Abstract
Background
Vitamin D inadequacy is widespread in children and adolescents worldwide. The present study was undertaken to assess the vitamin D status in active children living in a sunny climate and to identify the main determinants of the serum concentration of 25-hydroxyvitamin D (25-OHD).
Methods
This cross-sectional study included 225 children aged 7–15 years practicing sports in a football academy. Anthropometric measures were performed to calculate body mass index (BMI), fat mass, and maturity status. A nutritional enquiry was performed including 3-day food records and food frequency questionnaire. Plasma 25-OHD and insulin were assessed by immunoenzymatic methods ensuring categorization of vitamin D status and calculation of insulin sensitivity/resistance indexes. A logistic regression model was applied to identify predictors for vitamin D inadequacy.
Results
Vitamin D deficiency (25-OHD<12 µg/L) was observed in 40.9% of children and insufficiency (12<25-OHD<20 µg/L) was observed in 44% of children. In a multivariate analysis, vitamin D deficiency and insufficiency were associated with a lower dietary intake of vitamin D, proteins, milk, red meat, fish, and eggs. However, no significant relationship was observed with maturation status, adiposity, or insulin resistance.
Conclusions
Tunisian children and adolescents are exposed to a high risk of vitamin D inadequacy despite living in a sunny climate. Circulating 25-OHD concentrations are related to the intake of vitamin D food sources but not to maturation status or body composition. Ensuring sufficient and safe sun exposure and adequate vitamin D intake may prevent vitamin D inadequacy in children from sunny environments.
Accumulating evidence suggests that optimal vitamin D status throughout the life span may be important in maintaining bone health, and protecting against many chronic conditions, such as infectious, autoimmune and cardiovascular diseases, diabetes, and cancer (Citation1, Citation2). Vitamin D derives from skin synthesis under the influence of ultraviolet light and from food. Its status in the body depends on sun exposure, which varies according to latitude, season, skin color, and clothing, as well as on vitamin D provided by diet or supplements. Plasma 25-hydroxyvitamin D (25-OHD) reflects both skin synthesis and the supply from the diet or supplements. It is regarded as the best indicator of vitamin D status (Citation3).
A high prevalence of vitamin D inadequacy was observed in children and adolescents in several regions of the world (Citation4–Citation10). This high prevalence is mainly attributed to insufficient sun exposure and low intake of vitamin D-rich foods. It was also suggested that the pandemic increase in obesity in children contributes to this high frequency. Adiposity is supposed to be a risk factor for vitamin D deficiency (Citation9, Citation11) (Citation12). Nevertheless, the relationship between adiposity and low 25-OHD during childhood remains uncertain and the underlining mechanisms are still hypothetic (Citation7, Citation8) (Citation13, Citation14). There was also a suggestion of a relationship between vitamin D deficiency and insulin resistance, a recognized feature of obesity (Citation15, Citation16). But the evidence of such association is still inconclusive (Citation17, Citation18).
In sunny environments, most vitamin D in the body would be provided from skin synthesis and minimally derived from food (Citation3). Children living in sunny areas are expected to achieve adequate vitamin D status on the assumption that ample sun exposure covers the needs for this vitamin (Citation3). However, this condition could be influenced by factors such as time spent outdoors, exposed area of the skin, skin color, and body composition, as well as dietary intake. The present study was undertaken to examine plasma 25-OHD in active children who undergo reasonably outdoor activities and to test the effect of adiposity, insulin sensitivity, and diet on vitamin D status in these children.
