Abstract
Porphyromonas gingivalis, a keystone pathogen in chronic periodontitis, has been found to associate with remote body organ inflammatory pathologies, including atherosclerosis and Alzheimer’s disease (AD). Although P. gingivalis has a plethora of virulence factors, much of its pathogenicity is surprisingly related to the overall immunosuppression of the host. This review focuses on P. gingivalis aiding suppression of the host’s adaptive immune system involving manipulation of cellular immunological responses, specifically T cells and B cells in periodontitis and related conditions. In periodontitis, this bacterium inhibits the synthesis of IL-2 and increases humoral responses. This reduces the inflammatory responses related to T- and B-cell activation, and subsequent IFN-γ secretion by a subset of T cells. The T cells further suppress upregulation of programmed cell death-1 (PD-1)-receptor on CD+cells and its ligand PD-L1 on CD11b+-subset of T cells. IL-2 downregulates genes regulated by immune response and induces a cytokine pattern in which the Th17 lineage is favored, thereby modulating the Th17/T-regulatory cell (Treg) imbalance. The suppression of IFN-γ-stimulated release of interferon-inducible protein-10 (IP-10) chemokine ligands [ITAC (CXCL11) and Mig (CXCL9)] by P. gingivalis capsular serotypes triggers distinct T cell responses and contributes to local immune evasion by release of its outer membrane vesicles. In atherosclerosis, P. gingivalis reduces Tregs, transforms growth factor beta-1 (TGFβ-1), and causes imbalance in the Th17 lineage of the Treg population. In AD, P. gingivalis may affect the blood–brain barrier permeability and inhibit local IFN-γ response by preventing entry of immune cells into the brain. The scarcity of adaptive immune cells in AD neuropathology implies P. gingivalis infection of the brain likely causing impaired clearance of insoluble amyloid and inducing immunosuppression. By the effective manipulation of the armory of adaptive immune suppression through a plethora of virulence factors, P. gingivalis may act as a keystone organism in periodontitis and in related systemic diseases and other remote body inflammatory pathologies.
There are 3,000 years of history suggesting the oral influence, particularly of periodontitis on the general health of human subjects (Citation1). In periodontitis, Porphyromonas gingivalis represents a keystone pathogen causing microbial and immune dysbiosis (Citation2). Although P. gingivalis has a number of potent virulence factors (Citation3), much of its pathogenicity is due to its ability to subvert the host’s immune defenses (Citation4). Previously, we reviewed how P. gingivalis can affect innate immunity in periodontitis, in cardiovascular disease, and in Alzheimer’s disease (AD) by modulating the inflammasome (Citation5, Citation6) and neutrophil functions (Citation7). We have also discussed whether P. gingivalis and other oral microorganisms have a role in the development of AD (Citation5, Citation8). The aim here is to review how P. gingivalis may affect adaptive immunity in periodontitis () and related systemic diseases where atherosclerosis is the primary initiator of vascular pathology () and subsequently AD (). It has to be said that the adaptive and innate immune systems co-exist and provide co-stimulatory signals necessary for the adaptive immune system and vice versa. The adaptive immune arm of protection occurs in health, but during disease (periodontal and related systemic diseases) the beneficial effects appear to be reduced. As an example, CD4+ T-lymphocytes are an essential component in responses to microorganisms during infectious diseases as they orchestrate the functional activities of both the innate and adaptive immune system (Citation9). It will therefore not be possible to completely distinguish, in this review, between responses from the two different immune systems although the focus will be on P. gingivalis suppression of adaptive immunity in three inflammatory pathologies.
Fig. 1.
P. gingivalis and its presumed contribution to periodontitis via adaptive immune suppression. Following P. gingivalis infection, the outer membrane vesicles (OMVs) from P. gingivalis transfer LPS and gingipains to a lipid-binding site on an antigen presenting cell (AP cell). Gingipains erode cells cleaving CD14 and the immune cell receptor (RANKL [receptor activator of NF-κB ligand]) a TNF-related cytokine which binds to RANK, a protein expressed on the osteoclast progenitor cell activating an intracellular signaling cascade via NF-κB resulting in suppression of IL-2 secretion. Intact CD14 on membrane and soluble TLR4-MD2 (myeloid MD2= differentiation protein 2) can still function to promote the binding of LPS to the TLR4-MD2 complex and enlist intracellular cell signaling via NF-κB. The appropriate cytokine release or its suppression has implications on cellular/adaptive immune responses which involve host defective IgG.
Adaptive immune responses through activated CD14 T cells and secretion of immunoglobulins (Ig) by B cells constrain the subgingival biofilm or may give rise to disease progression. T cells can have both protective and destructive roles. Inhibition of IL-2: P. gingivalis can modify adaptive immune response through interaction with dendritic cells inducing a cytokine pattern favoring T-helper 17 (Th17) lineage and inhibiting the expression and accumulation of IL-2 which attenuates T cell proliferation and communication. Arg-gingipain (Agp) protease is involved in suppression of IL-2 which contributes to self-propagation of P. gingivalis in vivo. Th17/Treg imbalance: P. gingivalis can modify adaptive immune response by interaction with dendritic or antigen presenting cells (APC cells) which induce a cytokine pattern favoring the Th17 cell population. The imbalance is also promoted by P. gingivalis proteases because IL-1β is the most Th17 supporting cytokine and is the cytokine most resistant to P. gingivalis protease cleavage modification of Th17/Treg balance which occurs by Th17 cell response inhibition and increasing Treg-cell activation. IFN-γ suppresses upregulation of programmed cell death: Secretion of IFN-γ upregulation of programmed cell death – 1 (PD-1) – receptor on CD+ cells and its ligand PD-L1 on CD11b+-T cells. Since the PD-L1/PD-1 signaling pathway inhibits the T-cell response, the changes induced by P. gingivalis on the expression of these molecules could be a mechanism by which P. gingivalis suppresses T-cell immunity. RANK-RANKL, OPG: Activated lymphocytes expressing surface receptor activator of NF-κB ligand (RANKL) can bind to surface RANK expressed on hematopoietic progenitors of osteoclasts (osteoclast progenitors) activating a signal transduction cascade leading to osteoclastogenesis (in the presence of macrophage colony–stimulating factor, MCSF). This gives rise to activation and differentiation of functional osteoclasts and periodontal bone resorption. Osteoprotegerin (OPG), an inhibitor of RANKL–RANK interaction, produced by gingival fibroblasts, osteoblasts, and periodontal ligament fibroblasts, abrogates immune cell RANKL-dependent and destructive osteoclastic periodontal bone resorption. OPG can enhance bone formation.
