Abstract
Background
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is associated with cardiovascular morbidity and mortality, which can be improved by using continuous positive airway pressure (CPAP) therapy. However, the pathophysiological links between the two kinds of disease and the mechanism of the CPAP effect remain incompletely understood. We aimed to inquire into the myocardial involvement in this relationship. We suggested that serum brain natriuretic peptide (BNP) is sensitive enough to detect myocardial stress caused by OSAHS.
Design and methods
Sixty-four subjects without cardiovascular disease (21 controls, 24 normotensive OSAHS patients, and 19 hypertensive OSAHS patients) were analyzed for serum BNP at baseline and serially over 6 months. CPAP was applied to 23 patients with severe OSAHS.
Results
At baseline, the serum BNP levels were significantly higher (p=0.0001) in the OSAHS group (22.3±14.79 pg/ml) than in the control group (9.2±6.75 pg/ml). Increased serum BNP levels were significantly associated with mean transcutaneous oxygen saturation (SpO2) (p<0.0001), minimal SpO2 (p=0.002), oxygen desaturation index (p=0.001), and total sleep time spent with SpO2 lower than 90% (p=0.002). All patients with elevated BNP levels (≥37 pg/ml) had moderate or severe OSAHS (11/43 OSAHS patients). The more severe the OSAHS, the higher the BNP levels were. However, only the difference between severe and mild OSAHS was statistically significant (p=0.029). Hypertensive OSAHS patients had the highest baseline BNP levels (27.7±16.74 pg/ml). They were significantly higher (p=0.001) than in normotensive OSAHS patients (18±11.72 pg/ml) (p=0.039) and the controls (9.2±6.75 pg/ml). As compared with baseline, treatment with CPAP significantly decreased BNP levels in both hypertensive and normotensive OSAHS patients (respectively, from 36±16.10 to 29.7±14.29 pg/ml, p<0.001, and from 20±10.09 to 16±8.98 pg/ml, p<0.001). In contrast, the BNP levels slightly increased in the controls (from 9.2±6.75 to 9.5±7.02 pg/ml, p=0.029), but there was no statistically significant difference in comparison with the baseline value. The effect of CPAP on BNP levels was more marked in patients with higher baseline BNP levels and those with the most prolonged nocturnal desaturation (p=0.001, r=0.65). It was also more marked in hypertensive OSHAS patients (p=0.015, r=0.72) in comparison with normotensive OSAHS patients (p=0.03, r=0.62).
Conclusion
BNP seems to be sensitive enough to detect myocardial stress caused by OSAHS. As such, it is a potential marker for screening of preclinical cardiovascular damage in patients with untreated OSAHS. Application of CPAP decreases levels significantly in normotensive and particularly in hypertensive OSAHS. These findings are consistent with previous results suggesting the potential benefits of CPAP on cardiovascular outcome in OSAHS patients.
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a common sleep-related breathing disorder that occurs in about 4–7% of the general adult population (Citation1). It is characterized by recurrent episodes of complete or partial upper airway obstruction during sleep, resulting in oxygen desaturation, sleep fragmentation, and daytime sleepiness (Citation2).
Recent studies have shown a large body of epidemiologic evidence linking the OSAHS with important cardiovascular morbidity and mortality (Citation3). However, the underlying pathophysiologic mechanisms are not fully understood, nor is the potential role of cardiac biomarkers in these states. Recently, there has been growing interest in the potential relationship between OSAHS and brain natriuretic peptide (BNP) regulation (Citation4).
BNP is a cardiac neuro-hormone secreted predominantly by the left ventricular myocytes in response to volume expansion and pressure overload (Citation5). Because OSAHS has been suggested as a probable cause of increased ventricular load and therefore ventricular distension during periods of apnea, it has been proposed that BNP levels could be useful as a diagnostic and prognostic marker in patients with OSAHS (Citation6). Previous studies on the association between biomarkers and the presence and severity of OSAHS have revealed conflicting results (Citation7). While some studies reported increasing levels of BNP in patients with OSAHS (Citation8) and reduced BNP levels with continuous positive airway pressure (CPAP) treatment (Citation9), others have not found any association (Citation10). In addition, most studies are small and based on symptomatic patients referred to sleep clinics. Therefore, the association between OSAHS and BNP levels in the general population remains largely unknown (Citation8).