Materials and methods
Subjects
The study included 225 boys aged 7–16 years, recruited from two centers of a football academy in the area of Tunis (latitude, 35°N). Children with liver, renal, or bone disease; intestinal malabsorption; or cancer; and those taking vitamin D supplements, anticonvulsant drugs, or systemic corticosteroids were not included. The study was carried out from January to March 2014, a period during which temperatures varied between 10°C and 22°C and the humidity ranged from 70 to 75%. Besides 2 h per week of outdoor physical activity as part of the school program, each child attends three weekly outdoor sessions in the football academy. The training sessions are scheduled from 5 PM to 6 PM on Friday and Saturday, and from 9 AM to 10 AM on Sunday. No child declared having used sun screen during the 3 months of observation. Skin color of each participant was determined by two investigators (IB and MF), together with one parent of the child, and classified as fair, corresponding to Fitzpatrick skin types 1 and 2, or dark, corresponding to Fitzpatrick skin types 3 and 4 (Citation19). Written parental permission was obtained for each participant. The Ethics Committee of Rabta Hospital approved the experimental protocol.
Experimental protocol
Anthropometrical and maturity status measures
Weight, height, and sitting height were measured with the subjects barefooted and lightly clothed. Body mass index (BMI) was calculated as weight per height squared (kg m−2). Participants were divided according to the World Health Organization (WHO) child growth standards for BMI in three groups; normal-weight group (BMI≤85th percentile), overweight group (85th percentile <BMI <97th percentile), and obese group (BMI>97th percentile) (Citation20). Triceps and subscapular skinfolds thickness was measured with Harpenden's skinfold calipers (Baty International, West Sussex, England). Body fat percentage was calculated using Slaughter's prediction equation (Citation21). Biologic maturity was assessed by incorporating anthropometric variables (weight, standing height, and sitting height) and was calculated using the equation of Mirwald (Citation22); maturity offset=−9.236+(0.0002708*leg length*sitting height)+(−0.001663*age*leg length)+(0.007216*age*sitting height)+(0.02292*weight by height ratio). This assessment is a non-invasive and practical approved method of predicting age in years from the peak height velocity (PHV) as a measure of maturity offset. For the purpose of data analysis, children were divided into three groups: pre-PHV (−3 to −1 year from PHV), around PHV (−1 to +1 year from PHV), and post-PHV (+1 to +3 years from PHV).
Dietary intake
Nutritional inquiry was completed for 174 children. The daily vitamin D intake in children's diet was assessed using a 3-day food record (including 2 weekdays and 1 day over the weekend), combined with a food frequency questionnaire (FFQ) that the parents mostly completed. A 35-items FFQ to quantify the consumption of nutrients naturally rich in vitamin D, such as fatty fish, meat, eggs, milk, and other dairy products. The questionnaire was developed based on a validated FFQ (Citation23). It was translated into Arabic language and pre-tested before use. The FFQ was modified to estimate vitamin D intake and consumption frequency across nine categories (never, 1 time/month, 1–2 times/week, 2–3 times/week, 3–4 times/week, 1 time/day, 2 times/day, 3 times/day, and ≥4 times/day). Ease of administration of this FFQ was enhanced by the use of a food photograph album of Tunisian food products that emphasized portion sizes. Four food groups were selected as follows: fish (50 g/serving), eggs (50 g/serving), red meat (50 g/serving), and milk (200 mL/serving). Consumption frequencies of milk and egg were standardized into servings per day and into servings per week for fish and red meat. The data about the mean daily intake of nutrients were processed using the professional Nutri Pro 7 program (Nutri Pro 7 software, CERDEN, Brussels, Belgium).
Biochemical analyses
Blood samples were collected following an overnight fast. Blood was centrifuged at 2000×g for 20 min and the plasma was frozen at −40°C until analysis (within 3 months). Plasma glucose, calcium, phosphorus, and C-reactive protein (CRP) were assessed on Architect C8000 analyzer (Abbott Laboratories, Abbott Park, IL), using the respective reagents kits. Plasma 25-hydroxyvitamin D (25-OHD) and insulin concentrations were measured by chemiluminescence immunoassay methods using the Liaison analyzer (DiaSorin Inc., Stillwater, MN) and the respective reagents kit. Vitamin D status was evaluated according to the standards of the Institute of medicine (IOM). Vitamin D deficiency, insufficiency, and sufficiency were defined as plasma 25-OHD concentrations below 12 µg/L, 12 to 20 µg/L, and over 20 µg/L, respectively (Citation24). Insulin sensitivity/resistance was assessed using two indexes; the homeostasis model assessment of insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI), according to the following equations (Citation25, Citation26): HOMA-IR=[(fasting insulin in µU/mL) − (fasting glucose in mg/dL)/405]; QUICKI=1/[log(fasting insulin in µU/mL)+log(fasting glucose in mg/dL)].
