Abstract
Background
Volatile oils obtained from lemon grass [Cymbopogon citratus (DC.) Stapf, Poaceae family] are used in traditional medicine as remedies for the treatment of various diseases.
Aims
In the present study, lemon grass essential oil (LGEO) was evaluated for its in vivo topical and oral anti-inflammatory effects, and for its in vitro antifungal activity using both liquid and vapor phases.
Methods
The chemical profile of LGEO as determined by gas chromatography–mass spectrometry analysis revealed two major components: geranial (42.2%), and neral (31.5%). The antifungal activity of LGEO was evaluated against several pathogenic yeasts and filamentous fungi using disc diffusion and vapor diffusion methods.
Results
LGEO exhibited promising antifungal effect against Candida albicans, C.tropicalis, and Aspergillus niger, with different inhibition zone diameters (IZDs) (35–90 mm). IZD increased with increasing oil volume. Significantly, higher anti-Candida activity was observed in the vapor phase. For the evaluation of the anti-inflammatory effect, LGEO (10 mg/kg, administered orally) significantly reduced carrageenan-induced paw edema with a similar effect to that observed for oral diclofenac (50 mg/kg), which was used as the positive control. Oral administration of LGEO showed dose-dependent anti-inflammatory activity. In addition, topical application of LGEO in vivo resulted in a potent anti-inflammatory effect, as demonstrated by using the mouse model of croton oil-induced ear edema. To our knowledge, this is the first such report to be published. The topical application of LGEO at doses of 5 and 10 µL/ear significantly reduced acute ear edema induced by croton oil in 62.5 and 75% of the mice, respectively. In addition, histological analysis clearly confirmed that LGEO inhibits the skin inflammatory response in animal models.
Conclusion
Results of the present study indicate that LGEO has a noteworthy potential for the development of drugs for the treatment of fungal infections and skin inflammation that should be explored in future studies.
Aromatic and medicinal plants are still a major part of alternative and traditional medicine in the developing countries. Numerous herbal therapies are currently widely used in medicine (Citation1, Citation2). The use of medicinal herbs as anti-inflammatory, antifungal, and analgesic drugs is common in Algeria. In most cases, the active molecules of the herbs are unknown. Studying the biological and pharmacological properties of medicinal plant extracts is a rational approach in our quest for new drugs Citation3–(Citation6) .
The use of medicinal plants is more common among people who have modest or no access to medical assistance. In Algeria, rose-scented geranium and lemon grass are used for the treatment of bacterial and fungal infections, and stomachache and toothache, respectively. Hence, a number of studies have been published whose aim was to evaluate the anti-inflammatory activity of the organic or aqueous extracts of these plants, either on human monocytes in vitro or on rodents in vivo (Citation1, Citation7).
Cymbopogon citratus (DC.) Stapf (Poaceae family), commonly known as lemon grass, is a perennial tropical grass with thin, long leaves and is one of the main medicinal and aromatic plants cultivated in Algeria. It is also cultivated mostly for its essential oil (EO) in tropical and subtropical regions of Asia, South America, and Africa (Citation8).
There is a large international demand for the EO of Cymbopogon species (Citation8). Lemon grass essential oil (LGEO) is extracted by steam distillation from the dried or fresh leaves of the plant. Steam distillation produces EO plus hydrosols or aromatic waters, which are often used against inflammatory diseases and microbial infectious Citation9–(Citation11) . LGEO has considerable commercial importance because it is used in the manufacture of fragrances, flavors, perfumery, cosmetics, detergents, and pharmaceuticals (Citation12, Citation13). Biological research has shown that the various chemical compounds in EO possess antibacterial, antifungal, analgesic, and mosquito repellent properties Citation14–(Citation17) .
In Asia and Africa, LGEO is used as antiseptic, antitussive, and anti-rheumatic and to treat backache, sprains, and hemoptysis. Infusions of its leaves are used in alternative medicine as sedative, antimicrobial, and anti-inflammatory. In some African countries, it is used to treat diabetes (Citation8).
The pharmaceutical potential of LGEO has been demonstrated in rodents by a well-designed study in which citral, a major component of EO, was combined with the non-steroidal anti-inflammatory drug, naproxen, and administered orally to laboratory rats. Compared to naproxen alone, the naproxen−citral combination produced similar anti-inflammatory action but with minimal gastric side effects (Citation18).
The Mitidja region of Algeria, where traditional herbal medicine thrives, is rich in medicinal flora (Citation4, Citation17). Traditional practices based on medicinal plants exist among various tribes in Northern Algeria (Citation4). Literature survey showed that very few studies have been carried out on the lemon grass plant grown in Algeria. It is consequently essential to investigate the chemical composition of LGEO and evaluate the therapeutic potential of its volatile components.
