Abstract
Background
In Tunisia, diabetes mellitus and hemoglobinopathies are major public health problems. Glycated hemoglobin (HbA1c) is recommended for long-term monitoring of diabetes mellitus, but the presence of hemoglobin variants may interfere with HbA1c measurement. The aim was to determine the prevalence of hemoglobin variants in Tunisian diabetics and optimize the monitoring of diabetics using HbA1c.
Methods
The study enrolled 9,792 Tunisian diabetic patients. HbA1c was measured by cation-exchange high-pressure liquid chromatography (HPLC). All the chromatograms were analyzed for the presence of Hb variants.
Results
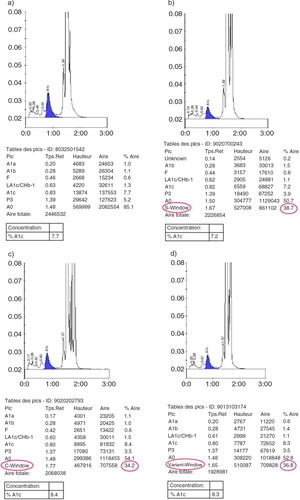
We identified 228 cases (2.33%) of Hb variants with D-10 HPLC (Bio-Rad): 191 with HbA/S trait, 27 with HbA/C trait, and 10 hemoglobin variants with the mention ‘Variant-Window’ on the chromatograms and subsequently identified as HbA/S on Variant I HPLC (Bio-Rad). Thus, the prevalence of HbS was 2.05%. We did not find any homozygous variant. All HbA1c results were reported to the treating physician.
Conclusions
To evaluate glycated hemoglobin in populations with a high prevalence of hemoglobinopathies, we should use the HPLC method, which is easy, economical, and reliable. Based on an algorithm, hemoglobin variants visualized on HPLC should be reported to the physician to improve the management of patients.
The prevalence of diabetes mellitus (DM) is increasing rapidly worldwide. The World Health Organization estimated that more than 366 million people will suffer from DM by the year 2030 (Citation1). For example, in 2011 about 14 million individuals were estimated to have diabetes in Africa, and this is expected to rise to 28 million by 2030 (Citation2). In Tunisia, the overall diabetes prevalence increased significantly during the last 15 years. This rate has more than doubled, reaching 9.90% in recent years (Citation3). This increasing trend of DM prevalence emphasizes the need to rely on glycated hemoglobin (HbA1c) for managing glycemic control and long-term monitoring. HbA1c has been shown to be directly related to the risk of developing diabetes complications. Treatment goals for HbA1c have been established, and this biomarker was recently recommended by the American Diabetes Association for DM diagnosis (Citation4). Therefore, accurate and precise measurement of HbA1c is extremely important (Citation5). Several studies indicated that there are differences in HbA1c among racial groups (Citation6). Herman et al. showed that HbA1c levels are different among racial and ethnic groups, and a particular threshold HbA1c value may not be appropriate for all ethnic groups because of the presence of hemoglobin variants (Citation7).
Hemoglobinopathies are associated with decreased erythrocyte survival and decreased glycohemoglobin percentages. Hemoglobinopathies are frequent in many parts of the world: more than 900 hemoglobin variants have been described, and at least 43.20% of all possible single-point mutations have been identified (Citation8). These inherited hemoglobin disorders are spread all around the world because of migration, and their high frequency and clinical severity in Africa make them a major public health problem (Citation9). The World Health Organization noted that more than 70% of all hemoglobin disorders are localized in Africa (Citation10). Fattoum (Citation11) reported that systematic surveys done in Tunisia show a prevalence of hemoglobinopathy carriers in Tunisia of 4.48%, reaching 12.5% in some regions. Many hemoglobin variants have minimal clinical significance and are sometimes a fortuitous finding when HbA1c is analyzed (Citation12). However, their presence may cause a spurious increase or decrease in the HbA1c result, depending on the methodology and the properties of the hemoglobin (Citation13, Citation14). Hemoglobin S (HbS) is the most common variant worldwide and is consequently found in a significant proportion of diabetic patients who are tested for HbA1c. Several studies investigated the prevalence of DM or of hemoglobinopathies in the Tunisian general population, but there is no study on the prevalence of hemoglobinopathies among the Tunisian diabetic population. The aim of this study was to determine the prevalence of hemoglobin variants in Tunisian diabetic patients and to optimize the monitoring of diabetics using HbA1c.