Statistical analysis
Data were analyzed using SPSS for Windows (version 18.0; SPSS Inc., Chicago, IL). Continuous variables were tested for normality using Kolmogorov-Smirnov test. Values are expressed as mean (SD) or median (inter quartile range, IQR) for continuous variables and as a percent for categorical variables. Comparisons between groups were performed using analysis of variance or the Mann–Whitney test for continuous variables and Pearson chi-square test or Fisher's exact test for categorical variables as appropriate. The association between continuous variables was tested using a Pearson correlation test. Unadjusted and multi-adjusted odd-ratios with 95% confidence intervals were calculated as an estimate of the risk of vitamin D deficiency/insufficiency for several potential risk factors. A binary logistic regression model was used to identify predictors for vitamin D deficiency, while adjusting for possible confounding factors. Adjustment was performed on maturation status (pre-PHV/around and post-PHV); body mass (normal-weight/overweight and obese); skin color (fair/dark); and dichotomous variables for fat mass (<20%/≥20%), HOMA-IR (<1.5/≥1.5); and the daily intake of vitamin D (<8.5/≥8.5 µg), milk (<500/≥500 mL), red meat (<100/≥100 g), fish (<100/≥100 g), and eggs (<50/≥50 g), defined as the respective continuous variables split at the median. The fit of logistic models was satisfactory. A two-tailed P-value less than 0.05 was considered statistically significant.
Results
The main characteristics of children according to body mass are shown in . Serum CRP and insulin concentrations and HOMA-IR and total energy intake were significantly higher and QUICKI lower in obese and overweight children compared to normal-weight children. Daily vitamin D intake was low and equivalent in the three groups. Plasma 25-OHD concentrations ranged between 3.80 and 31 µg/L, and did not differ according to body mass (). Vitamin D inadequacy was noted in about 85% of the children, with 40.9% having deficiency and 44% having insufficiency. Plasma 25-OHD was positively correlated with dietary intake of proteins (r=0.407, p<0.001), milk (r=0.542, p<0.001), red meat (r=0.282, p<0.001), fish (r=0.502, p<0.001), and eggs (r=0.512, p<0.001) (). However, no significant correlation was observed with PHV, BMI, fat mass, HOMA-IR, or QUICKI. Compared with vitamin-D-sufficient children, those with vitamin D deficiency or insufficiency showed lower intakes of proteins, milk, red meat, fish, and eggs (). In a multivariate analysis, vitamin D deficiency and insufficiency were associated with lower dietary intakes of vitamin D, proteins, milk, red meat, fish, and eggs. However, no association was observed with PHV, BMI, fat mass, HOMA-IR, or skin color ().
Fig. 1 Plasma 25-hydroxyvitamin D concentrations in Tunisian active children according to body mass (n=225).
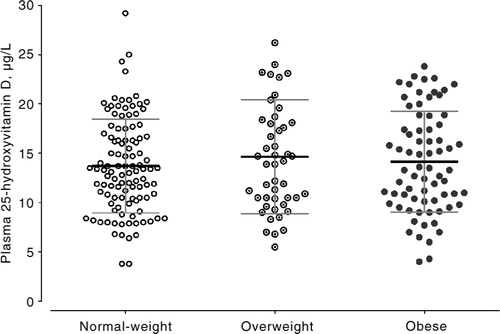
Fig. 2 Correlations of plasma 25-hydroxyvitamin D with daily intake of red meat, fish, eggs, and milk in Tunisian active children (n=174).