The chemical composition and pharmacological evaluation of LGEO has been the subject of several studies over the years. However, there are very few systematic studies on its antifungal activity. Furthermore, to our knowledge, there are no data in the published literature concerning the anti-inflammatory effect of LGEO.
The purpose of the present study was to evaluate the antifungal activity of LGEO against several pathogenic yeasts and filamentous fungi using disc diffusion and vapor diffusion methods. Furthermore, the in vivo topical and acute anti-inflammatory effects were assessed using croton oil-induced ear edema and carrageenan-induced paw edema in mice, respectively. In addition, we describe the identification of the chemical constituents of LGEO by gas chromatography–mass spectrometry (GC-MS) analyses.
Material and methods
Plant material
Lemon grass was cultivated in the field station of Medicinal and Aromatic Plants of ‘Extral Bio’ Company (Chiffa, Blida city, Algeria). The leaves were collected in May 2013 and dried in the shade at room temperature. Identification of the species was confirmed by the Botanical Laboratory in the National Institute of Agronomy (Algiers, Algeria). Herbarium specimens were deposited in the same department.
EO extraction
LGEO was distilled from the leaves by steam distillation. The total quantity of fresh plant used was 550 kg. The plant material was loaded in the still and stacked in layers to permit appropriate delivery of the steam. The method consists of passing steam at low pressure through an alembic filled with aromatic herbs. The steam captures the volatile components in the plant, which then pass through a cold-water refrigerated serpentine. The distillate contains a mix of hydrosol vapor and EO, which are separated using a Florentine vase. This method allows the collection and separation of LGEO and hydrosol (floral water). LGEO is then dried over sodium sulfate and stored in clean brown glass bottles in a controlled temperature chamber until needed. The oil content in the C. citratus was calculated as percentage yield, which is determined as the percent of the ratio of weight of oil to weight of lemon grass leaves used to extract it.
GC-MS analysis
GC analysis was conducted using a Hewlett-Packard 6870 (Agilent Technologies, Palo Alto, California, USA) gas chromatograph equipped with a flame ionization detector and HP-5MS capillary column (30 m×0.32 mmi.d., 0.25 µm film thickness). Oven temperature was held at 45°C for 8 min and then raised to 250°C at a rate of 2°C/min and held at 250°C for 16 min. Detector and injector temperatures were 280 and 250°C, respectively. Carrier gas was nitrogen at a flow rate of 1.2 ml/min in split mode 1:20 with an injection volume of 1 µl of LGEO. Quantitative data were obtained electronically from the flame ionization detector area percent data without the use of correction factors.
GC-MS analysis was conducted using a Hewlett-Packard 6870 gas chromatograph interfaced with a Hewlett-Packard Mass Spectrometer 5973 (Agilent Technologies, Palo Alto, California, USA) equipped with an HP-5MS capillary column (30 m × 0.32 mm, i.d. 0.25 µm film thicknesses). The column temperature was held at 45°C for 8 min and then increased to 250°C at a rate of 2°C/min and held at 250°C for 16 min. For GC–MS detection, an electron ionization system with ionization energy of 70 eV was used over a scan range of 29–550 m/z. Helium was used as the carrier gas at a constant flow rate of 1 ml/min in split mode 1:20 with an injection volume of 1 µl of LGEO. Detector and injector temperatures were 280 and 250°C, respectively. The ion source temperature was 175°C.
Identification and quantitative determination
The EO compounds were tentatively identified based on linear retention index (RI) and by comparison of mass spectra with MS data of reference compounds. The linear RIs were determined for all constituents by co-injection of the samples with a solution containing the homologous series of n-alkanes (C8–C24) (Fluka, Buchs/sg, Switzerland) on the HP-5MS column. The components were identified by matching their recorded mass spectra with the data bank Mass Spectra (Wiley 7N and NIST 2002 libraries) and further confirmed by comparison with values in the literature (Citation19). The relative proportions of the chemical constituents of LGEO were expressed as percentages obtained by peak area normalization.
Antifungal activities in liquid and vapor phases
The antifungal potential of LGEO in liquid and vapor phases against different yeasts and filamentous fungi was determined by the disc diffusion and disc volatilization methods (Citation13), respectively.
Yeast and filamentous fungi strains
The in vitro antifungal activity of LGEO was evaluated against eight yeast and seven filamentous fungi strains: eight pathogenic Candida strains comprising Candida albicans, C. parapsilosis, and C. tropicalis; five pathogenic Aspergillus strains (A. niger, A. terreus, A. flavus, and A. fumigatus); one Penicillium sp. strain; and one Mucor sp. strain. The isolates were obtained from patients with mucocutaneous and superficial fungal infections.