Symbols: ![]()
![Fig. 1. P. gingivalis and its presumed contribution to periodontitis via adaptive immune suppression. Following P. gingivalis infection, the outer membrane vesicles (OMVs) from P. gingivalis transfer LPS and gingipains to a lipid-binding site on an antigen presenting cell (AP cell). Gingipains erode cells cleaving CD14 and the immune cell receptor (RANKL [receptor activator of NF-κB ligand]) a TNF-related cytokine which binds to RANK, a protein expressed on the osteoclast progenitor cell activating an intracellular signaling cascade via NF-κB resulting in suppression of IL-2 secretion. Intact CD14 on membrane and soluble TLR4-MD2 (myeloid MD2= differentiation protein 2) can still function to promote the binding of LPS to the TLR4-MD2 complex and enlist intracellular cell signaling via NF-κB. The appropriate cytokine release or its suppression has implications on cellular/adaptive immune responses which involve host defective IgG. Adaptive immune responses through activated CD14 T cells and secretion of immunoglobulins (Ig) by B cells constrain the subgingival biofilm or may give rise to disease progression. T cells can have both protective and destructive roles. Inhibition of IL-2: P. gingivalis can modify adaptive immune response through interaction with dendritic cells inducing a cytokine pattern favoring T-helper 17 (Th17) lineage and inhibiting the expression and accumulation of IL-2 which attenuates T cell proliferation and communication. Arg-gingipain (Agp) protease is involved in suppression of IL-2 which contributes to self-propagation of P. gingivalis in vivo. Th17/Treg imbalance: P. gingivalis can modify adaptive immune response by interaction with dendritic or antigen presenting cells (APC cells) which induce a cytokine pattern favoring the Th17 cell population. The imbalance is also promoted by P. gingivalis proteases because IL-1β is the most Th17 supporting cytokine and is the cytokine most resistant to P. gingivalis protease cleavage modification of Th17/Treg balance which occurs by Th17 cell response inhibition and increasing Treg-cell activation. IFN-γ suppresses upregulation of programmed cell death: Secretion of IFN-γ upregulation of programmed cell death – 1 (PD-1) – receptor on CD+ cells and its ligand PD-L1 on CD11b+-T cells. Since the PD-L1/PD-1 signaling pathway inhibits the T-cell response, the changes induced by P. gingivalis on the expression of these molecules could be a mechanism by which P. gingivalis suppresses T-cell immunity. RANK-RANKL, OPG: Activated lymphocytes expressing surface receptor activator of NF-κB ligand (RANKL) can bind to surface RANK expressed on hematopoietic progenitors of osteoclasts (osteoclast progenitors) activating a signal transduction cascade leading to osteoclastogenesis (in the presence of macrophage colony–stimulating factor, MCSF). This gives rise to activation and differentiation of functional osteoclasts and periodontal bone resorption. Osteoprotegerin (OPG), an inhibitor of RANKL–RANK interaction, produced by gingival fibroblasts, osteoblasts, and periodontal ligament fibroblasts, abrogates immune cell RANKL-dependent and destructive osteoclastic periodontal bone resorption. OPG can enhance bone formation. Symbols: Display full size=suppression, Display full size=upregulated, Display full size=leads to, Display full size=contribution from, toll-like receptor 4 (TLR 4) and Display full size=from the osteoclast cell-surface receptor (RANK) and its membrane-bound ligand, Display full size=mRANKL or sRANKL (Receptor activator of nuclear factor-κB ligand), Display full size=P. gingivalis, Display full size=antibodies to P. gingivalis, Display full size=outer membrane vesicles (OMVs), Display full size=release of cytokines, Display full size=osteoprotegerin (OPG), Display full size=cell-surface receptor CD14, Display full size=RANK, a receptor expressed on the cell surface of osteoclast progenitor cells.](/cms/asset/9014dc79-a937-46ed-be8f-e32380d618d6/zjom_a_11821301_f0001_ob.jpg)
Fig. 2. P. gingivalis and its presumed contribution to atherosclerosis via adaptive immune suppression. The schematic shows an open blood vessel (black lines) with atherosclerotic plaque. Both the blood vessel and the atherosclerotic plaque contain classical immune cells (naïve and activated) with or without MHC class molecules and immunoglobulins (Y) in peripheral blood, and foam cells restricted to the atherosclerotic plaque. Cytokines in health are in balance for all immune cells. M, monocyte (naïve), MF, macrophage (activated), T cell (naïve), T cell (a) activated, DC (a) dendritic cells, B cell (a) activated, Y functional antibodies (pink border with black), non-functional antibodies (black). Increase in the serum P. gingivalis IgG confirms the involvement of adaptive/humoral immune responses of the host.
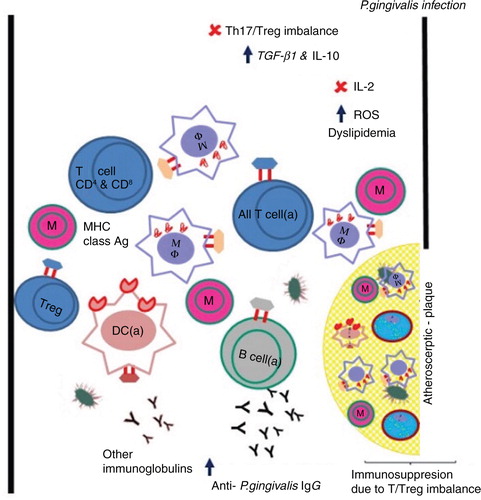
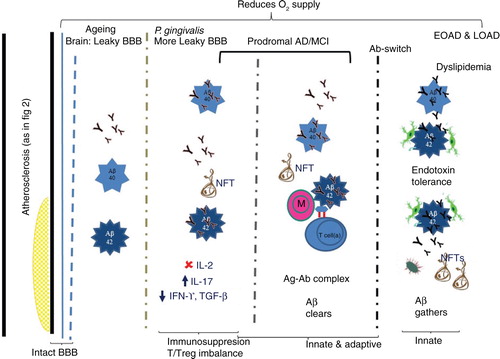
Fig. 3. P. gingivalis and its presumed contribution to AD via adaptive immune suppression. Vascular integrity with atherosclerotic plaque formation compromises blood flow and the available oxygen. Th17/Treg imbalance leads to immunosuppression. Insufficient cytokine levels (TGF-β and IFN-γ) mean that neurons in the hippocampal dentate gyrus cannot regenerate. During advancing age the blood-brain barrier (BBB) becomes leaky and if P. gingivalis infection persists, BBB becomes even more permeable. The greater opening of the BBB allows entry of larger plasma proteins into the brain. During aging, the brain shows largely Aβ1-40 deposition and few neurofibrillary tangles (NFTs). In early (prodromal/EOAD) and late-onset (LOAD) AD, copious amounts of Aβ1-40/1-42 deposits and NFTs occur. It is hypothesized that in prodromal/EOAD, Aβ deposits associate with few migrated monocytes and Tregs and functional antibodies. These are able to form antigen-antibody complexes (Ag-Ab complex) to clear Aβ deposits. In LOAD, an antibody switch takes place (IFN-γ imbalance), during which non-functional antibody is secreted, which binds Aβ, but does not promote its clearance by activated microglia from the brain. More NFTs also accumulate.
