Abstract
Medicines are health technologies that can translate into tangible benefits for numerous acute as well as chronic health conditions. A nation's pharmaceutical sector needs to be appropriately structured and managed in order to ensure a safe, effective and quality supply of medicines to society. The process of medicines management involves the sequential management of five critical activity areas; namely; registration, selection, procurement, distribution and use. Formalized and standardized management of all five critical activity areas positively influences the availability, quality and affordability of medicines and ultimately increases the reliability and quality of the national healthcare system.
Aim : The aim of this review is to examine the current structure and operation of medicines management (i.e. the pharmaceutical sector) in Libya.
Conclusion : In the Libyan healthcare system all five critical activity areas are compromised. Restructuring of the pharmaceutical sector in Libya is required in order to provide and sustain sound pharmaceutical services for Libyan society and improve the national public health outcomes.
Introduction
Medicines are health commodities that can translate into tangible benefits for numerous acute as well as chronic health conditions Citation1. The pharmaceutical sector of any nation is responsible for providing society with medicines and other pharmaceutical services. Flaws in the structure and management of the pharmaceutical sector can limit the population's access to quality pharmaceuticals, thereby depriving society of the health gains associated with these life-saving commodities.
The pharmaceutical sector is a multifaceted and technically complex arena that requires highly skilled personnel and a standardized operational infrastructure. Government should therefore hold the task of management of the pharmaceutical sector in order to ensure that it is institutionally sound and operates efficiently and in harmony with other healthcare sectors Citation2. Governments need to ensure that medicines are appropriately manufactured, supplied, and used Citation3 Citation4.
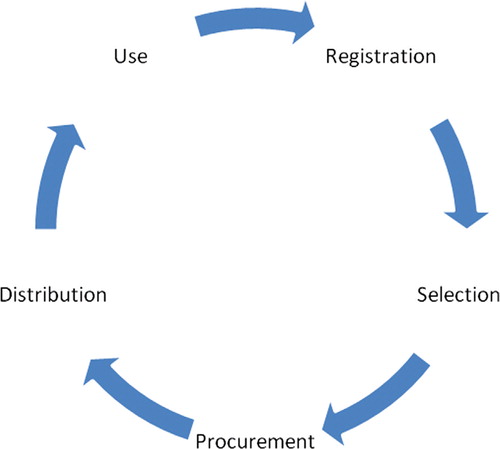
For the pharmaceutical sector to operate safely and efficiently the sequential operation of five primary areas of activity are required Citation3 Citation5: medicines registration, medicines selection, medicines procurement, medicines distribution, and medicines use (see ).
Libya is an upper middle income North African nation that strives for high standard healthcare services Citation6. Nevertheless, according to the World Health Organization (WHO) ‘Inappropriate management of drug supply and distribution’ is a major constraint facing health development in Libya Citation6. Evidence from local studies Citation7 Citation8 and the WHO Citation6 indicate that medicines management in Libya requires reconsideration. This paper will provide an overview in relation to each of the five critical areas of activity in the pharmaceutical sector and highlight processes that could be improved.
Medicines registration
Medicines registration is a critical regulatory function of the government (Ministry of Health) that should ensure that all medicines that are available within the nation's health system are safe, effective, and of good quality Citation2. The process of medicines registration evaluates the efficacy of a given medicine for a specific clinical indication as well as its safety and adverse effect profile Citation9. It is important to stress that the registration process should approve a particular product (dosage form and dosage strength) from a specific manufacturer only, as opposed to the singular generic active ingredient. In addition, medicines registration sets the standards that regulate the presentation and use of all medicinal products (e.g. labeling, storage conditions, marketing, warnings, and prescription requirements) Citation2 Citation5. Even though one generic medicine may be available nationally as many different products (i.e. dosage forms and strengths) from a variety of manufacturers, each of these individual products should undergo individual review and registration by the registering authorities Citation3.
It is also critical to stress that medicines registration should not be limited to the approval of new medicines, but should also include the periodical review of existing products on the national market Citation2.
Public health relevance of robust medicines registration
Weak, inefficient, or hasty non-systematic medicine registration systems increase the potential for infiltration of the national healthcare system with poor quality, irrational, substandard, or counterfeit medicines Citation10 Citation11 Citation12 Citation13 Citation14 . The public health consequences of substandard or counterfeit medicines can undermine the whole healthcare process and waste national healthcare resources Citation14. Substandard medicines impose a silent threat to healthcare systems that can lead to morbidity or, at worst, mortality. Antimicrobial medicines are of particular concern. Substandard quantities or unwanted inclusion of other antimicrobial medicines or contaminants in a medicinal product could cause treatment failure, have safety implications, or aggravate problems of antimicrobial resistance Citation12.