The aim of this study was to analyze the possible association between OSAHS during the night and levels of BNP the following morning in a community-based sample of patients. We also evaluated the effect of CPAP therapy on BNP levels.
Methods
Subjects and protocol
This was a prospective, controlled, cross-sectional study performed between September 2013 and September 2014 at the Hedi Chaker University Hospital of Sfax, Tunisia. The study was approved by the local ethics committee, and informed consent was obtained from each patient enrolled in the study. We included subjects older than 18 years with suspected OSAHS. Exclusion criteria included refusal to participate in the study, previously diagnosed sleep-disordered breathing, chronic obstructive pulmonary diseases or other severe lung disorders, a history of cerebrovascular or cardiovascular events, uncontrolled hypertension, psychiatric disorders, diabetes mellitus, hyperlipidemia, hypercholesterolemia, malignancy, inflammatory diseases, dysthyroidism, a history of recent facial trauma/operation, pregnancy, and the use of sedatives or muscle relaxants.
During the study, 456 patients were screened for OSAHS. Only 64 subjects met the inclusion/exclusion criteria and were enrolled in this study: 21 healthy normotensive subjects as controls, 24 normotensive OSAHS patients, and 19 hypertensive OSAHS patients.
Clinical assessment
Patient history was obtained with the help of a structured interview. We collected demographic variables (age, sex), current cigarette smoking status, history of preexisting diseases, and current drug use. Daytime sleepiness was estimated using the Arabic version of the Epworth sleepiness scale (ESS) (Citation10). In addition to standard physical examination, neck circumference, body weight, and height were measured. Body mass index (BMI) was calculated as kg/m2. Daytime blood pressure was measured in the right upper arm using an automatic oscillometric device after a rest of at least 10 min in a seated position. The main awake blood pressure was calculated based on three measurements on the day of admission. Patients were considered normotensive if they had no history of hypertension, their systolic blood pressure was ≤139 mmHg, and their diastolic blood pressure was ≤89 mmHg. Patients with a history of hypertension or systolic blood pressure ≥140 mmHg and diastolic ≥90 mmHg were classified as hypertensive (Citation11).
Nocturnal polygraph recording
All patients underwent overnight type III polysomnography (PSG) (Embla™ N7000, ResMed Corp., San Diego, CA, United States) according to standard techniques. Flow was monitored by an oronasal air pressure transducer (Embla™ N7000, ResMed Corp., San Diego, CA, United States), thoracic and abdominal respiratory effort by uncalibrated impedance plethysmography belts (Embla™ N7000, ResMed Corp., San Diego, CA, United States), pulse oximetry, and body position. Snoring was recorded by a microphone (Embla™ N7000, ResMed Corp., San Diego, CA, United States) placed on the anterior neck. A priority in the study was to make recommendations about avoiding caffeinated drinks and food, alcohol, and drugs that could interfere with sleep architecture. Respiratory parameters were scored by one doctor according to the standard criteria of the American Academy of Sleep Medicine (AASM). Based on the guidelines of the AASM published in 2012, obstructive apnea was defined as a ≥90% decrease in airflow relative to the basal amplitude persisting for at least 10 sec in the presence of thoracic and abdominal movements. Hypopnea was defined as a ≥50% decrease in the airflow amplitude relative to the baseline with an associated ≥3% oxygen desaturation or arousal persisting for at least 10 sec (Citation12). It was scored as an obstructive event when the inspiratory flow signal showed a flow limitation or snoring occurred. The numbers of apneic and hypopneic episodes per hour of estimated sleep time were reported as the apnea-hypopnea index (AHI). The presence of OSAHS was defined as an AHI ≥5 events per hour (Citation2). OSAHS was graded according to AHI as mild (5/h ≤AHI<15/h), moderate (15/h≤AHI<30/h), or severe (AHI ≥30/h). Patients without any evocative symptoms apart from snoring were classified as controls if polygraphic screening showed an AHI <5/h.