P. gingivalis
As mentioned earlier, P. gingivalis is described as a keystone pathogen in periodontitis meaning that following its initial colonization in the host, this bacterium can influence specific populations of bacterial species that reside in the same niche (Citation2). Although inflammation is a hallmark feature of gingivitis or periodontitis, P. gingivalis is not a potent inducer of inflammation, but rather a master of immune subversion and evasion (Citation10, Citation11). As an exemplar, P. gingivalis has a number of enzymes and surface membrane and capsular proteins to suppress the expression of neutrophil-recruiting chemokines (family of small cytokines, or signaling proteins secreted by cells), by eroding cells, cleaving CD14 and the immune cell receptor (receptor activator of NF-κβ ligand – RANKL), or inducing subversive cross-talk signaling between toll-like receptor (TLR-2) and other innate immune receptors like the C5aR anaphylatoxin receptor [reviewed in Refs. 2, 7]. Besides its atypical lipopolysaccharide (LPS) structure side groups (4-acyl monophosphates on lipid A), P. gingivalis LPS side groups can be poor agonists or strong antagonists of TLR4 (Citation10, Citation12). The ability of P. gingivalis to manipulate the host response and promote its chronic persistence in the periodontium also benefits from the presence of companion species and vice versa in subgingival plaque (Citation4). The microbial and immune dysbiosis, which it creates, occurs despite the fact that P. gingivalis is often only present in low numbers in diseased sites. Although the subversive effects of P. gingivalis mostly have been related to innate immunity (Citation2, Citation7) (Citation13), which is the host’s first defense met by microbial pathogens, it also has several ways to subvert the adaptive immune response within the periodontium (). Arteries and the demented brain also suffer the effects of P. gingivalis oral infection, albeit subtly, in the brain compared with atherosclerosis.
Table 1. Methods by which P. gingivalis suppresses adaptive immune responses
T cells
T cells constitute one arm of the adaptive immune system that is responsible for mounting a specific reaction against foreign antigens. They provide the impetus and signals for directing the cellular and antibody responses necessary to clear foreign pathogens and antigens.
CD4+ lymphocytes have been divided into distinct functional lineages called T-helper type 1 (Th1), Th2, Th17, and T-regulatory (Treg) cells (Citation9). T cells as well as B cells are found in inflamed periodontal tissues (Citation14, Citation15), but little is known about the role of the individual T-cell subsets in this oral condition. Studies on humans have concluded that Th1- and Th17-derived cytokines are released during the destructive periodontitis phase (active disease) and Th2- derived cytokines are released during the remission stage of periodontitis (Citation16–Citation20). Adaptive immune responses through activated CD4+ T cells and secretion of immunoglobulins (Igs) by B cells constrain the subgingival biofilm but may drive disease progression (Citation21, Citation22). T cells can therefore have both protective and destructive roles in the human host.
P. gingivalis can suppress adaptive immunity in a variety of ways (see ). In general, this may be protective against periodontal disease bone loss. However, in some cases, P. gingivalis or its antigens (and also other microorganisms) can directly stimulate a destructive response due to the presence of abundant T and B lymphocytes in diseased periodontal gingival tissues (Citation15). Activated lymphocytes express surface receptor activator of the RANKL and can bind to surface RANK expressed on hematopoietic progenitors of osteoclasts (osteoclast precursors). The binding of RANK to osteoclast progenitors activates a signal transduction cascade to initiate osteoclastogenesis in the presence of macrophage colony stimulating factor (MCSF), thus giving rise to activation and differentiation of functional osteoclasts and subsequent periodontal bone resorption (). For clarification, RANKL and RANK are separate proteins in a signaling pathway, and increased osteoclast development causes an imbalance with the number of osteoblasts resulting in increased bone resorption. Thus, it is clear that the dysregulated progressive immune responses in the periodontal inflammatory infiltrate can lead to robustly increased osteoclast frequency and activity (osteoclastogenesis) associated with active bone destruction. RANKL melds bone resorptive events with activated immune cell-mediated RANKL expression (Citation23, Citation24). It is the key intermediary linking immune cells to pathogenic bone loss in both rheumatoid arthritis (Citation25) and periodontal disease (Citation26, Citation27). Activated T cells and B cells have been linked to the pathway of immune cell involvement in pathogenic periodontal bone resorption by adoptive transfer of RANKL-expressing antigen-specific T or B cells in animal models of experimental periodontitis (Citation26, Citation28–Citation30) and also in human disease (Citation15, Citation27) (Citation30–Citation32). As mentioned earlier, B and T-lymphocytes are the primary cellular sources of RANKL expression in the bone resorptive lesions of periodontal disease. Whereas, in disease, the majority of these cells express RANKL, few, if any, immune cells from healthy tissue express RANKL (Citation15). Therefore, RANKL expressed by inflammatory immune cells are key molecules that regulate osteoclast recruitment and function (Citation24, Citation33).This indicates that RANKL is a requisite factor for the initiation of osteoclastogenesis in pathogenic bone resorption lesions. Thus, bone destruction can be caused by an inflammatory activation of the immune system in rheumatoid arthritis and in periodontitis (Citation34). However, several immunological interventions related to P. gingivalis suppression of adaptive immunity, for example, inhibition of IL-2 or inducing a cytokine pattern favoring the Th17 lineage impairment of inflammatory responses and T cell activation (see ) can alleviate RANKL-induced bone resorption and render destructive and/or protective effects.
Tregs are regulatory cells that provide immunological tolerance to the individual and actively restrain the immune system from distinguishing self from non-self (Citation35). Tregs control and suppress the activities of specific effector T cells (Teffs) and myeloid lineage cells such as dendritic cells, macrophages, and microglia that contribute to innate immunity (Citation35).
It is the balance between Th17 and Treg cells that controls inflammation and depends overall on the activation of several transcription factors which regulate the immune response through the secretion of pro- or anti-inflammatory cytokines. Forkhead box P3 (Foxp3) is a transcription factor that is necessary for the development and differentiation of T cells into Tregs. In T cell recognition and activation, CD4 and CD8 positive T cell surface molecules are important because they bind to their respective class I and class II major histocompatibility complex ligands on an antigen presenting cell.
Inhibition of IL-2
P. gingivalis can influence responses of T cell lineages to evade or suppress their adaptive responses (Citation18, Citation36) (Citation37). This is achieved by inhibiting the expression and accumulation of IL-2 (Citation38), which attenuates T cell proliferation and communication (Citation39). P. gingivalis secretes proteinases such as arg-gingipain (Rgp). Rgp protease is involved in the suppression of IL-2 accumulation which contributes toward the establishment and self-propagation of P. gingivalis in vivo. IL-2 accumulation is affected by P. gingivalis at the protein level and partially through suppression of activator protein 1 (AP-1) (Citation38). AP-1 is a transcription factor, which in this context, regulates IL-2 gene expression following stimuli from P. gingivalis infection.
Impairment of inflammatory responses and T-cell activation
T cells produce IL-10 which is a critical cytokine characterized by its anti-inflammatory properties. It is involved in the regulation of the host’s immune responses (Citation40) and plays a role in impeding resolution of infection (Citation41). It is an important cytokine produced during infection with P. gingivalis (Citation42–Citation44). Kobayashi et al. (Citation45) reported from studies on mice infected with P. gingivalis that gingival Tregs were essential players in the downregulation of inflammatory responses through their IL-10 production and others suggest that the anti-inflammatory effect of this cytokine may prevent bacterial clearance (Citation37). Kitamura et al. (Citation46) also found that P. gingivalis impairs T cell activation by cleaving CD4 and CD8 proteins on human T cells. The strategy of impeding T cell function can be used by P. gingivalis to evade the host immune system promoting the establishment and proliferation of this bacterium in the host.
