Cystatin and cystatin-derived peptides have antibacterial activity against the pathogen Porphyromonas gingivalis. Biol Chem. 1998; 379: 1371–5. [PubMed Abstract].
- Umemoto T, Naito Y, Li M, Suzuki I, Namikawa I. Growth inhibition of a human oral bacterium Porphyromonas gingivalis by rat cysteine proteinase inhibitor cystatin S. Lett Appl Microbiol. 1996; 23: 151–3. [PubMed Abstract].
- Bania J, Kubiak A, Wojtachnio K, Polanowski A. Pancreatic secretory trypsin inhibitor acts as an effective inhibitor of cysteine proteases gingipains from Porphyromonas gingivalis. J Periodontal Res. 2008; 43: 232–6. [PubMed Abstract].
- Snipas SJ, Stennicke HR, Riedl S, Potempa J, Travis J. Inhibition of distant caspase homologues by natural caspase inhibitors. Biochem J. 2001; 357: 575–80. [PubMed Abstract] [PubMed CentralFull Text].
- Barrett AJ, Rawlings ND. Evolutionary lines of cysteine peptidases. Biol Chem. 2001; 382: 727–33. [PubMed Abstract].
- Taiyoji M, Shitomi Y, Taniguchi M, Saitoh E, Ohtsubo S. Identification of proteinaceous inhibitors of a cysteine proteinase (an Arg-specific gingipain) from Porphyromonas gingivalis in rice grain, using targeted-proteomics approaches. J Proteome Res. 2009; 8: 5165–74. [PubMed Abstract].
- Taiyoji M, Yamanaka T, Tsuno T, Ohtsubo S. Potential value of a rice protein extract containing proteinaceous inhibitors against cysteine proteinases from Porphyromonas gingivalis, for managing periodontal diseases. Biosci Biotechnol Biochem. 2013; 77: 80–6. [PubMed Abstract].
- Daspher SG, Pan Y, Veith PD, Chen Y-Y, Toh ECY, Liu SW, etal. Lactoferrin inhibits Porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob Agents Chemother. 2012; 56: 1548–56.
- Gusman H, Grogan J, Kagan HM, Troxler RF, Oppenheim FG. Salivary histatin 5 is a potent competitive inhibitor of the cysteine proteinase clostripain. FEBS Lett. 2001; 489: 97–100. [PubMed Abstract].
- Toh ECY, Daspher SG, Huq NL, Attard TJ, O'Brien-Simpson NM, Chen Y-Y, etal. Porphyromonas gingivalis cysteine proteinase inhibition by κ-casein peptides. Antimicrob Agents Chemother. 2011; 55: 1155–61. [PubMed Abstract] [PubMed CentralFull Text].
- Ekici ÖD, Götz MG, James KE, Li ZZ, Rukamp BJ, Asgian JL, etal. Aza-peptide Michael acceptors: a new class of inhibitors specific for caspases and other clan CD cysteine proteases. J Med Chem. 2004; 47: 1889–92. [PubMed Abstract].
- Curtis MA, Opoku JA, Rangarajan M, Gallagher A, Sterne JAC, Reid CR, etal. Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect Immun. 2002; 70: 6968–75. [PubMed Abstract] [PubMed CentralFull Text].
- Kadowaki T, Baba A, Abe N, Takii R, Hashimoto M, Tsukuba T, etal. Suppression of pathogenicity of Porphyromonas gingivalis by newly developed gingipain inhibitors. Mol Pharmacol. 2004; 66: 1599–606. [PubMed Abstract].
- Kataoka S, Baba A, Suda Y, Takii R, Hashimoto M, Kawakubo T. A novel, potent dual inhibitor of Arg-gingipains and Lys-gingipain as a promising agent for periodontal disease therapy. FASEB J. 2014; 28: 3564–78. doi: 10.1096/fj.14-252130
- Nagahara T, Yokoyama Y, Inamura K, Katakura S, Komoriya S, Yamaguchi H, etal. Dibasic (amidinoaryl) propanoic acid derivatives as novel blood coagulation factor Xa inhibitors. J Med Chem. 1994; 37: 1200–7. [PubMed Abstract].