Medicines registration in Libya
Despite the fact that a medicines regulatory authority should be a vital component of any national health system, the WHO estimates that only one in six nations worldwide have fully functioning medicine registration systems; most of which are located in high-resourced nations Citation15. In developing nations, many barriers undermine the availability and/or the efficiency of medicine registration systems Citation13. According to Caudron et al. these barriers include a limited understanding of the significance of medicines registration as a key and critical regulatory step by national decision makers, the lack of essential infrastructure such as quality assurance laboratories, and appropriately trained human resources. These barriers have rendered registration of medicines in developing countries a process that is mainly based on document review and estimation by national decision makers. As stated previously, such deficiencies can undermine the entire healthcare sector and waste scarce national resources Citation13 Citation16.
In Libya, the Libyan Health Law act number 106 of 1973 and its explanatory notes of 1975 state that registration of medicines within the Libyan Ministry of Health should precede the availability of any medicine in the Libyan market. However, the act (and regulations) only offers a general statement and does not detail the technical requirements and requisites needed for sound medicines registration as recommended by the WHO Citation2. The limitations of medicines registration in Libya have been highlighted by Libyan researchers Citation7 Citation8 as well as the WHO Citation6. In 2003, Faitoori et al. noted the lack of an active registration process and the negative impact of this flaw on the quality of medicines in Libya Citation7. In 2005, Ekhshaibah also stated that medicine registration is inactive and is mainly hindered by a lack of independence, minimal infrastructure, and the lack of appropriately trained personnel Citation8. The WHO has stated that medicines registration in Libya not only lacks the availability of a defined independent body, but also operates with a limited technical capacity and infrastructure that is not sufficient for the optimal approval of the quality, safety, and efficacy of medicines in the Libyan healthcare system Citation6 Citation8 Citation15. The WHO also stated that appropriately equipped government quality assurance laboratories are lacking and quality assurance is mainly undertaken in some schools of pharmacy with limited expertise and operating facilities Citation6.
Although acknowledged as an area that requires improvement, there is no current available data in relation to how many products may be substandard in Libya (8), but media reports have indicated an alarming problem with medicines quality in Libya Citation1. In summary, medicines registration in Libya requires review, restructuring, and the implementation of a revised, thorough system in line with WHO guidelines. It is also important to reinforce the requirement of universal medicines registration for all subsidized and privately available medicines in Libya.
Medicines selection for government subsidy
Medicines vary in efficacy and price, therefore not every registered medicine is a candidate for government subsidy. A limited subset of registered medicines needs to be selected for the purpose of government subsidy Citation3 Citation4. Logically, this subset of medicines should be selected in reference to predefined criteria that ensure efficient coverage of the general pharmaceutical needs of the national population Citation2 Citation9. The WHO has created the Essential Medicines Concept (EMC) as a standardized framework that aids in appropriate medicines selection Citation17 Citation18.
The Essential Medicines Concept (EMC)
The EMC revolves around the selection of a subset of government subsidized medicines that are safe, clinically effective, and also cost effective. These government subsidized medicines should be available to treat the commonest national diseases and health conditions. The WHO has named this subset of medicines as ‘essential medicines’ Citation19. Essential medicines are: ‘those that satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness. Essential medicines are intended to be available within the context of functioning health systems at all times, in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. The implementation of the concept of essential medicines is intended to be flexible and adaptable to many different situations. Exactly which medicines are regarded as essential remains “a national responsibility”’ Citation17. The definition of essential medicines clearly identifies the public health relevance of selected medicines, evidence on efficacy and safety, comparative cost effectiveness, and the availability of essential medicines in the appropriate dosage forms as the baseline criteria of medicines selection for government subsidy schemes.
The list that encompasses national essential generic medicines has been named by the WHO as the National Essential Medicines List (NEML) Citation17. According to the WHO, the NEML should be the basis of medicines selection for public procurement (government subsidy schemes) as well as other medicines’ use activities in the public health system Citation20 Citation21.