Methods
Our study enrolled all 9,792 Tunisian diabetic patients consulting at or staying in Charles Nicolle Hospital, Tunis, Tunisia between January 2009 and December 2009. For all these patients, HbA1c was used as an index of mean glycemia over the preceding 2–3 months. Patients who show up repeatedly for HbA1c in the hospital were counted only once. Measurement of HbA1c was done by cation-exchange high-pressure liquid chromatography (HPLC) on a D-10 system (Bio-Rad Laboratories, Hercules, CA, USA) using reagents according to the manufacturer's instructions. This method quantifies HbA1c, which is defined as Hb A with glucose attached to the N-terminal valine beta chain, as a percentage of HbA1c: (HbA1c area/total HbA area of the chromatogram)*100. When HbS, HbC, or ‘Variant-Window’ was detected in a sample on HPLC D-10, a ‘corrected HbA1c value’ was automatically obtained by exclusion of the area of the variant from the total area of the chromatogram. Correction is necessary because retention times of glycated HbS and glycated HbC are similar to that of the major HbA fraction on the chromatogram, so these fractions coelute with HbA (Citation15).
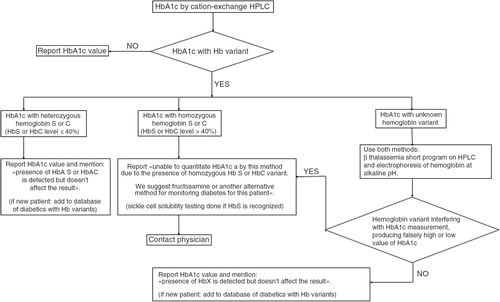
When a patient's chromatogram showed a hemoglobin variant during the measurement of HbA1c, it was reported to the ordering clinician, who then explained to the patient that he or she has a hemoglobin variant and requested approval to perform more tests on the blood sample. In that case, the blood sample was analyzed further using the β thalassemia short program on the HPLC Variant I analyzer (Bio-Rad Laboratories) in order to identify and quantify the variant. Whenever an HbS variant was found on a chromatogram, the presence of HbS was confirmed by sickle solubility testing. Hemoglobin variants whose retention time was not listed by the supplier on HPLC D-10 were called ‘unknown’, and those mentioned on the chromatogram by ‘Variant-Window’ had a retention time registered but their identification needed to be completed on HPLC Variant I. For both ‘unknown’ and ‘Variant-Window’, identification of the hemoglobin variant was completed by using two methods: β thalassemia short program on HPLC Variant I and electrophoresis of hemoglobin at alkaline pH. In accordance with guidelines for screening and diagnosis of hemoglobinopathies, presumptive identification was based on a minimum of two techniques based on different principles (Citation16).
Informed consent was obtained from all patients for whom Hb variants were determined by additional tests.
Results
The study enrolled 9,792 patients (4,291 males and 5,501 females); the male-to-female ratio was 0.78–1.00. Of the 9,792 patients, 9,564 (97.70%) had no hemoglobin variant (a). The chromatograms produced by the HPLC D-10 method showed the presence of hemoglobin variants in 228 cases (2.30%). Analysis of blood samples of hemoglobin variant carriers on Variant I identified 191 cases of heterozygous hemoglobin S (b) (confirmed by sickle solubility testing), 27 cases of heterozygous hemoglobin C (c), and 10 cases of heterozygous hemoglobin with a mention of ‘Variant-Window’ on the chromatogram (d). There were no cases of homozygous hemoglobin variants. For the 10 cases, the β thalassemia short program on HPLC Variant I and electrophoresis of hemoglobin at alkaline pH revealed that they were all heterozygous hemoglobin S. The 10 samples were positive in sickle solubility testing. Finally, we identified 201 cases of HbA/S and 27 cases of HbA/C. Thus, the prevalence of the HbS trait was 2.05%.