- Matsushita K, Imamura T, Tancharoen S, Tatsuyama S, Tomikawa M, Travis J, etal. Selective inhibition of Porphyromonas gingivalis growth by a factor Xa inhibitor, DX-9065a. J Periodontal Res. 2006; 41: 171–6. [PubMed Abstract].
- Potempa J, Pike R, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem. 1997; 378: 223–30. [PubMed Abstract].
- Bialas A, Grembecka J, Krowarsch D, Otlewski J, Potempa J, Mucha A. Exploring the Sn binding pockets in gingipains by newly developed inhibitors: structure-based design, chemistry and activity. J Med Chem. 2006; 49: 1744–53. [PubMed Abstract].
- Kadowaki T, Kitano S, Baba A, Takii R, Hashimoto M, Katunuma N, etal. Isolation and characterization of a novel and potent inhibitor of Arg-gingipain from Streptomyces sp. strain FA-70. Biol Chem. 2003; 384: 911–20. [PubMed Abstract].
- Travis J, Potempa J. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim Biophys Acta. 2000; 1477: 35–50. [PubMed Abstract].
- Krauser JA, Potempa J, Travis J, Powers JC. Inhibition of arginine gingipains (RgpB and HRgpA) with benzamidine inhibitors: zinc increases inhibitory potency. Biol Chem. 2002; 383: 1193–8. [PubMed Abstract].
- Eick S, Pfister W, Stürzebecher U, Jarema S, Stürzebecher J. Inhibitors of benzamidine type influence the virulence properties of Porphyromonas gingivalis strains. Acta Biochim Pol. 2003; 50: 725–34. [PubMed Abstract].
- Fröhlich E, Kantyka T, Plaza K, Schmidt K-H, Pfister W, Potempa J, etal. Benzamidine derivatives inhibit the virulence of Porphyromonas gingivalis. Mol Oral Microbiol. 2013; 28: 192–203.
- Imamura T, Matsushita K, Travis J, Potempa J. Inhibition of trypsin-like cysteine proteinases (gingipains) from Porphyromonas gingivalis by tetracycline and its analogues. Antimicrob Agents Chemother. 2001; 45: 2871–6. [PubMed Abstract] [PubMed CentralFull Text].
- Grenier D, Plamondon P, Sorsa T, Lee H-M, McNamara T, Ramamurthy NS, etal. Inhibition of proteolytic, serpinolytic, and progelatinase-b activation activities of periodontopathogens by doxycycline and the non-antimicrobial chemically modified tetracycline derivatives. J Periodontol. 2002; 73: 79–85. [PubMed Abstract].
- Genco CA, Odusanya BM, Potempa J, Mikolajczyk-Pawlinska J, Travis J. A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis. Infect Immun. 1998; 66: 4108–14. [PubMed Abstract] [PubMed CentralFull Text].
- Sela MN, Babitski E, Steinberg D, Kohavi D, Rosen G. Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin Oral Impl Res. 2009; 20: 496–502.
- Li L, Li Z, Chen D, Lu X, Feng X, Wright EC, etal. Inactivation of microbial arginine deiminases by L-canavanine. J Am Chem Soc. 2008; 130: 1918–31. [PubMed Abstract].
- Nakatsuka Y, Nagasawa T, Yumoto Y, Nakazawa F, Furuichy Y. Inhibitory effects of sword bean extract on alveolar bone resorption induced in rats by Porphyromonas gingivalis infection. J Periodontal Res. 2014; 49
- Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014; 16: 408. [PubMed Abstract] [PubMed CentralFull Text].
- Bodet C, Piché M, Chandad F, Grenier D. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J Antimicrob Chemother. 2006; 57: 685–90. [PubMed Abstract].
- Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007; 42: 589–92. [PubMed Abstract].
- La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 2010; 54: 1778–84. [PubMed Abstract] [PubMed CentralFull Text].
- Polak D, Naddaf R, Shapira L, Weiss EI, Houri-Haddad Y. Protective potential of non-dialyzable material fraction of cranberry juice on the virulence of P. gingivalis and F. nucleatum mixed infection. J Periodontol. 2013; 84: 1019–25. [PubMed Abstract].
- Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: a clinical pilot study. J Periodontal Res. 2002; 37: 433–8. [PubMed Abstract].