CPAP therapy
Nocturnal CPAP ventilation was offered to all patients with severe OSAHS using the S9 Elite (ResMed Ltd Bella Vita, NSW, Australia). CPAP titration was conducted with auto-titrating devices during a short hospitalization. Detailed explanation of CPAP use was provided, and control visits were conducted 1, 3, and 6 months after CPAP initiation. Compliance was assessed by the CPAP card data at each appointment. Benefits and side effects associated with CPAP therapy were also assessed. Device usage for >4 h per day and for >5 days a week was accepted as the only criterion of good compliance (Citation13).
Blood collection and analysis
Blood samples for BNP were taken using standard venipuncture between 7:00 and 9:00 a.m. after a 12-h fast and separated immediately after centrifugation at 4°C, 1,800×g for 20 min. Blood samples were obtained again after 6 months from those with CPAP therapy and those who received no treatment. The plasma levels of BNP were measured using a chemiluminescence immunoassay on the ADVIA Centaur Analyzer Siemens Healthcare Diagnostics (Saint-Denis, France). A value of BNP ≥37 pg/ml was regarded as elevated, in accordance with the reference value of the method.
Other examinations
Electrocardiogram, chest X-ray, and pulmonary function tests were performed for all OSAHS patients as well as for controls. Lung function parameters included forced vital capacity (FVC) and forced expiratory volume in 1 sec (FEV1). Each parameter was expressed as a percentage of the predicted value.
Statistics
Statistical analysis was performed using SPSS 17 (SPSS, Inc., Chicago, IL, USA) for Windows. Data are presented as frequencies, percentages, mean±standard deviation, or median. Differences between consecutive BNP values were assessed by a one-way analysis of variance test with a Bonferroni correction. A value of p<0.05 was considered significant.
Results
Clinical and sleep characteristics of the study population
Sixty-four subjects (mean age=44.4±10.90 years, 32 males/32 females, BMI=33.8±6.30 kg/m2) were included in the study: 21 in the control group (Group 1) and 43 in the OSAHS group (Group 2; AHI=28±16.95). OSAHS was severe in 17 patients (39.5%), moderate in 16 (37.2%), and mild in 10 (23.3%). Of the 43 patients with OSAHS, 24 were normotensive (Group 3), while 19 were hypertensive (Group 4).
The OSAHS and control groups were similar in age, sex distribution, BMI, smoking status, and blood pressure. In addition, patient demographics (age, sex, and smoking status) were similar in the three study groups. However, BMI was significantly higher in hypertensive OSAHS patients in comparison with normotensive OSAHS patients (p=0.032) but not compared with the controls. Based on the ESS, patients with OSAHS were significantly more somnolent than control subjects (p=0.013). Similarly, the mean ESS was significantly higher in hypertensive OSAHS patients than normotensive OSAHS patients (p=0.015). The highest AHI was observed in hypertensive OSAHS patients. The oxygen desaturation index (ODI) was significantly higher in OSAHS patients in comparison with controls (p=0.0001), but it was not significantly different between hypertensive and normotensive OSAHS. Spirometric measurements (FEV1, FVC, and FEV1/FVC) did not differ among the four groups. The baseline characteristics of the final study population are shown in the .
Table 1 Clinical characteristics, results of sleep studies, and spirometric measurements: comparison of OSAHS patients, normotensive and hypertensive patients, and controls
Levels of BNP and clinical data
Subjects were also divided into two groups based on whether the BNP level was above or below the cut-off value. At first presentation, the plasma levels of BNP in all subjects ranged from 1.9 to 57 pg/ml, with a median of 17.9±14.17 pg/ml.