Candida strains were identified using Auxacolor© yeast identification system (Sanofi Diagnostics Pasteur, Paris, France). Filamentous fungi were identified on the basis of their macro- and microscopic characteristics after sub-culturing on Sabouraud Dextrose Agar (SDA) medium. The fungal isolates were identified by standard microbiological methods and stored in SDA at 25°C. Before analysis, fungal species were cultured on SDA slants at 25°C until sporulation. Prior to susceptibility testing, each strain was inoculated on SDA with chloramphenicol to ensure optimal growth and purity. Inoculum were prepared in sterile saline from 48 h cultures of yeasts and standardized against 2 McFarland Standard to obtain suspensions containing approximately 108 CFU/ml.
Disc diffusion method
The agar disc diffusion method (A) was used for determination of the antifungal properties of LGEO (Citation13). All yeast and filamentous fungal strains were maintained on an agar slant at 25°C. Microbial media used for culture and multiplication of tested strains consisted of SDA with chloramphenicol. Inoculum were prepared from fresh cultures by suspending the microorganisms in sterile 0.9% NaCl and adjusting the density to 2 McFarland standard (108 CFU/ml) using Densimat (BioMérieux, France). Filter paper discs (9 mm in diameter, Schleicher and Schull GmbH, Dassel, Germany) were impregnated with three different volumes (20, 40, and 60 µl per disc) of LGEO and placed on the inoculated SDA plates. The plates were left at room temperature for 1 h and then incubated under aerobic conditions at 25°C for 72 h.
Fig. 1 Determination of the diameter of zone inhibition by (A) the disc diffusion method and (B) the vapor diffusion method (Citation19).
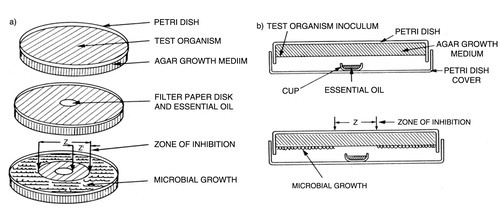
Antifungal activity was assessed by comparing the diameter of the growth inhibition zone (DGIZ) in millimeters (including disc diameter of 9 mm) for the test strains with that of controls. Antibiotic discs of amphotericin B (100 µg per disc) (Bio Rad Laboratories, France) and Hexomedine® solution (1%) (Sanofi Aventis, France) were used as reference positive controls. Discs without samples were used as a negative control.
Vapor diffusion method
Because EOs are volatile, methods that test their antimicrobial activities in their vapor phase have been outlined and modified (Citation20). A standard experimental set-up described by Tyagi and Malik (Citation13) was used.
The antifungal activity of LGEO in vapor phase was investigated by disc volatilization technique (B) at three different doses (20, 40, and 60 µl per disc). In brief, the surface of solidified SDA medium was inoculated with 0.1 ml of suspension of the fungal strains and allowed to dry. A paper disc (diameter 9 mm, Schleicher and Schull GmbH, Dassel, Germany) was laid on the inside surface of the upper lid and 20, 40, or 60 µl of lemon grass volatile oil was placed on each disc. The plate was then instantly inverted on top of the lid and sealed with parafilm to prevent the escape of LGEO vapor. Plates were incubated at 25°C for 72 h. The effectiveness of antifungal activity of LGEO was calculated by measuring the DGIZ (in millimeters) above the disc. Blank discs served as negative control.
In vivo anti-inflammatory activity assay
Animals
In total 60 male and female Swiss albino mice, weighing 25–27 g, were used. The animals were obtained from the Toxicological Laboratory of Saidal Antibiotical Company (Medea, Algeria).
The animals were grouped before the experiments and kept under standard conditions. A minimum of six animals was used in each group. They were housed in standard cages with free access to a standard commercial diet and water ad libitum. The animals were maintained under standard conditions of humidity (40–45%), temperature (23–28°C) with a 12-h light/dark cycle. All animal experimentations were performed according to guidelines of the Ethics Committee of Medical Sciences.
Carrageenan-induced paw edema in mice
The anti-inflammatory potential of LGEO was assessed by the carrageenan-induced paw edema test (Citation21). This method uses paw edema induced by 1% suspension of carrageenan in isotonic saline (w/v), injected at a dose of 0.1 ml per mouse into the sub-plantar region of the left hind paw.
