Abstract
Objective
Alteration of the antioxidant status in the kidneys may be related to lead (Pb) intoxication. The present study aimed to investigate the possible beneficial effect of thymoquinone (TQ), the major active ingredient of the volatile oil of Nigella sativa seeds, on Pb-induced renal antioxidant defense system impairment.
Methods
A total of thirty two healthy adult male Wistar rats were randomly divided into four equal groups as follows: a control group, which received no treatment; a Pb group, which was exposed to 2,000 ppm of Pb acetate in drinking water; a Pb-TQ group, which was cotreated with Pb plus TQ (5 mg/kg/day, per os); and a TQ group receiving only TQ. All treatments were applied for five weeks.
Results
TQ alone did not induce any significant changes in the antioxidant defense system. By contrast, Pb exposure significantly decreased reduced glutathione level and superoxide dismutase, glutathione peroxidase, catalase, and glutathione reductase activities in the renal tissue. Interestingly, supplementation with TQ significantly improved the affected antioxidant parameters.
Conclusion
Our data are the first to provide evidence on the protective effect of TQ against Pb-induced renal antioxidant capacity impairment and suggest that this component might be a clinically promising alternative in Pb nephrotoxicity.
Lead (Pb) is a very toxic, non-essential heavy metal. It is present in storage batteries, water pipes, plastic, glass, ceramics, paints, insecticides, and leaded gasoline. Exposure to Pb is impossible to avoid because it is ubiquitous and persistent in the environment. Pb poisoning has been reported since the discovery of Pb thousands of years ago, and it remains a major health issue worldwide. Air, water, soil, food, and consumer products are the major routes of human exposure to Pb. The current approved treatment for Pb poisoning is to administer chelating agents that form an insoluble complex with the metal and remove it from tissue. Nevertheless, these chelating agents have no effect on low levels of exposure and are incapable of removing metal from intracellular sites; in addition, they cause many undesirable side effects (Citation1).
After absorption, Pb accumulates primarily in the kidneys (Citation2), making it a critical target organ for Pb toxicity. Accumulated Pb in kidneys impairs the endogenous antioxidant defense system by inhibiting the main antioxidant enzymes (superoxide dismutase [SOD], glutathione peroxidase [GPX], catalase [CAT], glutathione reductase [GR], and glutathione S-transferase). It also depletes reduced glutathione (GSH), the most important intracellular non-enzymatic antioxidant (Citation3).
Medicinal plants nowadays are an important source of drug synthesis and at least a third of the current drugs are derived from plants (Citation4). The seeds of Nigella sativa are known to have healing potential and have been used for more than 2,000 years as folk medicine in the Middle East and Far East for a wide range of diseases (Citation5). They contain more than 30% fixed oil and 0.40–0.45% (w/w) volatile oil (Citation6).
Thymoquinone (TQ) (2-isopropyl-5-methyl-1,4-benzoquinone), first extracted by El-Dakhakhny (Citation7), is the main active and most abundant component (18.4–24%) of the essential oil of N. sativa seeds (Citation8). It is structurally homologous with the coenzyme Q, which is an important antioxidant in the electron transport chain. The compound can be readily synthesized in gram quantities by oxidation of thymol with hydrogen peroxide (Citation9). TQ oral administration is followed by slow absorption but relatively faster elimination (Citation10). It could lead to biotransformation due to the metabolizing activity of liver enzymes such as DT-diaphorase (a quinine reductase), which catalyzes the reduction of TQ into a dihydrothymoquinone (Citation11). This metabolite exhibits antioxidant properties stronger than those of TQ and similar to those of Trolox, which is considered a standard antioxidant compound (Citation12). In rats, the LD50 of TQ was 794.3 mg/kg and 57.5 mg/kg for oral and intraperitoneal administration, respectively (Citation13). TQ has various pharmacological effects such as antihypertensive (Citation14), anticancer (Citation15), antidiabetic (Citation16), anti-inflammatory (Citation17), and analgesic properties (Citation18). TQ is also reported to possess strong antioxidant properties (Citation19). The high biological activity and low systemic toxicity of TQ make it a promising alternative to conventional therapeutic drugs (Citation19).
The influence of TQ on Pb-induced nephrotoxicity has not previously been studied. Hence, the present study is the first to investigate the potential protective effect of TQ oral supplementation against Pb-induced renal antioxidant defense disruption.
Materials and methods
Chemicals
Pb acetate trihydrate ([C2H3O2]2Pb. 3H2O) and TQ (2-isopropyl-5-methyl-1,4-benzoquinone) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals used were of the best analytical grade.