Inhibition of IFN-γ production by T cells
Gaddis et al. (Citation37) showed in mice that IL-10 generated during the initial systemic exposure of host cells to P. gingivalis caused inhibition of the IFN-γ response by T cells. However, neutralization of IL-10 in naïve splenocytes and CD4+ and CD8+ T cells was more effective in increasing an IFN-γ response in cells that were primed with P. gingivalis. It therefore appears that IFN-γ increases macrophage-mediated opsonophagocytosis of P. gingivalis and other bacteria. Suppression of IFN-γ via IL-10 secretion could be another mechanism by which P. gingivalis protects itself and its co-habiting species and contributes to the chronicity of periodontal infection (Citation11).
Gaddis et al. (Citation37) also found in mice with intact TLR1 and TLR2 signaling that P. gingivalis induces the synthesis of high levels of IL-10 by CD11b+- T cells and CD4+ and CD8+ T cells. The downstream effect of IL-10 appears to inhibit IFN-γ production by CD4+ and CD8+ T cells. In this study, the fimA fimbriae of P. gingivalis were to a large extent responsible for the induction of this subversive TLR2/TLR1 signaling affecting IL-10 production (Citation37). This offers another mechanistic basis for how P. gingivalis can subvert T cell function.
Upregulation of PD-1 and its ligand PD-L1
Gaddis et al. (Citation37) reported that P. gingivalis infection mediated upregulation of programmed death 1 (PD-1) on CD+ cells and its ligand programmed death ligand 1 (PD-L1) on CD11b+-T cells. These effects appear to depend partially on IL-10 as they remained independent of TLR1/TLR2 signaling and/or the presence of fimA fimbriae. Since the PD-L1/PD-1 signaling pathway inhibits the T cell response, the changes induced by P. gingivalis on the expression of these molecules could be another mechanism by which P. gingivalis suppresses T cell immunity.
Downregulation of immune response–regulated genes
A proteomics microarray analysis in mice immunized with P. gingivalis outer membrane antigens indicated that P. gingivalis could undermine adaptive immunity by downregulating the expression of immune response–regulated genes in splenic CD4+ and CD8+ T cells (Citation47). A total of 1,141 and 1,175 genes, respectively, were downregulated, while only 5 and 288 genes, respectively, were upregulated. Sixty and 65 genes, respectively, were coded for immune response proteins or proteins important for the pathogenesis of periodontal disease. Among the downregulated genes were those encoding for cytokines, proteins involved in Ig binding, antigen presentation, innate immunity, extracellular matrix, and cell adhesion. It was clear from this study that P. gingivalis suppresses the T cell response in many ways including downregulating expression of genes which affect the CD3 complex, CD2 binding protein 2, and CD8 expression.
Inducing a cytokine pattern favoring the Th17 lineage
P. gingivalis can also modify the adaptive immune response through its interaction with dendritic cells that induce a cytokine pattern favoring the Th17 lineage. In the Moutsopoulos et al. (Citation36) investigation, the Th17 cell lineage was promoted by P. gingivalis proteases. This conclusion was reached because IL-1β released following P. gingivalis infection of primed antigen presenting (dendritic) cells appeared to be the most resistant cytokine in their study. This implied that P. gingivalis drives production of Th17-supporting cytokines (IL-1β, IL-6, IL-23) and not Th1-related cytokines such as IL-12 (Citation36). It would therefore appear that Th17/Treg cell responses play an important role in the development of chronic periodontitis and that their imbalance is involved in the pathogenesis of this disease.
Modulation of the Th17/Treg imbalance via P. gingivalis vaccination
The importance of the Th17/Treg imbalance in periodontitis was further supported by subcutaneous vaccination with P. gingivalis in a murine model. The results demonstrated that the vaccination inhibited the Th17 cell response and increased the Treg cell response affording protection to the mice from alveolar bone resorption and inflammation (Citation48). Similar results were obtained in the in vivo experiment with all-trans retinoic acid (ATRA) administration which modulated the Th17/Treg imbalance and protected against experimental periodontitis (Citation49). The likely mechanism modulating the Th17/Treg imbalance may therefore be inhibition of Th17 cell responses, increased Treg cell activation, and reduced RANKL expression on CD4+ T cells (Citation49).
In another murine model study performed by Wang et al. (Citation48), in which subcutaneous vaccination with P. gingivalis ameliorated periodontitis by modulating the Th17/Treg imbalance has been documented (Citation48). They reported that the expression levels of TGFβ-1 and IL-10 mRNA were significantly increased (p<0.01) implying an adverse role of these cytokines in modulating Th17/Treg imbalance. Furthermore, in the spleen, the ratio and number of Treg cells were significantly diminished (p<0.05) supporting an immunosuppressive role of these cytokines in their clonal expansion (Citation48). Accordingly, Th17 and Treg cells may be used to clarify the role of adaptive immune responses in periodontal disease instead of the originally proposed Th1/Th2 model (Citation19).
Suppression of IFN-γ stimulated release of IP-10, ITAC, and Mig
Jauregui et al. (Citation50) found that P. gingivalis did not induce the expression of the T cell chemokine interferon-inducible protein 10 (IP-10) or (CXCL 10) from neutrophils, peripheral blood mononuclear cells, or gingival epithelial cells. P. gingivalis also suppressed gamma interferon-stimulated release of IP-10, ITAC (CXCL11), and Mig (CXCL9) from epithelial cells. In addition, P. gingivalis inhibited IL-10 secretion in a mixed infection with the otherwise stimulatory Fusobacterium nucleatum. IP-10, ITAC, and Mig are ligands for the CXCR3 inflammatory chemokine receptor and may have important roles in T cell lymphopoiesis and the development of innate immunity and inflammation (Citation50). It was proposed that suppression of IP-10 and other Th1-biasing chemokines by P. gingivalis might destroy the balance of protective and destructive immunity in periodontal tissues, thereby extending the target range of chemokine paralysis (Citation51). Another consequence of P. gingivalis suppression of chemokine production might be the reduction of the overall antimicrobial activity since many chemokines exert antimicrobial activity similar to defensins in vitro (Citation52, Citation53).
Capsular serotypes trigger different T-lymphocyte responses
P. gingivalis occasionally synthesizes an extracellular capsule and different serotypes have been defined based on its capsular antigenicity. When dendritic cells were primed with different P. gingivalis K-serotypes, distinct T cell responses were elicited. While strains K1 (W83) and K2 (HG 184) induced a Th1/Th17 pattern immune response, K3 (A7A1-28), K4 (ATCC 49417), and K5 (HG 1690) induced a Th2 response. Accordingly, different human T lymphocyte responses can be triggered by different P. gingivalis capsular serotypes (Citation9). This supports results from a murine experimental model of periodontitis where a divergence of the systemic immune response following oral infection was observed with distinct strains of P. gingivalis and where IL-10 was associated with the least amount of alveolar bone loss (Citation54). Therefore, different strains of P. gingivalis vary in their ability to induce T cell responses in vivo.
Outer membrane vesicles contribute to local immune evasion
Local immune evasion of P. gingivalis was accomplished by its outer membrane vesicles (OMVs) which promoted monocyte unresponsiveness to live P. gingivalis (Citation55). Neutralization of IL-10 during OMV challenge partially restored monocyte responsiveness to P. gingivalis while full reactivity to this bacterium was regained by inhibiting the target of rapamycin (mTOR) intracellular signaling pathway which is the major signaling pathway for promoting TLR2-/TLR4-mediated tolerance in monocytes. It was speculated that continuous exposure to P. gingivalis OMVs can induce selective tumor necrosis factor (TNF) deficiency that makes microbial detection difficult and thus represents a possible local immune evasion strategy of P. gingivalis.