LGEO was diluted in 0.1% Tween 80 and administered 30 min prior to carrageenan injection. The control group received an equivalent volume of the vehicle. EO at the doses of 10, 40, 100 or 200 mg/kg and vehicle (0.1% Tween 80 in 0.9% NaCl solution) were administered orally 30 min before administering the edematogenic agent. Diclofenac (50 mg/kg, oral route) was used as a positive control group. Paw edema was determined before the application of the inflammatory substance, and every hour for 4 h after inducing acute inflammation. Foot pad thickness was estimated by a gauge calipers (Ozaki Co., Tokyo, Japan).
Mean values of treated groups were compared with those of a control group and analyzed statistically. Results are reported as means±standard deviation (SD) in millimeters. The percentage inhibition of the inflammatory response was calculated for each mouse by comparison to the negative control and calculated by the following formula:
where I (%)=percent inhibition of edema, ΔPt is the difference in paw thickness in the drug-treated group and ΔPc the difference in paw thickness in the control group.
Croton oil-induced ear edema
Topical anti-inflammatory activity was evaluated as inhibition of croton oil-induced ear edema in mice (Citation21). Four groups of four mice each were used for this test. Edema was induced on the left ear by topical application of 10 µl of acetone containing 5% croton oil to the dorsal and ventral aspects of the left ear of each mouse. The right ear remained untreated. Vehicle (10 ml/kg), different doses of the LGEO (5−10 µl per ear), or Voltaren® Emulgel 1% (diclofenac sodium topical gel (Novartis, France; 5 mg per ear)) were applied topically to the left ear about 30 min before the croton oil treatment. The diclofenac sodium served as a reference. The reaction was evaluated by measuring the increase in ear thickness with time. At the maximum of the edematous response, 4 h later, mice were sacrificed and a plug (5 mm in diameter) was removed from both treated (left) and untreated (right) ears. The edema response was measured as the weight difference between the two plugs.
LGEO anti-inflammatory activity was expressed as percentage of edema weight reduction in treated mice in comparison to the control group and calculated by the following formula:
where I (%)=percent inhibition of edema, ΔWt is the change in weight of ear tissue in the treated mice, and ΔWc the change in weight of ear tissue in the control mice.
Histopathology of ear tissue
The resulting inflammatory reaction was also evaluated by microscopic inspection. For morphological assessment of cutaneous inflammation, biopsies from control and treated ears of mice were collected at the end of the experiment. Samples were fixed in 10% neutral buffered formalin, routinely processed, sectioned at 6 µm using a microtome (LEICA RM 2125RT, Nussloch, Germany), and stained with Hematoxylin and Eosin (H & E). The tissues were examined under a light microscope (Olympus CX41) and inflammation was graded as mild (+), moderate (++), or severe (+++). Infiltration and polymorphonuclear (PMN) cell accumulation were also examined.
Statistical analysis
Data of the paw and the ear edema are reported as mean±SD. Comparison between groups was conducted by one-way analysis of variance (ANOVA) followed by Tukey's post hoc multiple comparison test. Differences between groups with P<0.05 were considered statistically significant. Statistical analysis was performed by probit analysis method using XLStats 2013 software (Pros statistical software, Addinsoft, Paris, France).
Results and discussion
Chemical composition of EO
We evaluated the volatile EO extracted from lemon grass, an aromatic herb that is commonly used in aromatherapy. LGEO has a light yellow color, a lemony scent, and an extraction yield of 0.6% (v/w) when distilled from the fresh aerial parts of the plant, as was done in the present study. It is well-known that grass plants produce terpenoidal hydrocarbons and EOs that can be grouped as medicinal, industrial, and perfumery, depending on their chemical composition.
GC-MS analyses revealed the presence of 23 compounds representing 90.6% of the total oil. The major components were geranial (42.2%), neral (31.5%), and β-myrcene (7.5%). Geranyl acetate (4.3%) and isopulegol (1.4%) were minor but significant components (). The quality of lemon grass is generally determined by its citral content. Citral (3,7-dimethyl-2,6-octadienal) consists of the cis-isomer geranial and the trans-isomer neral. These two oxygenated monoterpenes are present in our LGEO of about 73.3%. Also, LGEO had a high content of oxygenated monoterpenes and low amounts of monoterpene hydrocarbons (9.54%), sesquiterpene hydrocarbons (0.79%), and oxygenated sesquiterpenes (0.33%). Therefore, LGEO extracted by steam distillation may be classified as a ‘Citral Chemotype’. Comparing the chemical constituents of this LGEO with the published data (15−17), its composition seems to be similar to that of Indian oils, with the same content of neral and geranial, whereas the African oils contain high levels of myrcene. Bhattacharya et al. (Citation22) published the chemical composition of EO extracted from several species of lemon grass (Cymbopogon flexuosus, C. pendulus, C. pendulus). The quantity of neral was similar among the species analyzed (33−36%), whereas the amount of geranial varied from 45.0 to 54.5%.