Levels of BNP and polygraph data
Patients with increased levels of BNP were significantly older than those with normal levels (p=0.03). In contrast, low or high levels of BNP were not associated with sex ratio, BMI, smoking status, ESS, blood sugar blood levels, or creatinine clearance. Increased levels of BNP were significantly more frequent among patients with OSAHS (25.6%) in comparison with the control group (0%) (p=0.011). Similarly, the serum BNP levels were significantly higher in the OSAHS group (22.3±14.79 pg/ml) than in the controls (9.2±6.75 pg/ml) (p=0.0001). In addition, increased serum BNP levels were significantly associated with mean transcutaneous oxygen saturation (SpO2) (p<0.0001), minimal SpO2 (p=0.002), ODI (p=0.001), and total sleep time with SpO2<90% (TST90) (p=0.002) ().
Table 2 Baseline clinical and polygraph data according to baseline BNP levels
BNP levels by OSAHS classification are shown in . All patients with high BNP levels had moderate or severe OSAHS (11/43 OSAHS patients) (). There was also a dose – response relationship between increasing severity of sleep apnea and elevated BNP. However, only the difference between severe and mild OSAHS was statistically significant (p=0.029).
Fig. 1 Distribution of BNP by OSAHS severity. OSAHS, obstructive sleep apnea-hypopnea syndrome; BNP, brain natriuretic peptide.
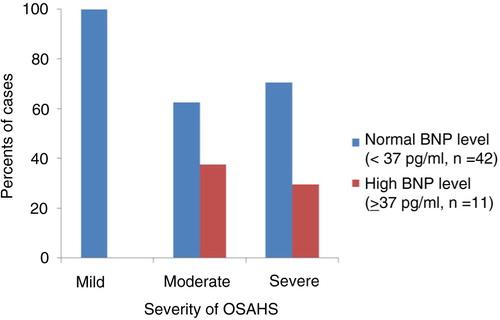
Table 3 OSAHS classification according to baseline BNP levels (pg/ml)
Baseline characteristics of the AHI groups are shown in . There were no significant differences between the two groups in age, sex ratio, smoking status, BMI, average blood pressure, ESS, spirometric indices, or creatinine clearance. In participants with high AHI (≥5/h), mean SpO2 and minimal SpO2 were significantly lower (p<0.001), while blood sugar levels were significantly higher (p=0.029), as well as mean BNP level (22.3±14.79 pg/ml vs. 9.2±6.75 pg/ml among participants with an AHI<5, p<0.0001). This association between high baseline AHI and higher baseline BNP levels remained after adjustment for confounders of blood sugar levels.
Table 4 Baseline patient characteristics according to baseline AHI
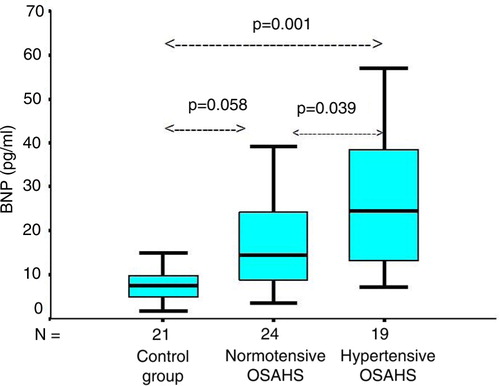
Hypertensive OSAHS patients had the highest baseline BNP level (27.7±16.74 pg/ml). It was significantly higher than in normotensive OSAHS patients (18±11.72 pg/ml) (p=0.039) and in controls (9.2±6.75 pg/ml) (p=0.001). Normotensive OSAHS patients had higher baseline BNP levels than controls, but there was no statistically significant difference between the two groups (p=0.058) ().