- Gadagi JS, Chava VK, Reddy VR. Green tea extract as a local drug therapy on periodontitis patients with diabetes mellitus: a randomized case-control study. J Indian Soc Periodontol. 2013; 17: 198–203. [PubMed Abstract] [PubMed CentralFull Text].
- Hattarki SA, Pushpa SP, Bhat K. Evaluation of the efficacy of green tea catechins as an adjunct to scaling and root planing in the management of chronic periodontitis using PCR analysis: a clinical and microbiological study. J Indian Soc Periodontol. 2013; 17: 204–9. [PubMed Abstract] [PubMed CentralFull Text].
- Chava VK, Vedula BD. Thermo-reversible green tea catechin gel for local application in chronic periodontitis: a 4-week clinical trial. J Periodontol. 2013; 84: 1290–6. [PubMed Abstract].
- Kudva P, Tabasum ST, Shekhawat NK. Effect of green tea catechin, a local drug delivery system as an adjunct to scaling and root planing in chronic periodontitis patients: a clinicomicrobiological study. J Indian Soc Periodontol. 2011; 15: 39–45. [PubMed Abstract] [PubMed CentralFull Text].
- Zhao L, La VD, Grenier D. Antibacterial, antiadherence, antiprotease, and anti-inflammatory activities of various tea extracts: potential benefits for periodontal diseases. J Med Food. 2013; 16: 428–36. [PubMed Abstract].
- Makimura M, Hirasawa M, Kobayashi K, Indo J, Sakanaka S, Taguchi T, etal. Inhibitory effect of tea catechins on collagenase activity. J Periodontol. 1993; 64: 630–6. [PubMed Abstract].
- Hosokawa Y, Hosokawa I, Shindo S, Ozaki K, Matsuo T. (-)-Epigallocatechin-3-gallate inhibits CC chemokine ligand 11 production in human gingival fibroblasts. Cell Physiol Biochem. 2013; 31: 960–7. [PubMed Abstract].
- Okamoto M, Sugimoto A, Leung KP, Nakayama K, Kamaguchi A, Maeda N. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral Microbiol Immunol. 2004; 19: 118–200. [PubMed Abstract].
- Cai Y, Kurita-Ochiai T, Hashizume T, Yamamoto M. Green tea epigallocatechin-3-gallate attenuates Porphyromonas gingivalis-induced atherosclerosis. Pathog Dis. 2013; 67: 76–83. [PubMed Abstract].
- Löhr G, Beikler T, Podbielski A, Standar K, Redanz S, Hensel A. Polyphenols from Myrothamnus flabellifolia Welv. inhibit in vitro adhesion of Porphyromonas gingivalis and exert antiinflammatory cytoprotective effects in KB cells. J Clin Periodontol. 2011; 38: 457–69.
- Devenyi AG, Plaut AG, Grundy FJ, Wright A. Post-infectious human serum antibodies inhibit IgA1 proteinases by interaction with the cleavage site specificity determinant. Mol Immunol. 1993; 30: 1243–8. [PubMed Abstract].
- Frandsen EV, Kjeldsen M, Kilian M. Inhibition of Prevotella and Capnocytophaga immunoglobulin A1 proteases by human serum. Clin Diagn Lab Immunol. 1997; 4: 458–64. [PubMed Abstract] [PubMed CentralFull Text].
- Kirkeby L, Rasmussen TT, Reinholdt J, Kilian M. Immunoglobulins in nasal secretions of healthy humans: structural integrity of secretory immunoglobulin A1 (IgA1) and occurrence of neutralizing antibodies to IgA1 proteases of nasal bacteria. Clin Diagn Lab Immunol. 2000; 7: 31–9. [PubMed Abstract] [PubMed CentralFull Text].
- Mistry D, Stockley RA. IgA1 protease. Int J Biochem Cell Biol. 2006; 38: 1244–8. [PubMed Abstract].
- Yokoyama K, Sugano N, Rahman AK, Oshikawa M, Ito K. Activity of anti-Porphyromonas gingivalis egg yolk antibody against gingipains in vitro. Oral Microbiol Immunol. 2007; 22: 352–5. [PubMed Abstract].
- Yokoyama K, Sugano N, Shimada T, Shofigur RA, Ibrahim el-SM, Isoda R, etal. Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J Oral Sci. 2007; 49: 201–6. [PubMed Abstract].