Atherosclerosis
A link between periodontitis and cardiovascular disease has been proposed (Citation56, Citation57) and in 2012 the American Heart Association released a statement supporting an association between periodontal disease and atherosclerotic vascular disease (ASVD) that is independent of known confounders (Citation58). Like periodontitis, atherosclerosis is a complex condition with a suspected microbial etiology in which P. gingivalis is attracting increasing attention for its possible role in accelerating disease progression (Citation59, Citation60). Unsurprisingly, therefore, pathogenesis of atherosclerosis is associated with both innate and adaptive immune responses. Maekawa et al. (Citation61) claimed that oral infection with P. gingivalis accelerates atheroma formation by shifting the lipid profile of the host. High titers of antibodies to P. gingivalis have been detected in patients with cardiovascular disease and stroke (Citation62). P. gingivalis invades cardiovascular cells and tissues [reviewed by Olsen and Progulske-Fox (Citation63)] and probably contributes to the progression of atherosclerosis (Citation48, Citation61) (Citation64) as depicted in .
Reduction of Tregs and TGF-β1
It is reported that in patients manifesting atherosclerosis, P. gingivalis infection reduced the number of Tregs compared with a control cohort without atherosclerosis (Citation65). Also the concentration of TGF-β1 was diminished in patients infected with P. gingivalis. TGF-β1 has an important role in the development of Tregs. As an example, gastric cancer cells can induce Treg development via producing TGF-β1 through which cross-talk between the tumor and immune cells might regulate anti-tumor immune response (Citation66). The Treg population was decreased in patients with type II fimA of P. gingivalis compared with those with other types of fimbriae. Therefore, P. gingivalis infection may be associated with dysregulation of Tregs in atherosclerosis with type II fimA as the predominant genotype.
Th17/Treg imbalance
Atherosclerosis is a chronic inflammatory disease that is regulated by T lymphocyte subsets. According to Cai et al. (Citation64), P. gingivalis infection increases Th17 responses during development of periodontitis. As for periodontitis, development of atherosclerosis was originally thought to be affected by the Th1/Th2 balance (Citation67). However, this view has changed in light of recent literature reporting that a Th17/Treg imbalance is present in patients suffering from acute coronary syndrome as well (Citation68). Since the Th17/Treg balance controls inflammation, it is plausible to suggest that the balance of these two T cell subsets may be important in both plaque destabilization and onset of this syndrome. Tregs generate large amounts of TGF-β isoforms and IL-10 and these proteins have an important role in the pathogenesis of atherosclerosis by repressing immune cell function. Cai et al. (Citation64) found that Th17 cells and Th17-related molecules are increased in the spleen and the heart compared with Th1 cells and Treg cells during atherosclerosis induced by P. gingivalis in ApoE-/- mice. This suggested a potential role of proinflammatory Th17 cell responses in the formation and progression of atherosclerosis.
P. gingivalis targets IL-2 production
As mentioned earlier, Khalaf and Bengstsson (Citation38) reported that viable P. gingivalis cells targeted IL-2 expression at the protein level. Reactive oxygen species production and Ca2+ were increased in response to P. gingivalis while the activity of transcription factors AP-1 and NF-κB fell below basal levels. T cells were not able to maintain a stableIL-2 accumulation. Rgp proteinases were probably involved in suppression of IL-2 accumulation since IL-2 was partly restored by leupeptin, but not by cathepsin inhibitor as described elsewhere (Citation38). Accumulation of IL-2 is also affected by P. gingivalis at the protein level and partially through suppression of AP-1 protein (Citation38). It was suggested that the change in IL-2 levels and thereby alteration of the adaptive immune response might contribute to progression of the inflammatory state in atherosclerosis due to the role of IL-2 in the clonal expansion of regulatory T cells.
Alzheimer’s disease
Periodontitis is implicated as a plausible risk factor for cognitive deficit via cardiovascular disease because of the systemic inflammatory burden from infections. Our unpublished data from ApoE-/- mice infected with P. gingivalis mono-infections suggest that increased carbonyl protein–related oxidative stress is present in their cerebral microvasculature following induction of experimental periodontitis and post atherosclerotic lesion appearance. Deteriorating vascular pathology could be a plausible factor in reducing the supply of sufficient oxygen to the brain (). In addition, our unpublished findings from in vitro studies indicate that Rgp proteases that are intrinsically associated with the outer surface membrane vesicles of P. gingivalis are capable of eroding cellular communication between cells. For example, shedding of surface membrane-bound CD14 receptor from neuroblastoma cell lines and loss of tight junction proteins of endothelial cells as observed from our studies in ApoE-/- P. gingivalis infected mice brains. These factors may render the blood–brain barrier (BBB) permeable to endotoxins and expose the brain cells to intrinsic and extrinsic inflammatory mediators. The central nervous system (CNS) is free from lymphatic vessels and dendritic cells and hence in AD, it is largely the innate immune system that is considered to be involved in the neuropathology. In AD, naïve T cell infiltration is not widely recognized whereas microglial cell and astroglial cell activation and their antigen presenting cell functions are accepted. It is fast becoming apparent that the strict regulation of CNS immune privilege is disturbed during aging (Citation69), and this impairment allows micro bleeds to occur and the entry of neurotoxic serum proteins into the hippocampus becomes an inevitable consequence. The hippocampus is an area related to learning and storage and retrieval of memory and its failure leads to functional loss and clinical symptoms of dementia with abnormal aggregated hallmark protein amyloid beta (Aβ) plaques and phosphorylated tau-coated neurofibrillary tangle formation. Although the innate immune system has widely been implicated in the inflammatory aspects of AD, the role of the adaptive immune arm has only been considered since the genome-wide association studies highlighted that the inability for microglia to clear Aβ from the brain may relate to several immune system genes (Citation70).
Immune reactions that affect neurodegeneration may occur from within or outside the CNS although little is known about the influence of peripheral immune cell populations in AD brains either way. Migration of antigen-specific CD4+ T cells from the periphery to the CNS with consequent immune cell interactions with resident glial cells can affect neuroinflammation and neuronal survival (Citation35). The relative numerical and functional dominance of the effector or regulatory T cells are believed to determine the destructive or protective mechanisms of these interactions.
Immune deficiency may impair clearance of Aβ from the brain
Recently, profound effects of peripherally derived neutrophils and Tregs were demonstrated in AD pathogenesis (Citation71, Citation72). To test the hypothesis that adaptive immunity affects AD pathogenesis, an immune-deficient transgenic AD mouse model (Rag-5xfAD) that lacked T cells, B cells, and natural killer (NK) cells, that is, an adaptive immune response, was found to restrain the pathogenesis of AD by modulating microglial function (Citation73). These mice exhibited a greater than twofold increase in Aβ. Therefore, adaptive immune cell populations seem to have an important role in restraining AD pathology (summarized in ). Gene expression analysis of the brain indicated altered innate and adaptive immune pathways such as changes in cytokine/chemokine signaling and decreased Ig-mediated processes.