Fig. 2 The BNP levels in hypertensive OSAHS were significantly higher than in normotensive OSAHS patients (18.05±11.73 pg/ml) (p=0.039) and controls (9.26±6.75 pg/ml) (p=0.001). Normotensive OSAHS patients had higher baseline BNP levels in comparison with controls, but there was no statistically significant difference between the two groups (p=0.058). Boxes represent values within the interquartile ranges. Whiskers represent the data range, and the lines across the boxes represent the median values. The bottom and top of the boxes are the 25th and 75th percentiles, respectively. OSAHS, obstructive sleep apnea-hypopnea syndrome; BNP, brain natriuretic peptide.
Effect of CPAP therapy
Twenty-seven patients who were treated with CPAP were reexamined after a follow-up of 6 months. The integrated hour meter showed that only 23 patients used CPAP regularly. Four patients had poor CPAP compliance and were excluded from the CPAP trial.
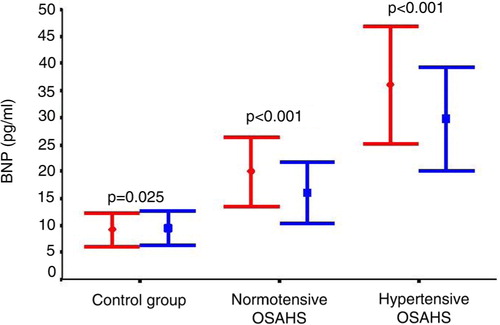
Compared with baseline, treatment with CPAP significantly decreased BNP levels in hypertensive OSAHS patients (from 36±16.1 to 29.7±14.29, p<0.001) and in normotensive OSAHS patients (from 20±10.09 to 16±8.98 pg/ml, p<0.001). In contrast, BNP levels slightly increased in controls (from 9.2±6.75 to 9.5±7.02 pg/ml, p=0.029), but there was no statistically significant difference from the baseline value ().
Fig. 3 Effect of CPAP treatment in hypertensive (n=11) and normotensive (n=12) OSAHS patients compared with controls (n=21). OSAHS, obstructive sleep apnea-hypopnea syndrome; BNP, brain natriuretic peptide; CPAP, continuous positive airway pressure. Red: baseline levels; blue: levels after 6 months of CPAP therapy.
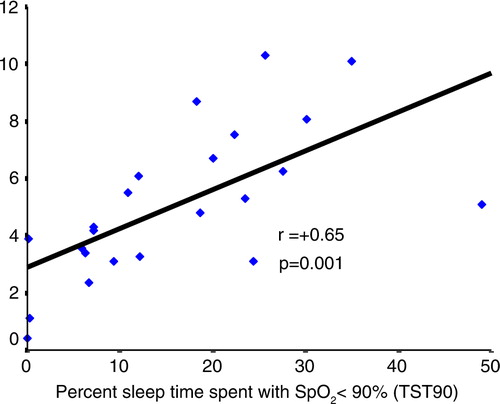
The changes in BNP levels (ΔBNP) were more marked in OSAHS with higher baseline BNP levels. As the hypertensive OSAHS patients had the highest baseline BNP, they had the magnitude of the CPAP effect (p=0.015, r=0.72) in comparison with normotensive OSAHS patients (p=0.03, r=0.62) (). Moreover, we found a significant association between the change in BNP levels and baseline TST90 (p=0.001, r=0.65) (). In contrast, we did not find any association with ESS, AHI, or ODI.
Fig. 4 In linear regression, the changes in BNP levels in 23 patients OSAHS after 6-month CPAP treatment were more important in OSAHS patients with higher baseline BNP levels. Note that hypertensive OSAHS patients had the higher delta BNP (T6–T0) (p=0.015, r=0.72) in comparison with normotensive OSAHS patients (p=0.03, r=0.62). OSAHS, obstructive sleep apnea-hypopnea syndrome; BNP, brain natriuretic peptide; CPAP, positive airways pressure. Blue: hypertensive OSAHS; red: normotensive OSAHS.