- Gibson FC III, Genco CA. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect Immun. 2001; 69: 7959–63. [PubMed Abstract] [PubMed CentralFull Text].
- Yonezawa H, Ishihara K, Okuda K. Arg-gingipain a DNA vaccine induces protective immunity against infection by Porphyromonas gingivalis in a murine model. Infect Immun. 2001; 69: 2858–64. [PubMed Abstract] [PubMed CentralFull Text].
- Miyachi K, Ishihara K, Kimizuka R, Okuda K. Arg-gingipain A DNA vaccine prevents alveolar bone loss in mice. J Dent Res. 2007; 86: 446–50. [PubMed Abstract].
- Nakagawa T, Saito A, Hosaka Y, Ishihara K. Gingipains as candidate antigens for Porphyromonas gingivalis vaccine. Keio J Med. 2003; 52: 158–62. [PubMed Abstract].
- O'Brien-Simpson NM, Paolini RA, Reynolds EC. Rgp-Kgp peptide-based immunogens provide protection against Porphyromonas gingivalis challenge in a murine lesion model. Infect Immun. 2000; 68: 4055–63. [PubMed Abstract] [PubMed CentralFull Text].
- Kuboniwa M, Amano A, Shizukuishi S, Nakagawa I, Hamada S. Specific antibodies to Porphyromonas gingivalis lys-gingipain by DNA vaccination inhibit bacterial binding to hemoglobin and protect mice from infection. Infect Immun. 2001; 69: 2972–9. [PubMed Abstract] [PubMed CentralFull Text].
- Rajapakse PS, O'Brien-Simpson NM, Slakeski N, Hoffmann B, Reynolds EC. Immunization with RgpA-Kgp proteinase-adhesion complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infect Immun. 2002; 70: 2480–6. [PubMed Abstract] [PubMed CentralFull Text].
- Sharma D, Prasad S, Karthikeyan BV. Vaccination against periodontitis: the saga continues. Expert Rev Vaccines. 2007; 6: 579–90. [PubMed Abstract].
- Inagaki S, Ishihara K, Yasaki Y, Yamada S, Okuda K. Antibody responses of periodontitis patients to gingipains of Porphyromonas gingivalis. J Periodontol. 2003; 74: 1432–9. [PubMed Abstract].
- Nakayama K. Molecular genetics of Porphyromonas gingivalis: gingipains and other virulence factors. Curr Protein Pept Sci. 2003; 4: 389–95. [PubMed Abstract].
- Booth V, Ashley FP, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996; 64: 422–7. [PubMed Abstract] [PubMed CentralFull Text].
- Kelly CG, Booth V, Kendal H, Slaney JM, Curtis MA, Lehner T. The relationship between colonization and haemagglutination inhibiting and B cell epitopes of Porphyromonas gingivalis. Clin Exp Immunol. 1997; 110: 285–91. [PubMed Abstract] [PubMed CentralFull Text].
- O'Brien-Simpson NM, Pathirana RD, Paolini RA, Chen YY, Veith PD, Tam V, etal. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J Immunol. 2005; 175: 3980–9. [PubMed Abstract].
- Muramatsu K, Kokubu E, Shibahara T, Okuda K, Ishihara K. HGP44 induces protection against Porphyromonas gingivalis-induced alveolar bone loss in mice. Clin Vaccine Immunol. 2011; 18: 888–91. [PubMed Abstract] [PubMed CentralFull Text].
- Yasaki-Inagaki Y, Inagaki S, Yamada S, Okudo K, Ishihara K. Production of protective antibodies against Porphyromonas gingivalis strains by immunization with recombinant gingipain domains. FEMS Immunol Med Microbiol. 2006; 47: 287–95. [PubMed Abstract].
- Yonezawa H, Kato T, Kuramitsu HK, Okuda K, Ishihara K. Immunization by Arg-gingipain A DNA vaccine protects mice against an invasive Porphyromonas gingivalis infection through regulation of interferon-γ production. Oral Microbiol Immunol. 2005; 20: 259–66. [PubMed Abstract].
- Tenorio EL, Klein BA, Cheung WS, Hu LT. Identification of interspecies interactions affecting Porphyromonas gingivalis virulence phenotypes. J Oral Microbiol. 2011; 3: 8396.