Table 1 Chemical composition of the essential oil extracted from Cymbopogon citratus by steam distillation
Plant clones especially rich in citral have been developed for the cosmetics and chemical industries. According to Akhila (Citation8), there are two types or clones of this aromatic herb species on the basis of the relative composition of the EO − the East Indian type with high amounts of myrcene (38%) and low citral (≈7%), and the West Indian type with little or no myrcene (0–12%) and a high citral content (up to 86%). Such differences in the chemical composition have been attributed to a range of factors, including geographical location, climatic conditions, time of harvest, age of the plant, and the method of distillation (Citation14).
Antifungal activity
Disc diffusion assay
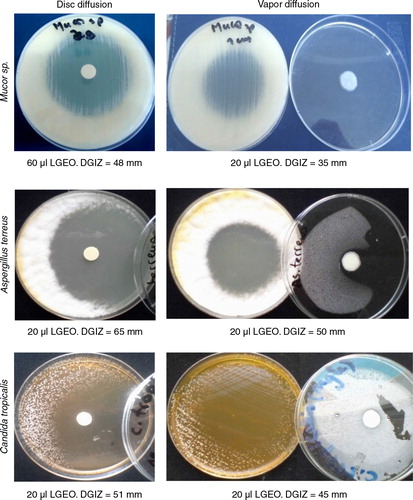
The agar disc diffusion method is a standard technique extensively used for the fast screening of natural extracts and EO for antiseptic effects. To the best of our knowledge, the antifungal activity of LGEO derived from the Algerian plant has never been reported prior to this study. We evaluated the antifungal activity of LGEO against 15 isolates of yeast and filamentous fungi strains in vitro at three different volumes. The resultant DGIZ for yeast and filamentous fungi are given in . Negative control discs showed no antifungal inhibitory against any of the tested microorganisms. Compared to amphotericin B and hexomedine solution, LGEO exhibited a potent inhibitory effect against Candida species, especially C. albicans (Ca2) and C. parapsilosis (Cp1). Among the molds, A. niger and Penicillium sp. were the most susceptible strains: the application of 20 µl of LGEO resulted in a DGIZ of 90 mm. The zone of growth inhibition expanded with the increasing LGEO volume. Using 60 µl of LGEO, the DGIZ was 45, 59, and 90 mm in the case of C. albicans (Ca4), A. niger (As2), and A. fumigatus, respectively. On the other hand, C. parapsilosis (Cp2) was the most resistant strain.
Table 2 Susceptibility of filamentous fungi and yeast to Lemon grass volatile oil and to classic antifungal drugs
Several reports indicate that the antimicrobial activity of an EO is related to its chemical composition (Citation23). In the present work, the antifungal property of LGEO could be due to two major monoterpenic aldehydes (geranial and neral). Other grass oils rich in these oxygenated compounds were previously found to have effective antifungal actions (Citation14, Citation15) (Citation17). Furthermore, previous studies also suggest that the fungicidal action of volatile oils could be explained by the presence of high amounts of oxygenated monoterpenes (Citation23). Geranial and neral are the most active components of LGEO and have a wide spectrum of antimicrobial properties against Gram-positive and Gram-negative bacteria and fungi. However, it is difficult to link the antifungal activity to particular compounds in a complex mixture of an EO. Some authors suggest that the strong inhibitory effect of LGEO against Candida species may result from synergism among the monoterpenes and other important compounds present in the EO, such as cymene, terpinene, and linalool (Citation2, Citation16) (Citation24).
Vapor diffusion assay
The antifungal potential of the LGEO was also evaluated in the vapor phase. The DGIZ resulting from the contact with LGEO vapors is reported in . As observed in the liquid phase, the DGIZ due to the vapors of LGEO increased with increasing quantity of the LGEO. For all the tested fungal strains, the DGIZ resulting from exposure to LGEO vapors was considerably superior to that resulting from a similar quantity of LGEO in the liquid phase (). This was especially evident at higher doses (60 µl per disc). In view of these findings, it is surprising that despite the publication of several important papers on the antifungal activity of LGEO in the liquid phase, the antifungal potential of the vapor phase has been largely ignored. The yeasts C. albicans and C. tropicalis were inhibited completely by the LGEO vapors at 60 µl per disc. Our results clearly demonstrate the superior antifungal effectiveness of LGEO in the vapor phase. This may be related to the difference in the compositions of the two phases, with the vapor phase being richer in the more volatile compounds (Citation13).