The process of medicines selection for the NEML should be formalized by allocation of the task of medicines selection to a defined committee that is equipped with the appropriate technical skills. Appropriate technical skills include: current knowledge of clinical studies and therapeutics, adverse drug reactions, national morbidity and mortality shifts, and pharmaco-economics and cost evaluation studies Citation9. The process of medicines selection should also be open and dynamic, i.e. the NEML should undergo periodical review in order to keep up with advances in therapy and national morbidity shifts Citation5. In addition, a sound national health information system is a necessary prerequisite to enable the compilation and review of a country's most prevalent diseases and conditions Citation6 Citation22 Citation23.
The WHO Model List of Essential Medicines (WMLEM)
Since the actual task of medicines selection for a NEML is technically complex and requires an array of resources and skilled professionals that may not be available in developing countries Citation3 Citation5 Citation9, the WHO has identified a subset of medicines that are safe, effective, and cost effective for the world's most common diseases and conditions. This subset of medicines is listed in the WHO Model List of Essential Medicines (WMLEM) and the list has been regularly updated since 1977. Essential medicines listed in the WMLEM represent the minimum number of medicines that are recommended to be available within any fully functioning healthcare system and should be the foundation of any NEML Citation17 Citation18 Citation24.
Medicines selection in Libya
In the past, the National Pharmaceutical and Medical Equipment Company (NPMEC) was responsible for medicines selection for public procurement as well as distribution to public healthcare facilities in Libya in accordance with Act number 69 of 1972, for regulating the drug trade Citation7. Historically, medicines were selected for public procurement (full government subsidy of medicines) from the national standard list of medicines, commonly known as the Libyan Pharmaceutical List (LPL). The LPL was originally formulated by the Libyan Ministry of Health and is a list of all registered medicines in the Libyan healthcare system. For several decades, the LPL was employed by the NPMEC for public procurement in order to cater for the subsidized pharmaceutical needs of Libyan society with safe and quality medicines that did not financially overburden healthcare by allowing open purchases of medicines from the international market.
However, the selection of medicines in Libya, as represented by the LPL, was flawed at multiple levels Citation7 Citation8. The LPL was too vast, since it included all registered medicines in the Libyan healthcare system; therefore, regular procurement of all medicines listed on the LPL was impractical and imposed a burden on the Libyan healthcare budget. As a result, officials allocated with the task of procuring medicines had to select a limited subset of medicines for public procurement. However, their selection method was unsystematic and based on local estimations of need. This non-systematic approach ultimately affected the reliability and consistency of medicine supply to national public health facilities. In addition, the LPL was obsolete since it encompassed several superseded and unnecessary medicines Citation8 Citation15. This was largely the result of an inefficient medicines registration system that hindered addition or deletion of any medicines that were listed on the LPL. It is also important to note that the actual contents and details of the LPL were not freely available for scrutiny by Libyan health professionals, particularly physicians Citation7.
In 2003, local researchers recommended the implementation of a NEML in order to rectify the problem of medicines management in Libya Citation7. According to local information Citation8 and information from the WHO Citation6, the National Committee of Drugs was formulated in 2003 and entrusted with the task of reviewing the LPL. Local information showed that this task was held back by the lack of national expertise on appropriate medicines selection Citation8. In 2005, a draft list was published on the WHO website.Footnote1 However, this list encompassed several irrational and superseded medicines. In 2006, the LPL was cancelled and replaced by a new Libyan national standard list of medicines – the Libyan Pharmaceutical List of Essential Medicines (LPLEM). According to the Department of Pharmacy and Medical Equipment in the Libyan Ministry of Health, the LPLEM encompassed only essential medicines (rather than all registered medicines in the health system) as advocated by the WHO. The LPLEM continues to be the current Libyan national standard list for general procurement (full government subsidy of medicines).
The overview of the history of medicines selection in Libya indicates that the process of medicines selection for public procurement purposes in Libya lacks a declared methodical procedure that follows the WHO guidelines and that is available for scrutiny by Libyan healthcare professionals. In addition, review of the Libyan standard reference list has never been undertaken at regular temporal intervals since it requires a directive from the Libyan cabinet Citation7. Such directives are not automatically generated, therefore a regular review of the list has been haphazard. The process of medicines selection in Libya thus requires reconsideration by national decision makers.
Medicines procurement
The procurement activity involves the task of the actual purchasing of medicines in an efficient manner. The interaction between drug manufacturers and the national healthcare system takes place at this critical interface Citation3 Citation5 Citation9. Procurement involves a spectrum of duties such as quantification of national pharmaceutical needs, bulk purchasing, establishment of bidding contests, technical analysis of offers, allocation of financial resources, payments, receipts of purchased medicines, quality assurance checks, and stock management. These activities also need to be standardized and carried out in a systematic manner in order to ensure sound and efficient procurement of medicines Citation9. Sound procurement requires a robust NEML, reliable data on actual consumption of medicines in national healthcare facilities, personnel trained in appropriate procurement practices, and sufficient financial resources in order to appropriately quantify and purchase national pharmaceutical needs Citation3 Citation5 Citation9.