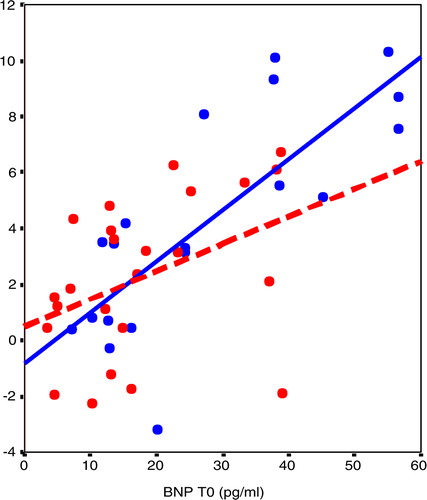
Fig. 5 In linear regression, the changes in BNP levels in 23 patients OSAHS after 6-month CPAP treatment were more pronounced in OSAHS patients with prolonged nocturnal desaturation (TST90) (r=+0.65, p=0.001). OSAHS, obstructive sleep apnea-hypopnea syndrome. BNP, brain natriuretic peptide; CPAP, continuous positive airway pressure.
Discussion
Is OSAHS associated with elevated serum levels of BNP?
In this study, we showed that the serum BNP level was significantly increased in patients with OSAHS in comparison with controls. The two groups were closely matched for almost all confounding variables such as age, BMI, blood pressure, and renal function. The higher blood sugar level in the OSAHS group was the only difference. After adjustment, a significant correlation between BNP level and OSAHS was identified. We also show that the elevated level of BNP is positively correlated with the severity of OSAHS, independent of obesity. Our results indicate that BNP is sensitive enough to detect myocardial stress caused by OSAHS and could be used as a sensitive marker for underlying preclinical cardiovascular changes in patients with OSAHS (Citation6).
Our finding is in accordance with some previous studies. Kita et al. (Citation14) reported increased levels of BNP during sleep in patients with OSAHS. However, cardiovascular diseases were not considered and the study group was small (12 males and 2 females). Similarly, in a community-based sample of 349 women, Ljunggren et al. (Citation8) found a relationship between the severity of sleep apnea during the night and the levels of plasma BNP in the morning that cannot be explained by known confounding factors. The same conclusion was reached by Kaditis et al. (Citation15) in a study on children with OSAHS investigated with overnight PSG and compared with simple snorers.
Many hypotheses have been suggested to explain how OSAHS might contribute as a stimulus of BNP release. It is well known that BNP is primarily secreted by the left ventricular myocytes in response to cardiac wall stretching. Hypoxia has also been reported to induce BNP secretion (Citation16). In contrast, we know that exaggerated negative intrathoracic pressure and its repetitive fluctuations during nightly apnea episodes markedly increases ventricular transmural pressure and left ventricular afterload, as well as the end-systolic and end-diastolic volume during sleep. Indeed, all of them together may give rise to both pressure load and volume expansion (Citation17). Furthermore, sustained activation of the sympathetic nervous system and intermittent nocturnal hypoxemia may also contribute. Repetitive rise in blood pressure may also add by inducing myocyte damage and increasing blood BNP levels (Citation18). Based on these findings, some authors, such as Maeder et al. (Citation19), have claimed that the high level of BNP is a convenient marker for detecting cardiovascular damage. Nevertheless, there is still uncertainty about whether OSAHS affects BNP, as several other studies have reported different data. In a prospective study on 60 consecutive patients, Hübner et al. (Citation6) did not find any correlation between N-terminal pro-brain natriuretic peptide (NT-pro-BNP) and AHI or other sleep-related indices. In multiple regression analysis, NT-pro-BNP was significantly correlated with left ventricular ejection fraction, creatinine clearance, and the presence of arterial hypertension but not with AHI. Likewise, Cifçi et al. (Citation20) evaluated 33 patients with OSAHS and did not find statistically significant increases in the levels of serum BNP. Consequently, they concluded that OSAHS does not induce enough myocardial damage to increase serum BNP levels. Tasci et al. (Citation9) found that OSAHS did not affect BNP levels even in hypertensive OSAHS patients, in contrast to our study. Recently, Maeder et al. (Citation7) showed that significant OSAHS is associated with a more pronounced overnight reduction in BNP. However, they could not find any significant differences in NT-pro-BNP between patients with moderate or severe OSAHS and those with mild or no OSAHS.