Animals
Healthy adult (4-month-old) male Wistar rats, weighing 200–230 g, obtained from the Tunisian Society of Pharmaceutical Industries, were used in this study. The animals were housed in plastic cages (free from any source of chemical contamination) with free access to tap water (free from Pb) and standard diet. The rats were kept at 22±3°C, in a natural light/dark cycle, with 55% humidity and a ventilation system. The experiments were started after the animals were allowed to adapt to the laboratory conditions for a week. All experimental procedures in this study were in full compliance with the European Council Directive (86/609/EEC) and approved by the institutional bioethics committee.
Experimental design
After the acclimation period, the rats were randomly divided into four groups of eight animals each and were treated for 5 weeks as follows: the control group received tap water; the Pb group received an aqueous solution containing 2,000 ppm of Pb acetate (0.2% w/v) (Citation20–Citation22); the Pb-TQ group was cotreated with Pb (as in the Pb group) plus TQ (5 mg/kg bw/day); and the TQ group received tap water and was given TQ (5 mg/kg bw/day) (Citation23–Citation25). TQ was administered by gastric tube daily between 8:00 and 9:00 am. At the end of the treatment period, the animals were euthanized by exsanguination through cardiac puncture under diethyl ether anesthesia.
Tissue collection
The kidneys were removed quickly from the rats, cleared of adhesive tissue, washed in ice-cold 0.9% (w/v) NaCl solution, and frozen at −80°C until assayed.
Biochemical assays
The kidney tissue was homogenized in 10 volumes of ice-cold phosphate-buffered saline (136.75 mM NaCl, 2.68 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4) and the homogenates were centrifuged at 3,500×g for 15 min at 4°C. The supernatant fractions were collected and used in biochemical analysis.
SOD (Citation26), GPX (Citation27), and GR (Citation28) activities were determined in the kidney homogenates using commercial kits (Randox Laboratories Ltd., Crumlin, UK). CAT activity was determined according to the ferrithiocyanate method of Cohen et al. (Citation29). The GSH level was determined spectrophotometrically using the method previously described by Ellman (Citation30).
Statistical analysis
The results were expressed as mean±SEM. Comparisons between the groups were performed by Student's t-test. Differences were considered statistically significant at p<0.05.
Results
Antioxidant enzyme activities
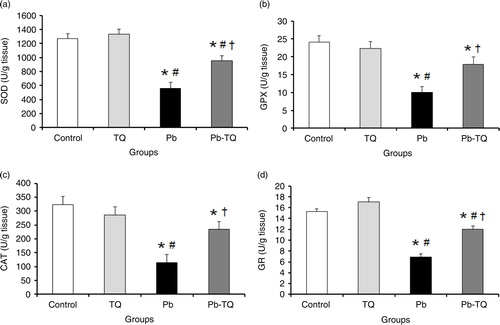
As shown in A through D, the kidney activities of SOD, GPX, CAT, and GR in the rats receiving TQ alone were not significantly different (p>0.05) from those of the control group; following Pb treatment, the activities of these enzymes were significantly decreased (p<0.05) by 56.11, 58.69, 64.7, and 54.78%, respectively. Interestingly, TQ coadministration significantly attenuated (p<0.05) the deleterious effect of Pb on the activities of these antioxidant enzymes. In fact, in the rats cotreated with Pb and TQ, the renal activities of SOD, GPX, CAT, and GR significantly increased (p<0.05) by 70.57, 78.85, 106.5, and 73.91%, respectively, in relation to the Pb-intoxicated rats.
Fig. 1 Effects of lead (Pb), thymoquinone (TQ), and their coadministration on the kidney activities of superoxide dismutase (SOD, a), glutathione peroxidase (GPX, b), catalase (CAT, c), and glutathione reductase (GR, d) in rats after five weeks. Values are expressed as mean±SEM of eight animals. Student's t-test: *p<0.05 versus control; # p<0.05 versus TQ-treated rats; † p<0.05 versus Pb-treated rats.
GSH levels
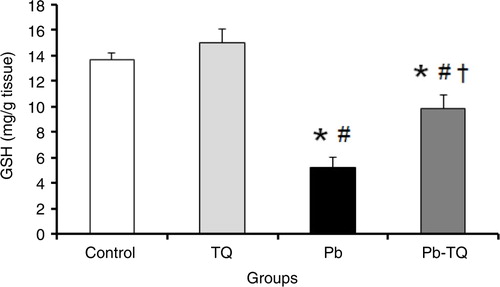
The results presented in indicate that the administration of TQ alone had no significant effect (p>0.05) on kidney GSH levels compared to those of the control rats. By contrast, Pb exposure caused a significant decrease (p<0.05) of about 61.8% in the concentration of this non-enzymatic antioxidant in relation to the control rats. This effect was significantly mitigated (p<0.05) by 55.16% when the Pb-treated animals simultaneously received TQ.