Medicines procurement in Libya
In the Libyan healthcare system, procurement is undertaken via reference to the national standard reference list, currently the LPLEM. However, minimal information is available in relation to the actual procurement process. According to the available evidence, procurement in the Libyan health system is not a temporally standardized process and occurs via annual or biennial tenders according to random estimation of national needs Citation7 Citation8. Appropriate procurement is also hindered by the lack of a systematic process of quantification of medicines as well as the lack of properly trained personnel in relation to the technical details of appropriate procurement techniques and practices Citation7. Medicines procurement in Libya requires indepth attention as it is not undertaken in concordance with current WHO guidelines.
Medicines distribution
The main function of a national medicines distribution system is to ensure efficient delivery of purchased pharmaceuticals to national warehouses and dispensing outlets. Efficient distribution systems must have storage facilities – including refrigeration units – that fulfill WHO recommendations in order to ensure that medicines are stored and transported in the appropriate conditions that preserve the therapeutic qualities of medicines. Security measures should also be in place in order to minimize the risk of theft and medicines seepage outside the national public healthcare system. In addition, it is important to ensure the accurate flow of inventory information as a critical aspect of medicines distribution in order to make certain that stocks are appropriately managed and supplied Citation2 Citation3 Citation9.
Medicine distribution in Libya
According to Faitoori et al., distribution in the Libyan pharmaceutical sector of the Libyan healthcare system has endured several limitations that hinder efficient performance Citation7. Such limitations include, but are not limited to, weak transportation systems, obsolete inventory management systems (medicines expire in storage), lack of coordination between storage and transportation systems, lack of computerized information systems, and weak security and monitoring systems that lead to seepage of medicines outside the healthcare system. Medicines distribution processes in Libya also require review and reorganization in order to comply with current WHO guidelines.
Medicines use
Effective medicines outcomes are ultimately the most important aspect of medicine use. It is here that patients experience either the benefits or the detriments of the pharmaceuticals provided by the national healthcare system. This activity area is the most difficult to assess since it involves the interplay of physicians, pharmacists, patients, and others who may have conflicting interests that can compromise medicines use in the national healthcare system Citation5 Citation9. The WHO recognizes inappropriate (irrational) use of medicines as a common practice worldwide Citation15. According to the WHO, more than half of all medicines are prescribed, dispensed, or sold inappropriately, and that only about half of all patients use medicines in a correct manner. The WHO has proposed a framework on the rational use of medicines as an essential component of the EMC Citation21. According to the WHO, medicines use should be formalized, standardized, and governed by regulatory as well as educational interventions Citation21. The problem of irrational medicines use exists in both low resource as well as high resource countries Citation15. However, the problem is more significant in developing countries due to many aggravating contextual factors that hinder the appropriate (rational) use of medicines Citation25.
Standardization of medicines use is an important component of national medicine policies Citation2 and modern healthcare delivery; since misuse, overuse or under use of medicines can impose a silent but serious threat to public health and, as a result, wastes the limited national resources Citation15. A NEML that has been formulated in concordance with WHO guidelines is central to the rational use of medicines as it improves medicines availability and enhances all educational efforts (for health professionals as well as patients) on the rational use of medicines. Liang et al. also recommended ten approaches that can be utilized by national decision makers in developing countries to standardize the medicines use process Citation20. These approaches include the implementation of a NEML that is linked to robustly developed standard treatment guidelines, drug information centers that provide health professionals with up-to-date independent drug information as well as other training and managerial measures.
It is also important to mention that the problem of irrational use of medicines requires collaborative efforts from all stakeholders in the pharmaceutical sector (physicians, pharmacists, government officials (the Ministry of Health), national medical associations, academia, and others) Citation26, therefore, measures and initiatives for the rational use of medicines should be administrated at the government level.
Medicines use in Libya
The WHO asserts that activities on the rational use of medicines in Libya need to be assessed and developed Citation6. The availability of information on patterns of use of medicines has been hindered by the general scarceness of published research in Libya Citation6 Citation27. ‘Absence of health systems research as an integral part of national health development can be considered as ‘one of the major constraints that faces health development in Libya’ Citation6.
