Abstract
Traditional medicines, in particular herbal products, have been used abundantly over the years in curing several diseases. Pharmacological interactions of herbal products with modern drugs, however, remain to some extent unknown. Herein, we examined whether co-administration of Faizol Ubat Batuk (FUB), a mixture of aqueous extract of different plants, modifies the metabolism of aminopyrine, a conventional analgesic drug, in rat liver. We used rat hepatocytes outfitted by collagenase perfusion technique. Determination of aminopyrine n-demethylase activity was performed using the Nash colorimetric method, by measuring the amount of formaldehyde produced. Compared to control treatment, FUB significantly increased the hepatic metabolism of aminopyrine in healthy adult male rats. In contrast, the hepatic metabolism of aminopyrine in adult female rats was decreased. Besides, a biphasic effect in n-demethylase activity was observed in young male rats treated with FUB. In a subsequent experiment, FUB did not change the metabolism of aminopyrine in streptozotocin (STZ)-diabetic adult male rats. In conclusion, administration of FUB could affect phase I aminopyrine metabolism in rat heptocytes. In addition, the effects of FUB on hepatic n-demethylase activity were gender and disease dependent.
Traditional remedies have been used for thousands of years to maintain health. Nowadays they are still used either alone or in conjunction with modern drugs. The majority of populations take traditional medicines following experiences adopted by elders and ancestors. Most of the people believe that natural products are good and wholesome, whereas synthetic products are bad and poisonous. Hence, WHO reported that traditional medicines should be integrated into primary care in order to offer the best means of achieving the goal of health-care for the entire population Citation1 Citation2.
However, the majority of traditional medicines, either obtained from one plant or mixture of plants, need to be investigated scientifically in order to understand and to appreciate the experience obtained from their traditional uses. In general, herbal preparations, consumed by the public, contain a mixture of plant extracts. Hence, the active ingredients are unclear. Accordingly, the therapeutic outcomes and the adverse effects are achieved from the summation of all those ingredients present inside the herbal product. So, in view of the progress of modern medicine, not only the synthetic drugs but also the herbal drugs have to fulfill the international requirements on safety and efficacy Citation3. Consequently, analysis of the herbal products is of more immediate relevance with respect to the health benefit or risk to consumers.
Herb–drug interactions are able to induce an enhancement or decrease in efficacy of co-administered drugs. Pharmacokinetic interactions have been studied, and in vivo and in vitro experiments indicated that the altered drug concentrations by the co-administered herbs may be attributable to the induction or inhibition of hepatic drug-metabolizing enzymes, in particular cytochrome P450 Citation4. Aminopyrine, a model drug, is an analgesic drug and is currently rarely used due to its dangerous side effects. Aminopyrine n-demethylase enzyme is classified as n-dealkylation reaction, which belongs to one of the oxidation processes in the phase I hepatic drug metabolism reaction Citation5. The present study aims to investigate the acute (1-day treatment) effect of Faizol Ubat Batuk (FUB), a mixture of 11 different extracts of certain Malay plants, and it is traditionally used to treat common cold diseases () on the hepatic aminopyrine metabolism in healthy and diabetic rat hepatocytes.
Materials and methods
Chemicals
All chemicals used were of analytical grade purity. Collagenase (type IV), streptozotocin (STZ), and aminopyrine were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The FUB was purchased from Faizol Industri, sdn. Bhd. Kuala Kangsar, Malaysia. The compositions of FUB were as follows: Duan Perya 5%; Daun Asam Jawa 10%; Buah Kembang Semangkuk 10%; Buah Bemban 5%; Lobak Ubi 20%; Agar-agar 14%; Rumput Laut 5%; Menthol 1%; Umbut Pisang 5%; Getah Angur 10% and Madu 15%.
Experimental animals and induction of diabetes
Healthy adult male and female (18–20 weeks old; 210–290 g; n=6 each group) and young male (8–10 weeks old; 85–103 g; n=6) rats of the Sprague Dawley (SD) strain were used. The rats were obtained from the animal house, Pharmacy Discipline, Universiti Sains Malaysia (USM), Pinang, Malaysia. The animals were housed in macrolon cages in laminar flow cabinet, 2–4 animals per cage, under standard environmental condition, and provided with food (Gold Coin) and water ad libitum. Animal care and use were conducted in accordance with the Animal Ethics Committee of USM, Malaysia, 1998.