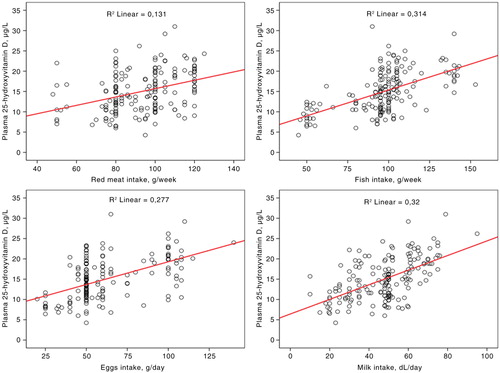
Fig. 3 Daily intake of proteins, red meat, fish, eggs, and milk according to the vitamin D status in Tunisian active children (n=174).
Table 1 Clinical, nutritional, and biochemical characteristics of children according to body mass (n=225)
Table 2 Plasma 25-hydroxyvitamin D and multi-adjusted odd-ratios for vitamin D deficiency/insufficiency in children according to confounding variables
Discussion
This study showed that vitamin D inadequacy is common among active Tunisian children and is associated with a low intake of vitamin D food sources, but not with maturity status, adiposity, or insulin resistance. The high prevalence of vitamin D inadequacy in Tunisian children is consistent with similar findings in other parts of the world (Citation4–Citation10). This finding is somewhat surprising since these are active children who live in a sunny environment and engage in a reasonable level of outdoor activities. However, a number of factors may be behind this finding. Our study had been conducted during the winter when plasma 25-OHD levels are usually at their nadir (Citation3). Because of cool environmental temperatures, children were wearing clothes that covered the trunk and limbs, minimizing the skin area exposed to the sun's rays and preventing vitamin D synthesis. Also, the training sessions were held in the early mornings or late afternoons when the sun's rays are least efficient for vitamin D synthesis (Citation7, Citation27). Although dark skin color is a risk factor for hypovitaminosis D, obviously, that distinction is of little importance when most of the skin areas are covered with the clothing. However, the most important factor behind the observed vitamin D inadequacy in most children is probably the inadequate intake of dietary vitamin D sources. Our data demonstrate that active children living in sunny environments may still have vitamin D inadequacy, which underlines the importance of monitoring plasma 25-OHD concentrations in children. The present study revealed the low dietary vitamin D intake in most children. In all the children participating in the present study, the daily oral vitamin D intake was well below the US Institute of Medicine Recommended Daily Allowance (RDA) of 15 mcg (Citation24). There is no dietary recommendation for vitamin D for children and adolescents in Tunisia, as it is assumed that sun exposure will ensure an adequate vitamin D status. Our finding of low plasma 25-OHD levels in 85% of the participants highlights the need for evidence-based dietary recommendations for Tunisian children. Our study also showed a clear relationship between vitamin D deficiency/insufficiency and low intake of vitamin D food sources (i.e. fish, meat, milk, and eggs). These findings are in line with the study of Areum et al. (Citation10) showing a positive correlation of serum 25-OHD with the consumption of vitamin D food sources in Korean adolescents.
In sunny areas, although sun exposure is the major source of vitamin D in the body, vitamin D inadequacy may occur. This suggests that concomitant appropriate dietary intake is required. The assumption that, in sunny environment, sun exposure alone may provide adequate plasma 25-OHD levels is often false. When sun exposure is limited as a consequence of low-sunshine seasons, pollution, dark skin, or clothing, the dietary intake of vitamin D may be the more significant contributor to vitamin D status. In these conditions, lack of dietary intake may increase the risk of vitamin D inadequacy.