We identified one study published in 1988 that investigated the prescribing patterns in six out-patient clinics in Tripoli Citation28. The study found that anti-infective medicines were prescribed at a high rate (85% of studied prescriptions). Vitamins (63%), analgesics (61%), antihistamines (35%), medicines acting on the gastrointestinal tract (19%), and hematinics (11%) were also commonly prescribed to patients. Another study published in 2003 identified a potential medication use problem (over prescribing of antibiotics and pain-killers), however, this problem was embedded in an availability of medicines in Libyan public healthcare facilities review Citation7. More research examining the current quality of medicines’ use in Libya is critical and will assist in improving healthcare in Libya Citation2 Citation25.
Also of note, the website of the Libyan Board of Medical SpecialitiesFootnote2 does not currently include any educational activities in relation to the rational use of medicines as advocated by the WHO. In addition, specific practice guidelines, including therapeutic guidelines on the appropriate use of medicines are currently lacking at all levels of the healthcare system Citation6. Inter professional collaboration and evidence-based decision making are essential components for activities on the rational use of medicines and modern healthcare delivery Citation21 Citation26. The Libyan healthcare system needs to encourage professional collaboration and evidence-based decision making Citation6. Overcoming such constraints is the first step toward the rational and effective use of medicines in Libya.
Transparency in medicines management
In order to ensure sound performance of the pharmaceutical sector, most activities in medicines management need to operate in a transparent manner that allows scrutiny by national healthcare professionals and other stakeholders Citation5 Citation9. For instance, national medicines regulatory authorities (registration systems) need to be formalized and applied in an open and reliable manner that complies with WHO guidelines. There is no place for personal judgment or inappropriate practices that imperil the quality of medicines or their use in the national healthcare system Citation2 Citation3 Citation5 Citation9.
Transparency is also an indispensable component of the selection activity for medicines management. Information in relation to the committee entrusted with the task of selecting medicines for the NEML should be publicly disclosed (on a website or similar channels) in order to ensure accountability and avoid inappropriate practices that endanger the quality and availability of medicines in the national healthcare system Citation5 Citation9 Citation17.
It is also important to mention that the stability of the pharmaceutical sector is a key component that secures long-term planning and sustainability of pharmaceutical services in any health system Citation2 Citation3. Stability has been a major issue that has undermined development, review, and improvement of the Libyan pharmaceutical sector Citation7 Citation8.
Conclusion
The sound and systematic management of the pharmaceutical sector is a critical requisite for the appropriate supply and use of medicines in the national healthcare system. In order to improve the pharmaceutical sector, the processes involved in medicines management need to be clearly defined in the Libyan national healthcare system. This will require extensive resources, thoughtful planning, and technical expertise. In addition, scarceness of research evidence and review in relation to all activities undertaken within the pharmaceutical sector is an area that can and needs to be addressed by appropriate research.
Recommendations
The Libyan management of the pharmaceutical sector does not measure up to the standards recommended by the WHO. A key reason for the suboptimal performance of the pharmaceutical sector in Libya is the lack of stability, appropriate infrastructure, and well-trained personnel. Urgent attention and support from national decision makers is required for the optimal performance of the sector, which is critical to the healthcare of Libyan society.
We therefore recommend:
Effective adoption of the WHO guidelines in relation to all five critical activities in the pharmaceutical sector.
Encouraging research projects that will assess and, ultimately improve, the Libyan pharmaceutical and healthcare system.
Adoption of transparent measures in all five critical activities in order to enhance performance and accountability.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Notes
1Available from: http://www.emro.who.int/emp/Publications_Details.asp?ID = 251.
2Available from: http://www.lbmlibya.org/index.php.
References
- Mendis S, Fukino K, Cameron A, Laing R, Filipe A, Khatib O, et al.. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. 2007; 85: 279–88.
- WHO. How to develop and implement a national drug policy. 2nd ed.Geneva: WHO. 2001. Available from: http://apps.who.int/medicinedocs/pdf/s2283e/s2283e.pdf [cited 4 July 2010].
- Quick JD, editor. Managing drug supply: the selection, procurement, distribution, and use of pharmaceuticals. 2nd ed.Kumarian Press books on international development. West HartfordCT: Kumarian Press. 1997.
- Birkett DJ, Mitchell AS, McManus P. A cost-effectiveness approach to drug subsidy and pricing in Australia. Health Aff (Millwood). 2001; 20: 104–14.
- Cohen J. Global corruption report. Corruption in the pharmaceutical sector. ; 2006. Available from: http://www.transparency.org. [cited 4 July 2010].