Is BNP decreased by nasal CPAP in OSAHS?
The most important finding of our study is that CPAP application decreased BNP levels significantly in OSAHS patients, suggesting that CPAP therapy improves cardiovascular function (Citation6). To our knowledge, this is the second time that CPAP application has been shown to produce a long-term reduction of BNP levels in healthy OSAHS patients. Kita et al. (Citation14) were the first to demonstrate that CPAP gradually decreases the nocturnal BNP profile from evening to morning in severe OSAHS patients. None of the patients in that study had clinical signs of heart or renal failure or lung disease, and they did not have any apparent edema. More recently, Tasci et al. (Citation9) observed that the NT-pro-BNP in OSAHS patients is significantly decreased after the first night of CPAP application. Here too, the study did not include patients with congestive heart failure, confirmed either clinically or echocardiographically. Zhao et al. (Citation21) reached the same conclusion. However, this study was conducted in OSAHS patients with congestive heart failure.
The mechanism of the effect of CPAP on NT-pro-BNP levels is not clear. However, Tasci et al. (Citation9) suggested that the main mechanism might be the Mueller maneuver because the application of CPAP normalizes intrathoracic pressure, which decreases by up to 50 mmHg during apneas (Citation22). This barometric change leads to a normalization of increased systolic transmural pressures and left ventricular afterload. Curiously, we found that the magnitude of the effect was greater in patients with higher baseline BNP levels, hypertensive patients, and those with the most nocturnal prolonged desaturation. These results suggest that increased cardiovascular risk factors are associated with a more marked effect of CPAP.
In contrast to our data, most studies did not find any effect of CPAP on BNP levels, neither short term nor long term. Maeder et al. (Citation7) showed that short-term CPAP used on 21 patients with moderate/severe OSAHS did not affect plasma concentrations of NT-pro-BNP before or after sleep, and changes during sleep did not significantly differ between the nights with and without CPAP. On the other hand, Hübner et al. (Citation6) observed an interesting long-term lowering effect on NT-pro-BNP levels by CPAP therapy in six patients with pathologically elevated levels before treatment. However, this observation was not statistically significant, probably due to the small subset group. Similarly, Colish et al. (Citation23) prospectively followed 47 patients with severe OSAHS under prolonged CPAP therapy. At baseline, the NT-pro-BNP levels were within normal limits for the entire population. At 3, 6, and 12 months of follow-up, there was no significant change in NT-pro-BNP levels compared with baseline. In agreement with these data, Cifçi et al. (Citation20) did not detect any significant difference in the levels of pro-BNP after 6 months of CPAP therapy. Only patients with moderate or severe OSAHS were included and none had congestive heart failure.
In view of all of these conflicting data, it is difficult to draw any clear conclusions about the acute and long-term effects of CPAP on BNP levels in OSHAS patients. Any result needs careful interpretation due to the possible role of other confounding variables (Citation6).
Limitations and strengths of this study
The strengths of this study include comparison of the OSAHS group to a control group with adjustment for relevant confounding covariates. Moreover, a group of patients with untreated OSAHS was compared with patients treated by CPAP. Additionally, all the blood samples were collected the morning after the nocturnal recording. The limitations of our study were the small size of the study group and the use of polygraph type 3 instead of standardized polysomnographic recording. Moreover, because we only evaluated patients with OSAHS without preexisting cardiac diseases, future studies are required to evaluate the effects of compliant CPAP therapy on BNP levels in patients with OSAHS and heart failure.
Conclusion
According to our results, BNP seems to be sensitive enough to detect myocardial stress caused by OSAHS. It might then be suggested as a potential marker for screening of preclinical cardiovascular damage in patients with untreated OSAHS. However, it should be noted that these findings are purely of an associative nature and do not imply cause and effect. Therefore, more research is needed before making any definitive conclusions.