The STZ-induced diabetes has been demonstrated to affect drug metabolism. Herein, hyperglycemia was induced by intravenous injection of STZ (60 mg/kg) in distilled water, via rat-tail. Increased blood glucose level was confirmed on the third day after administration of STZ. Animals (n=6) showed high blood glucose levels (higher than 300 mg/dl at fasting state), measured by using Extac Tech glucometer (Medisense, USA) Citation6.
Preparation of hepatocytes and liver enzyme assay
The protocol used for isolation of hepatocytes was performed by using the modified collagenase perfusion technique as previously described Citation7 Citation8 Citation9 . Rats received orally equal volume of serial dilutions of FUB in water, ranging from one tenth to one tenth million on day zero. The STZ-diabetic rats received FUB on day 4 post-STZ injection. Control rats were treated the same but had the FUB replaced with water. Twenty four hours after the administration of FUB, rats were anaesthetised with ether and the liver perfused in situ through the hepatic portal vein with calcium-free Hank's Balanced Salt Solution (HBSS) for 15 min. Hereafter, the collagenase buffer (HBSS supplemented with 4 mM calcium chloride and collagenase 0.5 mg/ml) was perfused until the liver appeared to have broken up. The liver was removed from the animal, and the cells were dispersed in calcium-free HBSS using steel forceps. The cell suspension was filtered through gauze and centrifuged at 200×g for 5 min (in a Hettich Universal Centrifuge) to clean the parenchymal cells of debris. The supernatant was drained out, and the cells were resuspended in incubation medium (HBSS supplemented with 1 g/L glucose, 100 mg/L MgSo4, 100 mg/L MgCl2 and 185 mg/L CaCl2; pH 7.4). The cells were then counted using a haemocytometer and assayed for viability using trypan blue exclusion method. The average yield was 5×107 cells/g liver, and viability was routinely greater than 90% for all preparations.
Then, the cells were plated out on 90-mm petri dishes (Medical Product, Malaysia) at a density of 6×103 cells/10 ml incubation medium as previously described Citation10. Aminopyrine dissolved in distilled water was added directly to the petri dish at a final concentration 250 mmol/L. The experiment was done in quadruplicates. Petri dishes were then shaken using a table top shaker (Belly Dancer, Stoval, USA) and incubated in CO2 incubator (Queue Systems Inc. Model 2710) for 18 min at 37°C Citation7 Citation8 Citation10. Aminopyrine n-demethylase activity was measured at 415 nm to determine the quantity of formaldehyde formed according to the colorimetric method of Nash Citation11.
Statistical analysis
Aminopyrine n-demethylase activity is expressed in nmol/min/million cells. Data are expressed as mean enzyme activity±SEM. The statistical significance of the differences was assessed using the Student's t-test. In all the cases, a probability value P < 0.05 was considered statistically significant. Analyses were performed by using GraphPad Prism (GraphPad Software Inc., version 3.0, San Diego, USA).
Results and discussion
In contrast to allopathic remedy where the content and dose of active ingredient in any dosage form is well quantitated, the pharmacological actions using herbal medical preparations are derived from the effects of the total ingredients. In the present study, findings indicated that co-administration of FUB, a multiple herbal ingredient concoction traditionally used to treat common cold diseases, changed the hepatic phase I metabolism of aminopyrine in rat liver.
Aminopyrine undergoes phase I n-demethylation in the liver in the presence of cytochrome P-450 n-demethylase. Studies in rat hepatocytes showed that the hepatic n-demethylase enzyme activity can be, indirectly, determined throughout the aminopyrine metabolism by measuring the quantity of formaldehyde formed Citation12. The present study showed that the metabolism of aminopyrine is changed in the presence of FUB. Compared to control-treated rats, FUB significantly increased the in vitro hepatic metabolism of aminopyrine in adult male rats by 30% (P < 0.05, ), indicating the induction of hepatic enzyme activities following treatment with this traditional medicine, FUB. However, this increase in n-demethylase activity was not dose dependent because increased FUB concentrations did not increase further the hepatic phase I metabolism of aminopyrine (). In addition, the effect of FUB on n-demethylase enzyme activity was gender dependent. In adult female rats, co-administration of FUB did significantly reduce (21% decrease, P < 0.05, ) the aminopyrine n-demethylase activity. Therefore, these results show that FUB alters aminopyrine metabolism, and that this effect is gender dependent.