- WHO. Country cooperation strategy for WHO and the Libyan Arab Jamahiriya 2005–2009. Regional Office for the Eastern Mediterranean. , Cairo: WHO. 2006. Available from: http://www.who.int/countryfocus/cooperation_strategy/ccs_lby_en.pdf [cited 4 July 2010].
- Faitoori A, Mgairbi Z, Alfakri M, Younis J, Hodana B, Bashir A, et al.. A study of the Libyan medicines situation. Libyan Central Bank. Libya, 2003
- Ekhshaibah E. Channels of medical supply in Libya. In:The fifth national pharmaceutical sciences conference; April22–24; 2005. Benghazi, Libya, 1–69.
- Cohen J. Improving transparency in pharmaceutical systems: strengthening critical decision points against corruption. Latin American and Caribbean Region, human development network. ; 2002. Available from: http://www.u4.no/pdf/?file = /themes/health/cohen_wb_paper_pharma2002.pdf [cited 4 July 2010].
- Gautam CS, Aditya S. Irrational drug combinations: need to sensitize undergraduates. Indian J Pharmacol. 2006; 38: 169–70.
- Poudel A, Palaian S, Shankar PR, Jayasekera J, Izham MIM. Irrational fixed dose combinations in Nepal: need for intervention. Kathmandu Univ Med J. 2008; 6: 399–405.
- Newton PN, Green M, Fernández F, Day N, White N. Counterfeit anti-infective drugs. Lancet Infect Dis. 2006; 6: 602–13.
- Caudron JM, Ford N, Henkens M, Mace C, Kiddle-Monroe R, Pinel J. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Trop Med Int Health. 2008; 13: 1062–72.
- Newton PN, Green MD, Fernández FM. Impact of poor-quality medicines in the developing world. Trends Pharmacol Sci. 2010; 31: 99–101.
- WHO. The world medicines situation. Geneva: WHO. 2004. Available from: http://apps.who.int/medicinedocs/en/d/Js6160e/ [cited 4 July 2010].
- Ravinetto RM, Cloëz S, Scouflaire-Mallet SM, Vandenbergh D. Poor-quality medicines in developing countries. Lancet Infect Dis. 2009; 9: 267–68.
- WHO. The selection of essential medicines. WHO policy perspectives on medicines. Geneva: WHO. 2002. Available from: http://whqlibdoc.who.int/hq/2002/WHO_EDM_2002.2.pdf [cited 4 July 2010].
- Hogerzeil HV. The concept of essential medicines: lessons for rich countries. BMJ. 2004; 329: 1169–72.
- Laing R, Waning B, Gray A, Ford N, Hoen E. 25 years of the WHO essential medicines lists: progress and challenges. Lancet. 2003; 361: 1723–29.
- Laing RO, Hogerzeil HV, Ross-Degnan D. Ten recommendations to improve use of medicines in developing countries. Health Policy Plan. 2001; 16: 13–20.
- WHO. Promoting rational use of medicines: core components. WHO policy perspectives on medicines No. 5. Geneva: WHO. 2002. Available from: http://apps.who.int/medicinedocs/pdf/h3011e/h3011e.pdf [cited 4 July 2010].
- Ellner A, Dillner L, Nash B, Godlee F. Essential medicines in the United States. BMJ Publishing Group. 2003. Available from: http://www.unitedhealthfoundation.org/Essential%20medicines%20-%20final.pdf [cited 4 July 2010].
- Okonjo-Iweala N, Osafo-Kwaako P. Improving health statistics in Africa. Lancet. 2007; 370: 1527–8.
- Reidenberg MM. World Health Organization program for the selection and use of essential medicines. Clin Pharmacol Ther. 2007; 81: 603–6.
- Le Grand A, Hogerzeil HV, Haaijer-Ruskamp F. Review article. Intervention research in rational use of drugs: a review. Health Policy Plan. 1999; 14: 89–102.
- WHO. The role of education in the rational use of medicines. Regional Office for South-East Asia. ; 2006. Available from: http://www.searo.who.int/LinkFiles/Reports_vision.pdf [cited 4 July 2010].
- Bakoush O, Al-Tubuly A, Ashammakhi N, Elkhammas E. PubMed medical publications from Libya. Libyan J Med. 2007; 2: 125–8.
- Naja SA, Idris M, Khan A. Drugs cost more at primary health clinics: an experience from Libya. Health Policy Plan. 1988; 3: 69–73.