The most important finding of our study is the decrease in daytime BNP levels after long-term CPAP application. This effect was more pronounced with higher baseline BNP levels. This finding provides further support for understanding how CPAP therapy can improve cardiovascular outcomes, as demonstrated by Marin et al. (Citation24). We also suggest that the BNP level can be useful for predicting the cardiac effects of CPAP therapy in the earlier stages of OSAHS without heart failure. Here again, because of the conflicting data in the literature, further studies on larger samples and rigorous methods are needed to identify potential applications that might be derived from the findings of the present study.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; 15: 136–43.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. 2014; Chicago, LI: American Academy of Sleep Medicine.
- Bradley TD, Floras JS. Obstructive sleep apnea and its cardiovascular consequences. Lancet. 2009; 373: 82–93.
- Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, etal. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003; 348: 1233–41.
- Boerrigter G, Costello-Boerrigter LC, Burnett JC Jr. Natriuretic peptides in the diagnosis and management of chronic heart failure. Heart Fail Clin. 2009; 5: 501–14.
- Hübner RH, El Mokhtari NE, Freitag S, Rausche T, Göder R, Tiroke A, etal. NT-proBNP is not elevated in patients with obstructive sleep apnea. Respir Med. 2008; 102: 134–42.
- Maeder MT, Strobel W, Christ M, Todd J, Estis J, Wildi K, etal. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem. 2015; 48: 340–6.
- Ljunggren M, Lindahl B, Theorell-Haglöw J, Lindberg E. Association between obstructive sleep apnea and elevated levels of type B natriuretic peptide in a community-based sample of women. Sleep. 2012; 35: 1521–7.
- Tasci S, Manka R, Scholtyssek S, Lentini S, Troatz C, Stoffel-Wagner B, etal. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin Res Cardiol. 2006; 95: 23–30.
- Riachy M, Juvelikian G, Sleilaty G, Bazarbachi T, Khayat G, Mouradides C. Validation of the Arabic version of the Epworth sleepiness scale: multicentre study. Rev Mal Respir. 2012; 29: 697–704.
- Blacher J, Lelong H, Kretz S, Yannoutsos A, Kondo T, Safar M. [JNC 8 is released … but this is not the JNC 8! New US guidelines for the management of hypertension]. Presse Med. 2014; 43: 1048–55.
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, etal. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012; 15: 597–619.
- Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, etal. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993; 147: 887–95.
- Kita H, Ohi M, Chin K, Noguchi T, Otsuka N, Tsuboi T, etal. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998; 7: 199–207.
- Kaditis AG, Alexopoulos EI, Hatzi F, Kostadima E, Kiaffas M, Zakynthinos E, etal. Overnight change in brain natriuretic peptide levels in children with sleep-disordered breathing. Chest. 2006; 130: 1377–84.
- Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008; 268: 59–93.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011; 57: 119–27.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995; 96: 1897–904.
- Maeder MT, Ammann P, Rickli H, Schoch OD, Korte W, Hürny C, etal. N-terminal pro-B-type natriuretic peptide and functional capacity in patients with obstructive sleep apnea. Sleep Breath. 2008; 12: 7–16.
- Cifçi N, Uyar M, Elbek O, Süyür H, Ekinci E. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath. 2010; 14: 241–4.
- Zhao ZH, Liu ZH, Luo Q, Xiong CM, Ni XH, Zhang J, etal. Positive pressure ventilation treatment reduces plasma levels of amino terminal-pro brain natriuretic peptide in congestive heart failure patients with sleep apnea. Circ J. 2006; 70: 572–4.
- Sharpey-Schafer EP. Hamilton WFDP. Effect of respiratory acts on the circulation. Handbook of physiology: a critical, comprehensive presentation of physiological knowledge and concepts. Section II: circulation. 1965; Washington, DC: American Physiological Society. 1875–86.
- Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, etal. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012; 141: 674–81.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365: 1046–53.