Fig. 2. Dose–response effect of FUB on aminopyrine metabolism in hepatocytes obtained from healthy adult male rats (•), healthy adult female rats (▪), healthy young male rats (▴), STZ-diabetic male rats (♦). Each dose done in quadruplicate, n=6 each. Control: hepatocytes obtained from matched animal group treated with distilled water. Values are expressed as percentage of the relevant control±SEM. * P < 0.05 compared to their respective control.
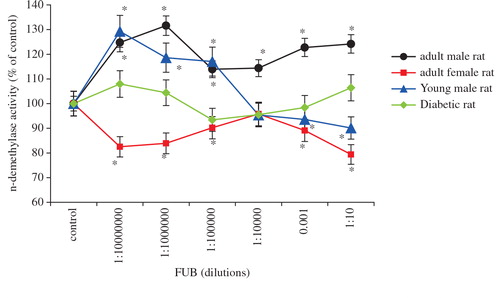
In young male rats, our data showed that FUB has biphasic effects, increased the enzyme activity at lower concentrations, while at higher concentrations FUB reduced the metabolism of aminopyrine in rat hepatocytes (). As can be seen from figure 2, young male rats responded similarly to adult male rats to FUB mainly at lowest concentrations at above 1:100,000 dilutions, indicating that the influence of FUB on aminopyrine metabolism is not affected by age. Nonetheless, as in normal and young male rats, the observed effects varied depending on the concentrations used age factor has a significant effect on FUB influence, mostly at highest concentration at beneath 1:10,000 dilutions, on aminopyrine metabolism. In contrast, difference in rat gender has a significant effect on FUB influence on hepatic phase I n-demethylase activity at all tested FUB dilutions ().
In a different study, our findings showed that 1-day oral administration of FUB showed little effect on the metabolism of aminopyrine in adult male STZ-diabetic rats compared to STZ-diabetic control rats (). The total amount of formaldehyde formed (in absolute value) is shown in . Therefore, in addition to being gender dependent, it seems that the influence of FUB on aminopyrine n-demethylase activity is also disease dependent since the n-demethylase activity being markedly enhanced in healthy rats while less affected in STZ-diabetic rats.
Table 1. In vivo effects of FUB on aminopyrine n-demethylase activity in healthy adult male and STZ-induced diabetic male SD rat hepatocytes.
The present study displayed that FUB differently affects the aminopyrine n-demethylase enzyme activity. However, it is not known which of the plant extract used in the concoction of FUB is responsible for those observed effects. Moreover, the mechanisms by which FUB changes the metabolism of aminopyrine are not known at present. Since in vitro incubation of FUB with rat liver hepatocytes did not change the metabolism of aminopyrine (data not shown), a direct affect of FUB on the n-demethylase enzyme activity is probably not at play. Hence, it seems likely that FUB changes the cytochrome P450 activity by indirect mechanisms. Moreover, it cannot be totally excluded that the in vivo effects of FUB are rather related to its actions on other physiological systems. Several studies have demonstrated that cytochrome P450 activity is changed after phosphorylation Citation13 Citation14. Therefore, it appears likely that while FUB, dose independently, changed the cytochrome P450 activity, FUB phosphorylated cytochrome P450 in male rats and de-phosphorylated cytochrome P450 in female rats. Our present data are in agreement with other studies, demonstrating that a change in aminopyrine metabolism is sex dependent Citation15 Citation16 Citation17 .
Age differences in pharmacokinetics and hepatic drug metabolism had been well documented in rodent models Citation18 Citation19. Similarly, de Wildt and his colleagues extrapolate those findings in human studies Citation20. In the present study, our findings are not completely in line with the studies Citation18 Citation19, since the metabolism of aminopyrine in adult male rats is not significantly different from that in young male rats, mainly at FUB lower concentrations. One likely explanation for this discrepancy may be that the other studies Citation18 Citation19 used a direct in vitro incubation of aminopyrine with herbal extract, whereas we treated our rats systemically with FUB. Various other studies have indicated that diabetes produce substantial changes in intracellular metabolism in most tissues including liver and play a significant role in drug metabolism Citation16 Citation21. In agreement with the literature, our findings showed that the hepatic metabolism of aminopyrine is slightly affected in diabetic rats while significantly enhanced in non diabetic (healthy) rats Citation22 Citation23. These findings provide further support for contributing this biological factor in drug metabolism.