Fig. 2 Inhibitory effect of lemon grass oil against fungal strains by (A) disc diffusion, and (B) vapor diffusion methods.
DGIZ: Diameter of growth inhibition zone; LGEO: lemon grass essential oil.
The use of the vapor phase has the additional advantages of ease of application and avoiding the need for direct contact with the oil. Furthermore, a smaller volume of LGEO is required to achieve the same level of inhibition. Our findings are in conformity with previous publications (Citation25). An 80% decrease in airborne microorganisms was achieved when the vapors of a LGEO blend were dispersed continuously for 20 h by a fragrance generator, suggesting its potential for use as air decontaminant in hospitals. The study conducted by Abe et al. (Citation12) revealed that LGEO significantly reduced the mycelial growth of the pathogenic yeast C. albicans, suggesting the potential value of LGEO in the treatment of cutaneous candidiasis. It can be demonstrated that LGEO vapors possess a strong inhibitory effect against C. albicans growth in addition to toxic morphological changes in fungal cellular structures and cell surface alterations. Such surface alterations and deformities reduce the ability of fungal cells to adhere to the skin and consequently decrease their infectiousness and virulence (Citation12, Citation13) (Citation15).
In vivo anti-inflammatory activity assay
Carrageenan-induced paw edema
Carrageenan-induced mouse paw edema is frequently used to determine the anti-inflammatory activity of diverse bioactive compounds, such as plant extracts and EO (Citation3, Citation7). It is also used in the development of new drugs to assess the effect of pro-inflammatory agents on the acute phase of inflammation. The anti-inflammatory effect of orally administered LGEO (10–200 mg/kg) was evaluated using the same paw edema model. As shown in , LGEO showed considerable anti-inflammatory activity. At 90 min after oral administration of LGEO, the degree of edema inhibition was similar for 10 and 100 mg/kg (82.75 and 86.2%, respectively). This level of edema inhibition was comparable to the level observed using 50 mg/kg oral doses of the standard reference drug diclofenac (86.2%). However, at 240 min LGEO exhibited stronger inhibition of carrageenan-induced paw edema, with 96.8 and 95.2% inhibition at 10 and 40 mg/kg, administered orally, respectively, compared to 88.9% obtained using the reference drug diclofenac (50 mg/kg, administered orally).
Table 3 Effect of lemon grass volatile oil on carrageenan-induced paw edema in mice
The exact mechanism of the anti-inflammatory effect of the LGEO is unclear. However, it has been suggested that several molecules contribute to the partial inhibition of the release of inflammation mediator substances. Some plant components, particularly aldehyde monoterpenes (geranial and neral) have been reported to be useful in the management of inflammatory processes (Citation26). Our findings are in conformity with those published in the literature for other volatile oils rich in monoterpenic aldehydes that showed an extraordinarily strong anti-edematogenic property (Citation7).
Citral, a major component of LGEO, has been shown to inhibit the production of IL-1β and IL-6 in lipopolysaccharide (LPS)-stimulated alveolar macrophages of normal mice (Citation11). EOs whose main components include citral, geranial, neral, and carvone have been found to inhibit the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α). Maruyama and coworkers also demonstrated the ability of EO to suppress TNF-α-induced neutrophil adherence responses (Citation9). Another study (Citation27) suggested that citral inhibited TNF-α in RAW 264.7 cells stimulated by LPS. Consistent with our data, anti-inflammatory and analgesic effects have also been suggested for diverse phytochemical extracts, polyphenolic fractions, and volatile oils of some other Cymbopogon species (Citation8).
In conclusion the results of the present investigation support the traditional usage of LGEO as an anti-inflammatory agent despite the need for more studies to better evaluate its pharmaceutical potential and understand its mechanism of action.
Croton oil-induced ear edema
Many medicinal plants possess topical anti-inflammatory properties. This effect is a common characteristic of many oxygenated monoterpenes (Citation9, Citation10). Because the LGEO demonstrated anti-inflammatory activity in vitro in isolated cell assay, this property was further evaluated by estimating the degree of inhibition of croton oil-induced ear edema in mice. Topical application of croton oil on the left ears of mice caused noticeable edema, as indicated by the increase in the ear plug weight of the left ear compared with the vehicle-treated right ear (data not shown). The results of topical pre-treatment with LGEO revealed dose-dependent reductions in croton oil-induced ear edema (). In comparison with diclofenac topical gel, LGEO produced a stronger and more effective anti-inflammatory activity in our experimental animal model. Prior topical application of LGEO reduced the edema response by 62.5% for 5 µl LGEO per ear and by 75% for 10 µl. Diclofenac sodium (5 mg per ear) produced a 56.2% inhibition of croton oil-induced inflammation and this effect was not statistically different from those observed with the maximum dose of LGEO (). To the best of our knowledge, this is the first report to demonstrate that LGEO possesses a significant topical anti-inflammatory activity in vivo. In accordance with our findings, Abe et al. (Citation9) reported that topical application of EO can limit the inflammatory symptoms of edema and neutrophil accumulation.