Neuroinflammation was also strongly exacerbated in the Rag-5xfAD mice as seen through a shift in microglial phenotype, increased cytokine production, and reduced phagocytic capacity (Citation73). In contrast to this, immune-intact 5xfAD mice showed elevated levels of non-amyloid reactive IgGs in association with microglia, and treatment of Rag-5xfAD mice or microglial cells with pre-immune IgG-increased Aβ clearance (). This supported the notion that the increase in AD pathology was driven not by altered Aβ production, but rather via its impaired clearance and strongly suggested that defective antibody-mediated clearance mechanisms may be involved. Therefore, immune deficiency in humans may impair clearance of Aβ from the brain.
Additionally, the loss of B cells, T cells and NK cells in the Rag-5xfAD mouse model considerably aggravated amyloid pathogenesis, but it also increased the inflammatory phenotype of microglia while decreasing their phagocytic activity (Citation73). Peripherally derived non-amyloid reactive IgG appeared to enter the brain via a route lacking the BBB (possibly the choroid plexus) and thereby increased microglial phagocytosis of Aβ in immune-intact mice. Adaptive immune cell populations were therefore thought to play an important role in restraining AD pathology. In contrast to this, depletion of B cells and their activation by T cells caused loss of adaptive-innate immunity cross-talk and accelerated progression of the disease (). The observations also indicated that alterations in peripheral immune functions, as those occurring with age, comorbid diseases, and genetic variation, could seriously affect development and progression of AD, as suggested by Olsen and Singhrao (Citation8, Citation74). Togo et al. (Citation75) proposed that the phenotype of T cells in the AD brain is activated but not fully differentiated. Nevertheless, vaccination studies suggested that T cells and B cells have a role in the pathogenesis of this dementing disease (Citation72).
Inhibition of IFN-γ response by T cells
P. gingivalis endotoxin (LPS) has been detected in the brain of AD patients (Citation76). TLR2-mediated IL-10 production, following the initial systemic exposure to P. gingivalis, inhibits the IFN-γ T cell response. We propose that this may be a mechanism to evade the host’s immune response in the brain pending further experimental confirmation. IFN-γ is reported to increase neurogenesis in wild-type mice and in a mouse model of AD (Citation77), and its lack due to P. gingivalis infection will contribute overall to greater neuronal loss as neurogenesis will be halted. From studies on mice, it was also suggested that IFN-γ plays a role in neuronal protection and repair and that this enhanced neurogenesis possibly represents the normal function of the immune system in controlling brain inflammation and repair (Citation77, Citation78).
Tregs’ immunosuppressive effect may be harmful
Tregs normally act to suppress excessive immune responses and protect against autoimmune disease onset (Citation79, Citation80). Notably, from AD mouse models, it seems that this immunosuppressive activity may be detrimental in impairing the adaptive immune system from responding to and restraining AD pathology (Citation73). Thus, when Tregs are depleted, Aβ pathology is dramatically reduced. This was found in 5xFAD mice where transient depletion of Foxp3+ Tregs or pharmacological prevention of their activity was followed by Aβ clearance, mitigation of neuroinflammatory response, and reversal of cognitive decline (Citation71). On the contrary, compounds that promote Treg differentiation and function increase AD pathology. If the observations from adaptive immune-deficient mice correlate with data from successful pharmacological prevention of Treg activity in human AD, this would identify a previously unknown but significant role for the adaptive immune system in this disease process.
Baruch et al. (Citation71) identified systemic Foxp3+ Treg-mediated immunosuppression as a negative player in AD pathology acting at least in part by reducing the choroid plexus IFN-γ availability necessary for gateway activity in orchestrating recruitment of leukocytes to the CNS. When a reparative immune response is required in the brain, the ability to mount this response is limited by systemically derived Tregs. Also, pharmacological approaches interfering with Foxp3+ Treg cell activity demonstrated the existence of this inverse relationship (Citation71). Furthermore, timely and transient depletion of Tregs was associated with a neuroprotective effect in 5xFAD transgenic mice.
Age and endotoxin tolerance cause immune modulation
Age-related changes in the adaptive immune system have been well documented such as altered cytokine patterns and a decline in antigen presenting function (Citation81) and decreased function of macrophages, NK cells, and lymphocytes (Citation82). Sun et al. (Citation83) found that endotoxin tolerance is a case of immune modulation and reprogramming rather than a global downregulation of cytokine expression and function. Their studies were based on endotoxin tolerance induced by P. gingivalis and Escherichia coli in young (2 months) and middle-aged (12 months) mice following a single or repeated LPS stimulation.
Impaired immune tolerance to P. gingivalis LPS was found to promote neutrophil migration and decreased apoptosis in a monocytic cell line (Citation84). Such a delay in neutrophil apoptosis might prolong the inflammatory response with increased potential for vital neuronal cell and tissue damage.
Concluding remarks
Like the innate immune system, P. gingivalis also affects adaptive immunity in several ways as indicated here for periodontitis and related systemic diseases like atherosclerosis and AD. Notably, P. gingivalis uses many of the methods to evade adaptive immunity in periodontitis as it does in systemic diseases and possibly AD. Therefore, it would not be surprising to suggest that P. gingivalis may have co-evolved with the host’s immune defense developing strategies to overcome protective host barriers and to modulate the host’s defense systems to its own advantage. This has been attributed to P. gingivalis being able to endure inflammation and also being able to exploit inflammation for its own sustenance and survival. In addition, P. gingivalis can invade periodontal, atherosclerotic, and brain tissue, thereby avoiding immune surveillance and maintaining its viability. By these efforts and through a plethora of other virulence factors, it may act as a keystone organism both in periodontitis and related systemic diseases and other remote body inflammatory pathologies including dementia.
Periodontal pathogenic bacteria play a key role in adaptive immune-mediated bone destruction by activated immune cell RANKL expression osteoclastogenesis (see section on T cells, and ). P. gingivalis can suppress this adaptive immune response which can also be protective [for review, see Ref. (Citation85)]. Immunological mechanisms to interfere with immune cell-mediated bone resorption have also been reviewed (Citation86). Thus, a new concept of periodontal pathogenesis therapeutic strategy utilizing suppressive adaptive immunity induced by P. gingivalis may reduce some immune-mediated periodontal disease bone resorption (Citation82) and ameliorate the disease (Citation87).
Thus, induction of P. gingivalis suppression of adaptive immunity can represent a unique type of immune therapy to ameliorate immune cell–mediated, RANKL-dependent osteoclastogenesis and periodontal bone resorption (Citation88). This novel induction of immune therapy and direct injection of anti-RANKL antibody (Citation89) and other antibody to pathogenesis factors (Citation90) point to the significance of immune therapy as a potentially powerful therapeutic antagonist of diseased periodontal bone resorption.
However, P. gingivalis is not alone in being responsible for disease development neither in periodontal nor in systemic diseases or dementia. Although it can modulate the growth of other bacteria in the subgingival biofilm, periodontitis and its related systemic diseases are not the effect of a single bacterium. Each bacterium in a cluster with quorum sensing-like properties may affect the responses induced by others and vice versa. Unfortunately, at present, we are far from understanding the complex microbial-host interactions or the inter-microbial interactions that promote either health or disease in vivo.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
Acknowledgements
Ingar Olsen acknowledges funding through the European Commission (FP7-HEALTH-306029 ‘TRIGGER’). Daniel J Smith is acknowledged for his help with the manuscript. Sim K. Singhrao acknowledges the continued financial support for her research by the University of Central Lancashire.