Fig. 2 Effects of lead (Pb), thymoquinone (TQ), and their coadministration on the kidney level of reduced glutathione (GSH) in rats after five weeks. Values are expressed as mean±SEM of eight animals. Student's t-test: *p<0.05 versus control; # p<0.05 versus TQ-treated rats; † p<0.05 versus Pb-treated rats.
Discussion
Pb is a pervasive environmental and industrial pollutant with no beneficial biological role, and its toxicity continues to be a major public health problem throughout the world. Recent studies point to the potential involvement of the cell's antioxidant capacity failure in the pathogenesis of Pb poisoning, suggesting that exogenous antioxidants may play an effective protective effect. In the present study, we adopted an in vivo experimental animal model to investigate whether TQ could maintain renal intracellular antioxidant reserves in Pb subchronic treatment.
The metalloproteins SOD, GPX, and CAT are the major antioxidant enzymes. Their activities were used to assess oxidative stress in cells. SOD catalyzes the dismutation of O2 ·− to H2O2 and O2. Because H2O2 is still harmful to cells, CAT and GPX further catalyze the decomposition of H2O2 to water. In the reaction catalyzed by GPX, GSH is oxidized to GSSG, which can then be reduced back to GSH by GR. In the present study, we found that treatment with Pb for 5 weeks significantly decreased the activities of SOD, GPX, CAT, and GR in the rat kidney. These results are in concordance with previous findings (Citation3, Citation31) (Citation32).
It has been shown that Pb directly alters antioxidant activities by irreversible direct binding to functional sulfhydryl (SH) groups of several enzymes such as SOD, GPX, CAT, and GR (Citation33). Because Pb interferes with the metabolism of essential trace elements such as copper, zinc, selenium, and iron needed for proper molecular structure and enzymatic activity (Citation2), the antioxidant enzymes could be a potential target for Pb toxicity. The decrease in antioxidant enzyme activities may be explained by the downregulation of antioxidant enzyme mRNA expression (Citation34).
GSH is a tripeptide-containing cysteine that has a reactive SH group with reductive potency. Accordingly, GSH plays a vital role in the protection of cells against oxidative stress. It can act as a non-enzymatic antioxidant by direct interaction of SH groups with reactive oxygen species, or it can be involved in the enzymatic detoxification reactions for reactive oxygen species, as a cofactor or a coenzyme. In agreement with recent investigations studying the effect of Pb in the kidneys of rats and mice (Citation35, Citation36), our data show that Pb treatment significantly lowered the renal GSH level.
As for antioxidant enzymes, Pb can damage GSH directly and/or indirectly. The reduction in concentration of GSH may be due to the high affinity of Pb to the SH groups of this tripeptide, thereby interfering with its antioxidant activity (Citation33). Pb can also decrease the level of GSH by inhibiting the activities of GSH metabolizing enzymes, such as GR, GST, and glucose-6-phosphate dehydrogenase, by blocking their SH groups (Citation37). Further, the reduction of GSH synthesis can be proposed as another explanation.
Despite extensive research now focusing on herbal products as alternative medicines, no evidence has been reported in the literature regarding the role of TQ against Pb-induced renal toxicity. In the present study, cotreatment of Pb-exposed rats with TQ significantly improved the altered antioxidant defense system in the kidneys.
Our results are in consonance with recent literature data indicating that oral supplementation of TQ (10 mg/kg/day, 15 days) protects rat kidneys against sodium arsenite–induced depletion of antioxidant enzyme activities (SOD, GPX, and CAT) (Citation38). Furthermore, Farag et al. (Citation39) reported that TQ (10 mg/kg/day, 28 days) prevented reduction in kidney SOD activity and GSH levels provoked by chronic treatment with cyclosporine A, an immunosuppressant drug, and by acute renal ischemia/reperfusion in rats. In addition, Samarghandian et al. (Citation40) reported that TQ enhances the declined renal antioxidant status in gentamicin-treated rats. TQ (10 mg/kg/day, 10 days, po) also reversed a renal decrease in CAT activity and GSH concentration in rats receiving methotrexate, an anticancer drug (Citation41).
The restoration of tissue antioxidant function by TQ clearly demonstrated in the current work could be attributed to its ability to upregulate antioxidant gene expression (Citation42, Citation43).