Table 4 Lemon grass aromatic oil prevents croton oil-induced ear edema in vivo
It is well established that skin inflammation is triggered and maintained by the interaction of diverse inflammatory cell populations that migrate to the site of inflammation in response to the release of soluble pro-inflammatory mediators such as leukotriene, prostaglandins, and cytokines (Citation3, Citation7). In aromatherapy, skin application of volatile oils in a full body massage or to limited parts of the body is highly desirable. Many EO are used as a curative management for inflammatory symptoms with lesional neutrophil accumulation: aphthous stomatitis (canker sores), rheumatoid arthritis, and lesional fungal or bacterial contagions (Citation1).
Mouse ear tissue morphology
We investigated H&E-stained ear sections from croton oil-induced animals. Croton oil application resulted in a marked increase in ear thickness with clear evidence of edema, epidermal hyperplasia, and substantial inflammatory cell infiltration in the dermis with accompanying connective tissue disruption (C and D). Topical application of LGEO reduced ear thickness and associated pathological indicators to an extent comparable to the positive control diclofenac (A and B). These findings constitute direct evidence that LGEO decreases the croton oil-induced ear edema and contact dermatitis.
Fig. 3 Histopathology sections of mice ear biopsies showing keratin, epidermal, dermal, muscle, and cartilage layers.
Hematoxylin-eosin stained sections were scored as mild (+), modest (++), and severe (+++) for edema and substantial inflammatory polymorphonuclear (PMN) cell infiltration in the dermis inflammation phase. (A) Lemon grass essential oil (LGEO) treatment (5 µl per ear); (B) Positive control (5 mg per ear of diclofenac sodium 1% gel, Voltaren Emulgel (Novartis, France)) treatment: edema (±); inflammatory cell infiltration (+), inflammation phase (±). (C) and (D) croton oil: edema (++); inflammatory cell infiltration (+++), inflammation phase (++). Magnification×100.
Ke: keratin; Ep: epidermal layer; De: dermal layer; Mu: muscle; Ca: cartilage layer; Od: edema; Pc: PMN cells infiltration.
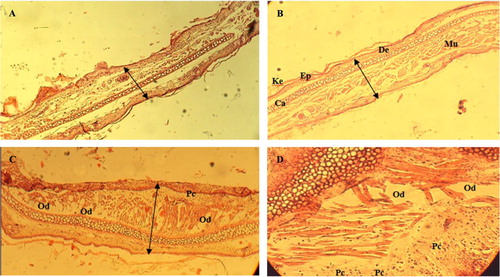
Microscopic examination confirmed the beneficial anti-inflammatory effects of the topical treatment with LGEO. Compared to the control group, the edema was dramatically reduced by prior topical treatment with LGEO (C and A). To the best of our knowledge, this is the first report to demonstrate that LGEO possesses a significant topical anti-inflammatory activity that is confirmed by histopathology investigation.
The data of our histopathology analysis are in accordance with previously reported findings using the carrageenan and croton oil-induced edema methods (Citation7, Citation10) (Citation21). Also, our results are in agreement with a study by Boukhatem et al. (Citation21), whose histological analysis revealed that rose-scented geranium oil inhibited the skin inflammatory process in vivo.
Conclusions
Our findings, together with previous studies, give strong support to the consideration of LGEO as a potentially valuable antifungal and anti-inflammatory agent for the prevention and treatment of acute inflammatory skin conditions. Furthermore, there is growing evidence that LGEO in vapor phase is an effective antifungal system that has several advantages over the liquid phase, such as greater potency so that lower doses are required for the same effect. The vapor phase makes it possible to use LGEO as air decontaminant in hospitals. The present study demonstrates the need for more investigations to identify the active chemical constituents of LGEO responsible for its therapeutic properties as well as their specific mechanisms of action.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007; 21: 308–23. [PubMed Abstract].
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol. 2008; 46: 446–75. [PubMed Abstract].
- Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asian Pac J Clin Nutr. 2006; 15: 143.