Discussion
The Diabetes Control and Complications Trial reference method for measurement of HbA1c was cation-exchange HPLC (Citation17). Our analysis using this method showed a prevalence of hemoglobin variants of 2.30% among all the diabetic patients attending the hospital during the 1-year period of the study. Moreover, the prevalence of HbS was 2.05%. This is in agreement with the prevalence of HbS found by Fattoum in the general population in Tunisia (1.90%) (Citation9). Recent detailed reviews on hemoglobinopathies in Tunisia reported that sickle cell disease is by far the most frequent hemoglobinopathy in North Africa; its average frequency in Algeria was 0.80–3.50% and in Morocco 1.20% (Citation18). Until now, there have been no data on the prevalence of hemoglobin variants in the diabetic populations in Africa. However, in Singapore, Saw et al. (Citation13) studied 5,628 chromatograms of diabetics and noted a prevalence of 2.30% of hemoglobin variants. As the program of measurement of HbA1c on D-10 system does not detect α and β thalassemia, we cannot compare our prevalence with their epidemiologic data.
The presence of hemoglobin variants among diabetics has a consequence for diabetic survey analysis. HbA1c is strongly correlated with complications of DM, so its measurement must be accurate and precise (Citation5). Although cation-exchange HPLC is the reference method, the presence of hemoglobin variants may interfere with HbA1c measurements, producing falsely high or low values when the hemoglobin variant or its glycated form cannot be separated from hemoglobin A or HbA1c (Citation13, Citation19). In contrast, heterozygous hemoglobin S and C samples do not affect the assay (Citation14). Ideally, we should first detect the presence of hemoglobin variants to decide if the HbA1c measurement can be accepted at face value (monitoring of HbA1c cannot be used if the patient is homozygous for a hemoglobin variant or compound heterozygote). In our study, the 228 cases of hemoglobin variants were carriers of HbS (Hb AS) or HbC (Hb AC), so we reported the HbA1c values. Elution time of HbS is sometimes not recognized by the HPLC D-10, and that is why for 10 samples the chromatogram indicates ‘Variant-Window’. However, when an interfering hemoglobin variant is visualized or suspected, samples should be analyzed by an alternative method that is not affected by hemoglobin variants. Fructosamine may be a better indicator of diabetes control when hemoglobin variants are associated with reduced red blood cell half-life and hence reduction of glycated hemoglobin (Citation13).
The presence of abnormal Hb cannot be detected by immunoassay or the enzymatic methods used to quantify HbA1c. Besides, these methods do not provide any information about the proportions of the different Hb fractions, which might be helpful in interpreting the results (Citation20). In managing diabetic patients, knowledge of hemoglobinopathies affecting HbA1c determination is essential because ignoring the presence of Hb variants could result in mismanagement of diabetes due to false HbA1c determinations (Citation21).
In Tunisia, the prevalence of HbS trait in the general population is 1.89% (Citation9), and we propose that diabetes be monitored by the use of a user friendly cation-exchange HPLC, such as the D-10. When diabetic patients homozygous for HbS or C or compound heterozygotes SC are identified, they should not be monitored by HbA1c because the life span of the red blood cells is altered (Citation19). The laboratory staff should add a footnote, ‘Unable to quantitate HbA1c by this method due to the presence of homozygous HbS or HbC variant or compound heterozygotes SC. We suggest fructosamine or another alternative’. This provides the physician with the information needed to properly manage such diabetic patients. The procedure currently used in our laboratory () explains to laboratory staff how to communicate an accurate value of HbA1c of diabetics to the physicians. Other authors, such as Behan et al. (Citation14), also recommend a standardized reporting format for HbA1c that includes notification of variant hemoglobins. In conclusion, we show that the prevalence of variant Hb in our diabetic population is high and that the monitoring of diabetic patients using HbA1c should be optimized. It is also desirable that laboratory reports on glycated hemoglobin be standardized.