The ability of TQ to correct the disrupted antioxidant system, as demonstrated in the present research, does not precisely mean that kidney oxidative stress can be decreased. Numerous previous data showed that this component has powerful free radical scavenging activity (Citation12), Citation14, Citation44–(Citation46), which may be related to the redox properties of the quinone structure of TQ molecule and to its unrestricted crossing of morphophysiological barriers to access subcellular compartments (Citation46). The effect of TQ on endogenous antioxidants has been relatively poorly studied. Thus, in the current work we are limited to some antioxidant markers.
In conclusion, our results clearly indicate that TQ oral supplementation, at a safe dose, protects against Pb-induced cellular antioxidant defense system depletion in rat kidneys. Our findings suggest that TQ may be a clinically promising agent in Pb nephrotoxicity.
Conflict of interest and funding
The authors declare that there are no conflicts of interest. This work was supported by funds allocated to the Genetic, Genotoxicity and Childhood Illness Research Unit (UR12ES10) by the Tunisian Ministry of Higher Education and Scientific Research.
Authors' contributions
AM and HBC collected the data, and AM also analyzed the data, designed the study, and wrote the paper.
Acknowledgements
The authors thank Prof. Mohsen Sakly (Laboratory of Integrative Physiology, Faculty of Sciences of Bizerte, Tunisia) for his help.
References
- Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010; 7: 2745–88. [PubMed Abstract] [PubMed CentralFull Text].
- Azoz HA, Raafat RM. Effect of lead toxicity on cytogenisity, biochemical constituents and tissue residue with protective role of activated charcoal and casein in male rats. Aust J Basic Appl Sci. 2012; 6: 497–509.
- Dewanjee S, Sahu R, Karmakar S, Gangopadhyay M. Toxic effects of lead exposure in Wistar rats: involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves. Food Chem Toxicol. 2013; 55: 78–91. [PubMed Abstract].
- Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med. 2008; 23: 854–9. [PubMed Abstract] [PubMed CentralFull Text].
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, etal. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013; 3: 337–52. [PubMed Abstract] [PubMed CentralFull Text].
- Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003; 17: 299–305. [PubMed Abstract].
- El-Dakhakhny M. Studies on the chemical constitution of Egyptian Nigella sativa L. seeds. The essential oil. Planta Med. 1963; 11: 465–70.
- Al-Saleh IA, Billedo G, El-Doush II. Levels of selenium, dl-α-tocopherol, dl-γ-tocopherol, all-trans-retinol, thymoquinone and thymol in different brands of Nigella sativa seeds. J Food Compos Anal. 2006; 19: 167–75.
- Dockal ER, Cass QB, Brocksom TJ, Brocksom U, Corrěa AG. A simple and efficient synthesis of thymoquinone and methyl p-benzoquinone. Synthetic Commun. 1985; 15: 1033–6.
- Alkharfy KM, Ahmad A, Khan RM, Al-Shagha WM. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur J Drug Metab Pharmacokinet. 2015; 40: 319–23. [PubMed Abstract].
- Nagi MN, Almakki HA. Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: possible role in protection against chemical carcinogenesis and toxicity. Phytother Res. 2009; 23: 1295–8. [PubMed Abstract].
- Khalife KH, Lupidi G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic Res. 2007; 41: 153–61. [PubMed Abstract].
- Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad. 2008; 20: 25–7. [PubMed Abstract].
- Khattab MM, Nagi MN. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother Res. 2007; 21: 410–14. [PubMed Abstract].
- El-Far AH. Thymoquinone anticancer discovery: possible mechanisms. Curr Drug Discov Technol. 2015; 12: 80–9. [PubMed Abstract].
- El-Ameen NMH, Taha MME, Abdelwahab SI, Khalid A, Elfatih F, Kamel MA, etal. Anti-diabetic properties of thymoquinone is unassociated with glycogen phosphorylase inhibition. Pharmacogn J. 2015; 7: 406–10.
- Taka E, Mazzio EA, Goodman CB, Redmon N, Flores-Rozas H, Reams R, etal. Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. J Neuroimmunol. 2015; 286: 5–12. [PubMed Abstract].
- Amin B, Hosseinzadeh H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. 2016; 82: 8–16. [PubMed Abstract].
- Darakhshan S, Bidmeshki Pour A, Hosseinzadeh Colagar A, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015; 95–96: 138–58.
- Çaylak E, Halifeoʇlu ̄. Effects of sulfur-containing antioxidants on malondialdehyde and catalase levels of liver, kidney and brain in lead-exposed rats. Turkiye Klinikleri J Med Sci. 2007; 27: 1–8.