The present study showed no association between plasma 25-OHD and adiposity as assessed either by BMI percentile or fat mass. Some previous studies reported an inverse relationship between plasma 25-OHD and adiposity (Citation7, Citation9) (Citation11, Citation28), while others found no association (Citation8, Citation13) (Citation14, Citation29). In a sample of Quebec youth, BMI was negatively associated with 25-OHD levels in girls but not in boys (Citation30). Hypothetic mechanisms of low plasma 25-OHD levels in obesity include sequestration of vitamin D in the fat depot, impaired mobilization from the fat depot, and reduced skin and dilution throughout the body. Other important factors in the obese include limited sun exposure due to few outdoor activities and reduced exposed skin area because of clothing (Citation31, Citation32). The latter mechanism may be of great importance in explaining hypovitaminosis D in obesity. This may explain the lack of an association between vitamin D and adiposity in our series. Indeed, the overweight or obese and normal-weight children included in this study spend as much time outdoors and wear similar clothes, and thus receive the same dose of sunshine. The relationship between adiposity and the low vitamin D status described in some studies may be related to short exposure to the sun, rather than an excess of body fat per se. In line with the lack of an association with adiposity, our study showed no association between plasma 25-OHD and insulin resistance, a condition usually associated with obesity. In fact, the relationship between vitamin D and insulin resistance is still a subject of debate (Citation17–Citation20, Citation33).
Our study has focused on a broad sample of children and adolescents, and its findings arose from multivariate analyses adjusting on several potential confounders for vitamin D status. The study has controlled for the time spent outdoors and thus on the amount of sun exposure, which is an important predictor of vitamin D status. Although sun exposure was not measured with precision, all participants have comparable outdoor activities and equivalent sun exposure. This group of children is probably typical of urban and suburban children from the Mediterranean region. The study has also limitations. The trans-sectional design prevents the evaluation of vitamin D status year-round, rendering the findings only suitable for the winter season. The socioeconomic rank of children was not properly identified. However, based on parents’ occupation, most participants have average to high socioeconomic status. Nutritional assessment was achieved in only 77% of participants and vitamin D intake was estimated using a non-Tunisian food database. Because the vitamin D content of foods is not given in the food composition database for Tunisia, the presumed concentrations of vitamin D were obtained from European food composition tables, which make estimations less precise. To overcome this issue, we considered the consumption of vitamin D food sources in Tunisian diet. The development and validation of a vitamin D database for Tunisian food is necessary to allow future estimations of vitamin D intake. Our study did not look for health problems that may be related to vitamin D deficiency, an aspect that is beyond the scope of the study. Further studies should address the health consequences of hypovitaminosis D in exposed populations.
In conclusion, Tunisian children and adolescents are exposed to a high risk of vitamin D inadequacy. They have inadequate sun exposure during winter despite living in a sunny climate. Vitamin D intake is also low in most children, due to little consumption of vitamin D food sources. Finally, circulating 25-OHD concentrations are related to vitamin D food sources intake, suggesting that dietary intake is a key contributor in vitamin D status when sun exposure is limited. Given the key role of vitamin D in growth and health in general, every measure should be undertaken to achieve sufficient vitamin D status in these children. These measures include education in order to ensure adequate and safe sun exposure and appropriate consumption of vitamin D-rich/fortified foods. Further research is needed to establish an optimal combination of sun exposure and food intake/supplementation ensuring year-round sufficient circulating 25-OHD in children and adolescents living in a sunny climate.
Conflict of interest and funding
The authors declare no conflicts of interest. The study was supported by Funds of Research Unit ‘05/UR08-08’ and Research Laboratory LR99ES11, Ministry of Higher Education and Scientific Research of Tunisia.
Acknowledgements
The authors thank the children, their parents, and the technical staff for their contributions.
References
- Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence?. Br J Nutr. 2003; 89: 552–72.
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004; 80(6 Suppl): 1678S–88S. [PubMed Abstract].
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266–81.
- Lehtonen-Veromaa M, Möttönen T, Irjala K, Kärkkäinen M, Lamberg-Allardt C, Hakola P, etal. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999; 53: 746–51.