Conclusion
The present study indicates that co-administration of FUB preparations was able to manipulate the hepatic metabolism of aminopyrine in rat liver. A possibility exists that interactions occur with other drugs that undergo hepatic phase-I n-demethylation process, such as imipramine, chlorpromazine, morphine, and phenothiazine, and uncertainly reduces their bioavailability. Moreover, it is apparent that gender and induced diabetes modifies the effect of FUB. A likely, but only at higher concentrations, the influence of FUB on aminopyrine metabolism was age dependent. Whether the results obtained are similarly reproduced in humans still needs further investigation.
Conflict of Interest and funding
Y.A.T was supported by a grant 4-1994 from the Libyan Ministry of Higher Education.
References
- World Health Organization. (2001). Legal status of traditional medicine and complementary/alternative medicine: a worldwide review. WHO Technical Report Series, Geneva, Switzerland.
- World Health Organization. (2002). WHO Traditional Medicine Strategy 2002–2005. WHO Technical Report Series, Geneva, Switzerland.
- Briggs D. The regulation of herbal medicines in Australia. Toxicology. 2002; 181–812: 565–70.
- Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of herbs with cytochrome P450. Drug Metab Rev. 2003; 35: 35–98.
- Yang X, Kulkarni AP. N-dealkylation of aminopyrine catalyzed by soybean lipoxygenase in the presence of hydrogen peroxide. J Biochem Mol Toxicol. 1998; 12: 175–83.
- Robert W, Flint J. Emotional arousal, blood glucose levels and memory modulation: three laboratory exercises in cognitive neuroscience. J Undergrad Neurosci Edu. 2004; 3: A16–23.
- Han C, Abas H, Sabariah I. Effect of Orthosiphon stamineus leaf extracts on hepatic cytochrome P450, UGT and GST activity in STZ-induced diabetic rats. JASA. 2009; 1: 1–8.
- Hussin A, Skett P. Lack of effect of insulin in hepatocytes isolated from streptozotocin-diabetic male rats. Biochem. Pharmacol. 1988; 37: 1683–6.
- Jagoda R, Josip R. Effects of vanadate on oleic acid induced insulin resistance in cultured rat hepatocytes. Diabetologia Croatica. 2004; 33: 17–21.
- Taher Y, Hussin A. In-vitro effect of a herbal product on aminopyrine metabolism in rat hepatocytes. Fitoterapia. 1998; LXIX: 265–68..
- Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953; 55: 416–21.
- Purwantiningsih, Abas HH, Kit L. Phase I drug metabolism study of the standardized extract of Eurycoma longifolia (TAF273) in rat hepatocytes. Int J Pharm Pharmac Sci. 2010; 2: 147–52..
- Oesch-Bartlomowicz B, Oesch F. Cytochrome-P450 phosphorylation as a functional switch. Arch Biochem Biophys. 2003; 409: 228–34.
- Oesch-Bartlomowicz B, Oesch F. Phosphorylation of cytochromes P450: first discovery of a posttranslational modification of a drug-metabolizing enzyme. Biochem Biophys Res Commun. 2005; 338: 446–49.
- Lin G, Cui Y, Liu X. Gender differences in microsomal metabolic activation of hepatotoxic clivorine in rat. Chem. Res. Toxicol. 2003; 16: 768–74.
- Skett P, Joels L. Different effects of acute and chronic diabetes mellitus on hepatic drug metabolism in the rat. Biochem. Pharmacol. 1985; 34: 287–9.
- Xu M, Wang G, Xie H, Li H, Huang Q, Wang R, et al.. Gender difference regarding schizandrin pharmacokinetics in rats. Eur J Drug Metab Pharmacokinet. 2008; 33: 65–8.
- Han CJ, Abas HH. Effect of the Orthosiphon stamineus benth on aminopyrine metabolism in rat hepatocytes. Malaysian J Pharmaceutical Sciences. 2007; 5: 25–32.
- Koch KM, Palmer JL, Noordin N, Tomlinson JJ, Baidoo C. Sex and age differences in the pharmacokinetics of alosetron. Br J Clin Pharmacol. 2002; 53: 238–42.
- de Wildt SN, Trevor NJ, Imti C. The effect of age on drug metabolism. Paediatr Perinat Drug Ther. 2003; 5: 101–6.
- Khalid M. Influence of overt diabetes mellitus on cyclosporine pharmacokinetics in a canine model. Exp Diabetes Res. 2009; 2009: 1–6.
- Cheng P, Morgan E. Hepatic cytochrome P450 regulation in disease states. Curr Drug Metab. 2001; 2: 165–83.
- Schenkman J. Induction of diabetes and evaluation of diabetic state on P450 expression. Methods Enzymol. 1991; 206: 325–31.