- Boukhatem MN, Saidi F, Hamaidi MS, Hakim Y, Mekarnia M. Culture et exploitation industrielle du géranium rosat (Pelargonium graveolens) en Algérie: état des lieux et perspectives. Phytothérapie. 2011; 9: 304–9.
- Taher YA. Antinociceptive activity of Mentha piperita leaf aqueous extract in mice. Libyan J Med. 2012; 7: 16205.
- Dub AM, Dugani AM. Antithrombotic effect of repeated doses of the ethanolic extract of local olive (Olea europaea L.) leaves in rabbits. Libyan J Med. 2013; 8: 20947. [PubMed Abstract].
- Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010; 15: 9252–87. [PubMed Abstract].
- Akhila A. Essential oil-bearing grasses: the genus Cymbopogon . 2010; New York: CRC Press.
- Abe S, Maruyama N, Hayama K, Ishibashi H, Inoue S, Oshima H, Yamaguchi H. Suppression of tumor necrosis factor-alpha-induced neutrophil adherence responses by essential oils. Med Inflamm. 2003; 12: 323–8.
- Alitonou GA, Avlessi F, Sohounhloue DK, Agnaniet H, Bessiere JM, Menut C. Investigations on the essential oil of Cymbopogon giganteus from Benin for its potential use as an anti-inflammatory agent. Inter J Aromather. 2006; 16: 37–41.
- Tiwari M, Dwivedi UN, Kakkar P. Suppression of oxidative stress and pro-inflammatory mediators by Cymbopogon citratus DC. Stapf extract in lipopolysaccharide stimulated murine alveolar macrophages. Food Chem Toxicol. 2010; 48: 2913–19. [PubMed Abstract].
- Abe S, Sato Y, Inoue S, Ishibashi H, Maruyama N, Takizawa T, etal. Anti-Candida albicans activity of essential oils including Lemongrass (Cymbopogon citratus) oil and its component, Citral. Jap J Med Mycol. 2002; 44: 285–91.
- Tyagi AK, Malik A. Morphostructural damage in food-spoiling bacteria due to the Lemon grass oil and its vapor: SEM, TEM, and AFM investigations. Evid Based Complement Alternat Med. 2012; 692625. [PubMed CentralFull Text] 2012.
- Negrelle RRB, Gomes EC. Cymbopogon citratus DC. Stapf: chemical composition and biological activities. Rev Bras Pl Med. 2007; 9: 80–92.
- Silva CDBD, Guterres SS, Weisheimer V, Schapoval EE. Antifungal activity of the Lemon grass oil and citral against Candida spp. Braz J Infect Dis. 2008; 12: 63–6.
- Tyagi AK, Malik A. Liquid and vapor-phase antifungal activities of selected essential oils against Candida albicans: microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement Alternat Med. 2010; 10: 65.
- Boukhatem MN, Kameli A, Ferhat MA, Saidi F, Tayebi K. The food preservative potential of essential oils: is lemongrass the answer?. J Verbr Lebensm. 2014; 9: 13–21.
- Ortiz MI, Gonzales-Garcia MP, Ponce-Monte HA. Synergistic effect of the interaction between naproxen and Citral on inflammation in rats. Phytomedicine. 2010; 18: 74–9. [PubMed Abstract].
- Adams RP. Identification of essential oil components by Gas Chromatography-quadrupole Mass Spectroscopy. 2007; Illinois: Allured.
- Zaika LL. Spices and herbs: their antimicrobial activity and its determination. J Food Saf. 1988; 9: 97–118.
- Boukhatem MN, Kameli A, Ferhat MA, Saidi F, Mekarnia M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J Med. 2013; 8: 22520. [PubMed Abstract].
- Bhattacharya AK, Kaul PN, Rajeswara Rao BR, Mallavarapu GR, Ramesh SI. Inter-specific and inter-cultivar variations in the essential oil profiles of lemongrass. J Essent Oil Res. 1997; 9: 361–4.
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000; 88: 308–16. [PubMed Abstract].
- Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J Antimicrob Chemother. 2001; 47: 565–73. [PubMed Abstract].
- Laird K, Phillips C. Vapor phase: a potential future use for essential oils as antimicrobials?. Lett Appl Microbiol. 2012; 54: 169–74. [PubMed Abstract].
- Pérez GS, Zavala SM., Arias GL, Ramos LM. Anti-inflammatory activity of some essential oils. J Essent Oil Res. 2011; 23: 38–44.
- Lin CT, Chen CJ, Lin TY, Tung JC, Wang SY. Anti-inflammation activity of fruit essential oil from Cinnamomum insularimontanum Hayata. Bioresour Technol. 2008; 99: 8783–7. [PubMed Abstract].