- Guillemant J, Le HT, Maria A, Allemandou A, Pérès G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int. 2001; 12: 875–9.
- El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, etal. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001; 107: E53.
- Khor GL, Chee WS, Shariff ZM, Poh BK, Arumugam M, Rahman JA, etal. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health. 2011; 11: 95.
- Sacheck J, Goodman E, Chui K, Chomitz V, Must A, Economos C. Vitamin D deficiency, adiposity, and cardiometabolic risk in urban schoolchildren. J Pediatr. 2011; 159: 945–50.
- Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013; 131: e152–61.
- Yu A, Kim J, Kwon O, Oh SY, Kim J, Yang YJ. The association between serum 25-hydroxyvitamin d concentration and consumption frequencies of vitamin d food sources in Korean adolescents. Clin Nutr Res. 2013; 2: 107–14.
- Rockell JE, Green TJ, Skeaff CM, Whiting SJ, Taylor RW, Williams SM, etal. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 y. J Nutr. 2005; 135: 2602–8. [PubMed Abstract].
- Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015; 16: 341–9.
- Stoian CA, Lyon M, Cox RG, Stephure DK, Mah JK. Vitamin D concentrations among healthy children in Calgary, Alberta. Paediatr Child Health. 2011; 16: 82–6. [PubMed Abstract] [PubMed CentralFull Text].
- González-Gross M, Valtueña J, Breidenassel C, Moreno LA, Ferrari M, Kersting M, etal. HELENA Study Group. Vitamin D status among adolescents in Europe: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr. 2012; 107: 755–64.
- Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am J Clin Nutr. 2010; 92: 1344–9.
- Pham NM, Akter S, Kurotani K, Nanri A, Sato M, Hayabuchi H, etal. Serum 25-hydroxyvitamin D and markers of insulin resistance in a Japanese working population. Eur J Clin Nutr. 2012; 66: 1323–8.
- Gannagé-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009; 160: 965–71.
- Gulseth HL, Gjelstad IM, Tierney AC, Lovegrove JA, Defoort C, Blaak EE, etal. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care. 2010; 33: 923–5.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988; 124: 869–71.
- de Onis M, Onyango AW, Borghi E, Garza C, Yang H. WHO Multicentre Growth Reference Study Group. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programs. Public Health Nutr. 2006; 9: 942–7.
- Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, etal. Skinfold equation for estimation of body fatness in children and youth. Hum Biol. 1988; 60: 709–23. [PubMed Abstract].
- Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002; 34: 689–94.
- Uenishi K, Ishida H, Nakamura K. Development of a simple food frequency questionnaire to estimate intakes of calcium and other nutrients for the prevention and management of osteoporosis. J Nutr Sci Vitaminol (Tokyo). 2008; 54: 25–9.
- Institute of Medicine (IOM). Dietary reference intakes for calcium and vitamin D. 2011; Washington DC: National Academies Press.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–9.
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, etal. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85: 2402–10.
- Alshahrani FM, Almalki MH, Aljohani N, Alzahrani A, Alsaleh Y, Holick MF. Vitamin D: light side and best time of sunshine in Riyadh, Saudi Arabia. Dermatoendocrinol. 2013; 5: 177–80.
- Valtueña J, González-Gross M, Huybrechts I, Breidenassel C, Ferrari M, Mouratidou T, etal. Factors associated with vitamin D deficiency in European adolescents: the HELENA study. J Nutr Sci Vitaminol. 2013; 59: 161–71.
- Aypak C, Türedi O, Yüce A. The association of vitamin D status with cardiometabolic risk factors, obesity and puberty in children. Eur J Pediatr. 2014; 173: 367–73.
- Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007; 86: 150–8. [PubMed Abstract].
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000; 72: 690–3. [PubMed Abstract].
- Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012; 20: 1444–8.
- Del Gobbo LC, Song Y, Dannenbaum DA, Dewailly E, Egeland GM. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta cell function in Canadian Cree. J Nutr. 2011; 141: 290–5.