References
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol. 2007; 13(Suppl 4): 3–10.
- Hajishengallis G, Darveau RP, Curtis MA. The keystone pathogen hypothesis. Nat Rev Microbiol. 2012; 10: 717–25. doi: http://dx.doi.org/10.1038/nrmicro2873.
- How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016; 7: 53. doi: http://dx.doi.org/10.3389/fmicb.2016.00053.
- Hajishengallis G. Immune evasion strategies of Porphyromonas gingivalis. J Oral Biosci. 2011; 53: 233–40. doi: http://dx.doi.org/10.2330/joralbiosci.53.233.
- Olsen I, Yilmaz Ö.Modulation of inflammasome activity by Porphyromonas gingivalis in periodontitis and associated systemic diseases. J Oral Microbiol. 2016; 8: 30385. doi: http://dx.doi.org/10.3402/jom.v8.30385.
- Olsen I, Singhrao SK. Inflammasome involvement in Alzheimer’s disease. J Alzheimers Dis. 2016; 54: 45–53.
- Olsen I, Hajishengallis G. Major neutrophil functions subverted by Porphyromonas gingivalis. J Oral Microbiol. 2016; 8: 30936. doi: http://dx.doi.org/10.3402/jom.v8.30936.
- Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer’s disease?. J Oral Microbiol. 2015; 7: 29143. doi: http://dx.doi.org/10.3402/jom.v7.29143.
- Vernal R, Diaz-Guerra E, Silva A, Sanz M, Garcia-Santz JA. Distinct human T-lymphocyte responses triggered by Porphyromonas gingivalis capsular serotypes. J Clin Periodontol. 2014; 41: 19–30. doi: http://dx.doi.org/10.1111/jcpe.12176.
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012; 91: 816–20.
- Hajishengallis G. Editorial: a toll gate to escape T cells. J Leukoc Biol. 2013; 93: 3–5. doi: http://dx.doi.org/10.1189/jlb.0912465.
- Coats SR, Jones JW, Braham PH, Do CT, Braham PH, Bainbridge BW, etal. Human toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4’-phosphatase activities. Cell Microbiol. 2009; 11: 1587–99. doi: http://dx.doi.org/10.1111/j.1462-5822.2009.01349.x.
- Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm. 2015; 2015: 137357. doi: http://dx.doi.org/10.1155/2015/137357.
- Teng YT. Protective and destructive immunity in the periodonticum. Part 2 – T-cell mediated immunity in the periodontium. J Dent Res. 2006; 85: 209–19.
- Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, etal. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006; 169: 987–98.
- Takeichi D, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000; 79: 1548–55.
- Vernal R, Dutzan N, Chaparro A, Puente J, Valenzuela MA, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005; 32: 383–9. doi: http://dx.doi.org/10.1111/j.1600-051X.2005.00684.x.
- Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007; 43: 14–40.
- Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008; 87: 817–28.
- Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011; 3: 5304. doi: http://dx.doi.org/10.3402/jom.v3i0.5304.
- Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999; 67: 2804–9.
- Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 2000; 68: 5864–8.
- Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a common link between osteoclastogenesis, lymph node formation and lymphocyte development. Immunol Cell Biol. 1999; 77: 188–93.
- Theill LE, Boyle WJ, Penninger JM. RANKL-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002; 20: 795–823.
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, etal. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999; 402: 304–9.
- Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, etal. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000; 106: R59–67.
- Taubman MA, Kawai T. Involvement of T lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001; 12: 125–35.
- Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000; 164: 2102–9.
- Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Min Res. 2004; 19: 155–64.
- Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006; 176: 625–31.
- Taubman MA, Ebersole JL, Smith DJ. Genco RJ, Mergenhangen SE. Association between systemic and local antibody and periodontal diseases. Host parasite interactions in periodontal diseases. 1982; Washington, DC: American Society for Microbiology. 283–98.
- Yoshie H, Taubman MA, Olson CL, Ebersole JL, Smith DJ. Periodontal bone loss and immune characteristics after adoptive transfer of Actinobacillus-sensitized T cells to rats. J Periodontal Res. 1987; 22: 499–505.
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999; 20: 345–57.
- Valverde P, Kawai T, Taubman MA. Potasium channel-blockers as therapeutic agents to interfere with bone resorption of periodontal disease. J Dent Res. 2005; 84: 488–99.
- Anderson KM, Olson KE, Estes KA, Flanagan K, Gendelman HE, Mosley RL. Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders. Transl Neurodegener. 2014; 3: 25. doi: http://dx.doi.org/10.1186/2047-9158-3-25.
- Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, etal. P. gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012; 39: 294–303. doi: http://dx.doi.org/10.1016/j.jaut.2012.03.003.
- Gaddis DE, Maynard CL, Weaver CT, Michalek SM, Katz J. Role of TLR2-dependent IL-10 production in the inhibition of the initial IFN-γ T cell response to Porphyromonas gingivalis. J Leukoc Biol. 2013; 93: 21–31. doi: http://dx.doi.org/10.1189/jlb.0512220.
- Khalaf H, Bengtsson T. Altered T-cell responses by the periodontal pathogen Porphyromonas gingivalis. PLoS One. 2012; 7: e45192. doi: http://dx.doi.org/10.1371/journal.pone.0045192.
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naïve CD4+CD25- cells to CD25+ Fox3+ regulatory T cells and for expansion of these cells. J Immunol. 2007; 178: 2018–27.
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19: 683–765.
- Filippi CM, von Herrath MG. IL-10 and the resolution of infections. J Pathol. 2008; 214: 224–30.
- Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003; 82: 632–5.
- Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004; 39: 432–41.
- Herminajeng E, Sosroseno W, Bird PS, Seymour GJ. The effects of interleukin-10 depletion in vivo on the immune response to Porphyromonas gingivalis in a murine model. J Periodontol. 2001; 72: 1527–34.
- Kobayashi R, Kono T, Bolerjack BA, Fukuyama Y, Gilbert RS, Fujihashi M, etal. Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J Dent Res. 2011; 90: 653–8. doi: http://dx.doi.org/10.1177/0022034510397838.
- Kitamura Y, Matono S, Aida Y, Hirofuji T, Maeda K. Gingipains in the culture supernatant of Porphyromonas gingivalis cleave CD4 and CD8 on human T cells. J Periodontal Res. 2002; 37: 464–8.
- Gemmell E, Drysdale KE, Seymour GJ. Gene expression in splenic CD4 and CD8 cells from BALB/c mice immunized with Porphyromonas gingivalis. J Periodontol. 2006; 77: 622–33. doi: http://dx.doi.org/10.1902/jop.2006.050211.
- Wang L, Guan N, Jin Y, Lin X, Gao H. Subcutaneous vaccination with Porphyromonas gingivalis ameliorates periodontitis by modulating Th17/Treg imbalance in a murine model. Int Immunopharmacol. 2015; 25: 65–73. doi: http://dx.doi.org/10.1016/j.intimp.2015.01.007.
- Wang L, Wang J, Jin Y, Gao H, Lin X. Oral administration of all-trans retinoic acid suppresses experimental periodontitis by modulating the Th17/Treg imbalance. J Periodontol. 2014; 85: 740–50. doi: http://dx.doi.org/10.1902/jop.2013.130132.
- Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun. 2013; 81: 2288–95. doi: http://dx.doi.org/10.1128/IAI.00264-13.
- Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis . Infect Immun. 1998; 66: 1660–5.
- Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001; 167: 623–7.
- Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, etal. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J Leukoc Biol. 2003; 74: 448–55. doi: http://dx.doi.org/10.1189/jlb.0103024.
- Marchesan JT, Morelli T, Lundy SK, Jiao Y, Lim S, Inohara N, etal. Divergence of the systemic immune response following oral infection with distinct strains of Porphyromonas gingivalis. Mol Oral Microbiol. 2012; 27: 483–95. doi: http://dx.doi.org/10.1111/omi.12001.
- Waller T, Kesper L, Hirschfeld J, Dommisch H, Kölpin J, Oldenburg J, etal. Porphyromonas gingivalis outer membrane vesicles induce selective TNF tolerance in a TLR4- and mTOR-dependent manner. Infect Immun. 2016; 84: 1194–204. doi: http://dx.doi.org/10.1128/IAI.01390-15.
- Mattila KJ, Valle MJ, Nieminen MS, Valtonen VV, Hietaniemi KL. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993; 103: 205–11.
- Matilla K, Valtonen NM, Rasi V, Huttunen J. Association between dental health and acute myocardial infection. BMJ. 1989; 298: 779–82.
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, etal. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012; 125: 2520–44.
- Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol. 2009; 36(Suppl 10): 15–19. doi: http://dx.doi.org/10.1111/j.1600-051X.2009.01417.x.
- Miyauchi S, Maekawa T, Aoki Y, Miyazawa H, Tabeta K, Nakajima T, etal. Oral infection with Porphyromonas gingivalis and systemic cytokine profile in C57BL/6.KOR-ApoEshl mice. J Periodontal Res. 2012; 47: 402–8. doi: http://dx.doi.org/10.1111/j.1600-0765.2011.01441.x.
- Maekawa T, Takahashi N, Tabeta K, Aoki Y, Miyashita H, Miyauchi S, etal. Chronic oral infection with Porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PLoS One. 2011; 6: e20240. doi: http://dx.doi.org/10.1371/journal.pone.0020240.
- Pussinen PJ, Alfthan G, Jousilathi P, Paju S, Tuomilehto J. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis. 2007; 193: 222–8. doi: http://dx.doi.org/10.1016/j.atherosclerosis.2006.06.027.
- Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. 2015; 7: 28788. doi: http://dx.doi.org/10.3402/jom.v7.28788.
- Cai Y, Kobayashi R, Hashizume-Takizawa T, Kurita-Ochiai T. Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch Oral Biol. 2014; 59: 1183–91. doi: http://dx.doi.org/10.1016/j.archoralbio.2014.07.012.
- Yang J, Wu J, Liu Y, Huang J, Lu Z, Xie L, etal. Porphyromonas gingivalis infection reduces regulatory T cells in infected atherosclerotic patients. PLoS One. 2014; 9: e86599. doi: http://dx.doi.org/10.1371/journal.pone.0086599.
- Yuan XL, Chen L, Zhang TT, Ma YH, Zhou YL, Zhao Y, etal. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-β1. World J Gastroenterol. 2011; 17: 2019–27. doi: http://dx.doi.org/10.3748/wjg.v17.i15.2019.
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, etal. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997; 389: 737–42.
- Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, etal. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008; 127: 89–97. doi: http://dx.doi.org/10.1016/j.clim.2008.01.009.
- Montagne A, Pa J, Zlokovic BV. Vascular plasticity and cognition during normal aging and dementia. JAMA Neurol. 2015; 72: 495–6. doi: http://dx.doi.org/10.1001/jamaneurol.2014.4636.
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, etal. Alzheimer genetic analysis group. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013; 368: 117–27. doi: http://dx.doi.org/10.1056/NEJMoa1211851.
- Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, etal. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015; 6: 7967. doi: http://dx.doi.org/10.1038/ncomms8967.
- Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, etal. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015; 21: 880–6. doi: http://dx.doi.org/10.1038/nm.3913.
- Marsh SE, Abud EM, Lakatos A, Karimzadeh A, Yeung ST, Davtyan H, etal. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc Natl Acad Sci USA. 2016; 113: E1316–25. doi: http://dx.doi.org/10.1073/pnas.1525466113/-/DC Supplemental.
- Singhrao SK, Harding A, Chukkapalli S, Olsen I, Kesavalu L, Crean S. Apolipoprotein E related co-morbidities and Alzheimer’s disease. J Alzheimers Dis. 2016; 51: 935–48. doi: http://dx.doi.org/10.3233/JAD150690.
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, etal. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002; 124: 83–92.
- Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 2013; 36: 665–77. doi: http://dx.doi.org/10.3233/JAD-121918.
- Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A. IFN-γ enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 2008; 22: 2843–52. doi: http://dx.doi.org/10.1096/fj.08-105866.
- Hohlfeld R. Neurotrophic cross-talk between the nervous and immune systems: relevance for repair strategies in multiple sclerosis?. J Neurol Sci. 2008; 265: 93–6. doi: http://dx.doi.org/10.1016/j.jns.2007.03.012.
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007; 8: 191–7.
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133: 775–87.
- Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002; 80: 243–84.
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004; 76: 291–9. doi: http://dx.doi.org/10.1189/jlb.1103592.
- Sun Y, Li H, Yang M-F, Shu W, Sun M-J, Xu Y. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. PLoS One. 2012; 7: 139224. doi: http://dx.doi.org/10.1371/journal.pone.0039224.
- Zaric S, Shelburne C, Darveau R, Quinn DJ, Weldon S, Taggart CC, etal. Impaired immune tolerance to Porphyromonas gingivalis lipopolysaccharide promotes neutrophil migration and decreased apoptosis. Infect Immun. 2010; 78: 4151–6. doi: http://dx.doi.org/10.1128/IAI.00600-10.
- Kajiya M, Giro G, Taubman MA, Han X, Mayer MP, Kawai T. Role of periodontal pathogenic bacteria in RANKL-mediated bone destruction in periodontal disease. J Oral Microbiol. 2010; 2: 5532. doi: http://dx.doi.org/10.3402/jom.v2i0.5532.
- Han X, Kawai T, Taubman MA. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol 2000. 2007; 45: 76–94.
- Taubman MA, Kawai T, Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J Clin Periodontol. 2007; 34: 367–9.
- Han X, Lin X, Yu X, Lin J, Kawai T, LaRosa KB, etal. Porphyromonas gingivalis infection-associated periodontal bone resorption is dependent on receptor activator of NF-κB ligand. Infect Immun. 2013; 81: 1502–9.
- Lin X, Han X, Kawai T, Taubman MA. Antibody to receptor activator of NF-κB ligand ameliorates T cell-mediated periodontal bone resorption. Infect Immun. 2011; 79: 911–17.
- Gaffen SL, Herzberg MC, Taubman MA, Van Dyke TE. Recent advances in host defense mechanisms/therapies against oral infectious diseases and consequences for systemic disease. Adv Dent Res. 2014; 26: 30–7. doi: http://dx.doi.org/10.1177/0022034514525778.
