1 Case presentation
A three-week-old boy with Down syndrome presented with poor feeding and jaundice. He had been born at term by normal delivery at home. The pregnancy had been uneventful and the mother had declined antenatal testing so the diagnosis of Down syndrome was made in the postnatal period. The infant required oxygen at birth and was admitted to the neonatal unit for a few days after birth. He started to become jaundiced after discharge. There was no family history of note and the infant has three siblings who are healthy. The mother is Rh D positive.
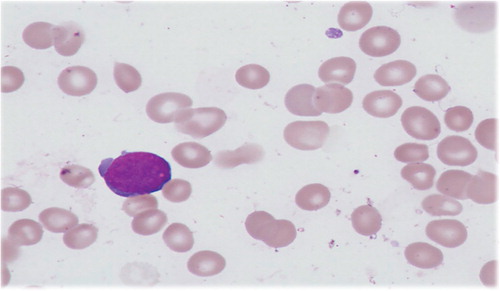
On examination the infant was jaundiced, with neither hepatosplenomegaly, nor signs of sepsis but had hypotonia typical of Down syndrome. Investigations revealed unconjugated jaundice with a negative direct antiglobulin test. A full blood count showed thrombocytopenia with a platelet count of 65×109/L, a white cell count of 8.6×109/L, neutrophil count of 1.9×109/L and haemoglobin level of 18.0 g/dL. Blast cells of medium to large size with cytoplasmic blebs were found on the blood film (see ). Immunophenotyping showed the blasts to have myeloid markers with expression of CD41 and CD61.
Figure 1. Photomicrograph of a typical TAM blast cell seen on a blood film (600× oil immersion objective).
The infant was treated with phototherapy and the jaundice improved. He was followed up closely with weekly full blood counts for two months.
2 Diagnosis
Transient abnormal myelopoiesis (ICD-O 9898/1).
He was reviewed in clinic after three months when he was found to be well and gaining weight. His full blood count showed the previously documented thrombopenia had normalised but neutropenia had persisted: haemoglobin 11.5 g/dL, white cell count 5.4×109/L, platelets 334×109/L and neutrophils 1.09×109/L. Vacuolated neutrophils, reactive lymphocytes and the occasional primitive cell were seen on blood film reflective of a reactive process. Within a further two months and at an age of six months his full blood count had normalised completely confirming that the diagnosis had been transient abnormal myelopoesis (TAM). He continues to have monthly full blood counts as 70% of acute myeloid leukaemia (AML) cases following TAM develop some form of cytopenia prior to developing an increasing blast percentage.
3 Discussion
TAM is the clonal proliferation of myeloid blast cells which affects an estimated 5–10% of infants with Down syndrome and trisomy 21 mosaicism Citation1. The majorities of infants with TAM are asymptomatic and picked up incidentally on routine testing. Most cases of TAM resolve over the first few months of life, but 13–33% may go on to develop AML within the first four years of life.
Neonates with Down syndrome are unique in having a predisposition to transient abnormal myelopoeisis Citation2. One of the reasons for this is thought to be that trisomy 21 affects foetal liver haematopoesis causing an increase in the megakaryocyte-erythroid progenitor frequency with common myeloid progenitors and a reduction in the granulocyte–monocyte progenitors. Increased clonogenicity affecting megakaryocyte–erythroid progenitors, granulocyte–monocytes and colony-forming unit-granulocytes has also been found Citation3. GATA-1 mutations, a transcription factor mutation integral to the normal development of erythroid, megakaryocytic and basophilic cell lines, have been associated with TAM and AML in Down syndrome Citation4. As such, TAM and myeloid leukaemia of Down syndrome provides an excellent model for leukaemogenesis Citation5.
TAM is difficult to diagnose and is not easily distinguished from AML. Microscopically, TAM is seen as an increase in the number of blasts on peripheral blood film with thrombocytopenia or other cytopenias. There may be fewer blasts in the marrow than peripheral blood.
Five-year survival after TAM is 85% and event-free survival 63% Citation1. The typical clinic picture of a patient requiring treatment is one of an unwell infant on intensive care with an oxygen or inotrope requirement or serious bleed. Often they have hepatomegaly. Factors associated with early death in TAM include a high white cell count, increased liver enzymes, failure to normalise the blood count, ascites, preterm delivery, bleeding diatheses and failure of spontaneous remission Citation1.
These events can occur in utero and be a cause of foetal death. Treatment with low-dose cytarabine in TAM patients with a high white cell count, thrombocytopenia or liver dysfunction has been found to have a favourable effect on five-year event-free survival Citation1. The dose chosen is usually 1–2 mg/kg intravenous or subcutaneous daily for 3–7 days Citation1. The diagnosis of TAM has prognostic importance as AML patients with a history of TAM have a better five-year event-free survival than those without Citation1.
4 Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- Klusmann JH, Creutzig U, Zimmermann M, Dworzak M, Jorch N, Langebrake C, et al.. Treatment and prognostic impact of transient leukaemia in neonates with Down syndrome. Blood. 2008; 111: 2991–8.
- Webb D, Roberts I, Vyas P. Haematology of Down syndrome. Arch Dis Child Fetal Neonatal Ed. 2007; 92: F503–7.
- Tunstall-Pedoe O, Roy A, Karadimitris A, de la Fuente J, Fisk NM, Bennett P, et al.. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood. 2008; 112: 4507–11.
- Ahmed M, Sternberg A, Hall G, Thomas A, Smith O, O'Marcaigh A, et al.. Natural history of GATA1 mutations in Down syndrome. Blood. 2004; 103: 2480–9.
- Vyas P, Roberts I. Down myeloid disorders: a paradigm for childhood preleukaemia and leukaemia and insights into normal megakaryopoiesis. Early Hum Dev. 2006; 82: 767–73.