- Çaylak E, Halifeoğlu İ, Aydin S, Telo S, Bulmuş Ö, Çelik H. The effects of sulfur-containing compounds on total antioxidant capacity levels of liver, kidney and brain in lead-exposed rats. Turkiye Klinikleri J Med Sci. 2007; 27: 823–8.
- Abdel Aal KM, Hussein AMR. Therapeutic efficacy of alpha lipoic acid in combination with succimer against lead-induced oxidative stress, hepatotoxicity and nephrotoxicity in rats. Ass Univ Bull Environ Res. 2008; 11: 87–99.
- Alsaif MA. Effect of thymoquinone on ethanol-induced hepatotoxicity in Wistar rats. J Med Sci. 2007; 7: 1164–70.
- El-Sayed WM. Upregulation of chemoprotective enzymes and glutathione by Nigella sativa (black seed) and thymoquinone in CCl4-intoxicated rats. Int J Toxicol. 2011; 30: 707–14. [PubMed Abstract].
- Kurt E, Dede S, Ragbetli C. The investigations of total antioxidant status and biochemical serum profile in thymoquinone-treated rats. Afr J Tradit Complement Altern Med. 2015; 12: 68–72.
- Arthur JR, Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985; 36: 1569–75. [PubMed Abstract].
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70: 158–69. [PubMed Abstract].
- Goldberg DM, Spooner RJ. Bergmeyen HV. Assay of glutathione reductase. Methods of enzymatic analysis. 1983; Deerfield Beach, FL: Verlag Chemie. 258–65. Vol. 3. 3rd ed.
- Cohen G, Kim M, Ogwu V. A modified catalase assay suitable for a plate reader and for the analysis of brain cell cultures. J Neurosci Methods. 1996; 67: 53–6. [PubMed Abstract].
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82: 70–7. [PubMed Abstract].
- El-Khishin IA, El-fakharany YMM, Abdel Hamid OI. Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicol Rep. 2015; 2: 824–32.
- Hasanein P, Teimuri-Far M. Protective effect of bioactive peptide carnosine against lead-induced oxidative stress in kidney of rats. Cell Mol Biol (Noisy-le-grand). 2015; 61: 8–14.
- Matović V, Buha A, Ðukić-Ćosić D, Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol. 2015; 78: 130–40.
- Liu CM, Zheng YL, Lu J, Zhang ZF, Fan SH, Wu DM, etal. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010; 29: 158–66. [PubMed Abstract].
- Agrawal S, Bhatnagar P, Flora SJ. Changes in tissue oxidative stress, brain biogenic amines and acetylcholinesterase following co-exposure to lead, arsenic and mercury in rats. Food Chem Toxicol. 2015; 86: 208–16. [PubMed Abstract].
- Wang Z, Yan Y, Yu X, Li W, Li B, Qin C. Protective effects of chitosan and its water-soluble derivatives against lead-induced oxidative stress in mice. Int J Biol Macromol. 2016; 83: 442–9. [PubMed Abstract].
- Sivaprasad R, Nagaraj M, Varalakshmi P. Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem. 2004; 15: 18–23. [PubMed Abstract].
- Sener U, Uygur R, Aktas C, Uygur E, Erboga M, Balkas G, etal. Protective effects of thymoquinone against apoptosis and oxidative stress by arsenic in rat kidney. Ren Fail. 2015; 38: 117–23. [PubMed Abstract].
- Farag MM, Ahmed GO, Shehata RR, Kazem AH. Thymoquinone improves the kidney and liver changes induced by chronic cyclosporine A treatment and acute renal ischaemia/reperfusion in rats. J Pharm Pharmacol. 2015; 67: 731–9. [PubMed Abstract].
- Samarghandian S, Azimi-Nezhad M, Mehrad-Majd H, Mirhafez SR. Thymoquinone ameliorates acute renal failure in gentamicin-treated adult male rats. Pharmacology. 2015; 96: 112–17. [PubMed Abstract].
- El-Sheikh AA, Morsy MA, Abdalla AM, Hamouda AH, Alhaider IA. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediators Inflamm. 2015; 2015: 859383. doi: http://dx.doi.org/10.1155/2015/859383.
- Ismail M, Al-Naqeep G, Chan KW. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010; 48: 664–72. [PubMed Abstract].
- Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah OA, Hafez MM, etal. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev. 2010; 3: 254–61. [PubMed Abstract] [PubMed CentralFull Text].
- Kruk I, Michalska T, Lichszteld K, Kladna A, Aboul-Enein HY. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere. 2000; 41: 1059–64. [PubMed Abstract].
- Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. 2002; 20: 143–51. [PubMed Abstract].
- Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003; 26: 87–98. [PubMed Abstract].