Conflict of interest and funding
We declare no conflict of interest in relation with this paper.
References
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27: 1047–53.
- Bos M, Agyemang C. Prevalence and complications of diabetes mellitus in Northern Africa, a systematic review. BMC Public Health. 2013; 13: 387.
- Bouguerra R, Alberti H, Salem LB, Rayana CB, Atti JE, Gaigi S, etal. The global diabetes pandemic: the Tunisian experience. Eur J Clin Nutr. 2007; 61: 160–5.
- American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011; 34: 11–61.
- Lin CN, Emery TJ, Little RR, Hanson SE, Rohlfing CL, Jaisson S, etal. Effects of haemoglobin C,D,E, and S traits on measurements of HbA1c by six methods. Clin Chim Acta. 2012; 413: 819–21.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, etal. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011; 34: e61–99.
- Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, etal. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007; 30: 2453–7.
- Kleinert PK, Schmidt K, Speer O, Schmugge M, Roschitzki B, Durka SS, etal. Mass spectrometry: a tool for enhanced detection of haemoglobin variants. Clin Chem. 2008; 54: 69–76.
- Fattoum S. Evolution of hemoglobinopathy prevention in Africa: results, problems and prospect. Mediterr J Hematol Infect Dis. 2009; 1: 2009005.
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008; 86: 480–7.
- Fattoum S. Hemoglobinopathies in Tunisia. An updated review of the epidemiologic and molecular data. Tunis Med. 2006; 84: 687–96.
- Clarke GM, Higgins TN. Investigation of hemoglobinopathies and thalassemias; review and update. Clin Chem. 2000; 46: 1284–90.
- Saw S, Loh TP, Yin C, Sethi SK. Identification of hemoglobin variants in samples received for glycated hemoglobin testing. Clin Chim Acta. 2013; 415: 173–5.
- Behan KJ, Storey NM, Lee HK. Reporting variant hemoglobins discovered during hemoglobin A1c analysis-common practices in clinical laboratories. Clin Chim Acta. 2009; 406: 124–8.
- Bouzid K, Bahlous A, Ferjani W, Kalai E, Ducrocq R, Ben Mami F, etal. Advantage of HbA1c assay by HPLC D-10 versus cobas integra 400 in a population carrier for HbS and HbC. Clin Lab. 2012; 58: 821–8.
- Ryan K, Bain BJ, Worthington D, James J, Plews D, Mason A, etal. Significant haemoglobinopathies: guidelines for screening and diagnosis. Br J Haematol. 2010; 149: 35–49.
- The DCCT Research Group. Feasibility of centralized measurements of glycated hemoglobin in the diabetes control and complications trial: a multicenter study. Clin Chem. 1987; 33: 2267–71.
- Haj Khelil A, Denden S, Leban N, Daimi H, Lakhdhar R, Lefranc G, etal. Hemoglobinopathies in North Africa: a review. Hemoglobin. 2010; 34: 1–23.
- Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001; 47: 153–63.
- Jaisson S, Desmons A, Renard B, Chevelle B, Leroy N, Gillery P. Analytical performances of a new enzymatic assay for hemoglobin A1c. Clin Chim Acta. 2014; 434: 48–52.
- Schnedl WJ, Krause R, Halwachs-Baumann G, Trinker M, Lipp RW, Krejs GJ. Evaluation of HbA1c determination methods in patients with hemoglobinopathies. Diabetes Care. 2000; 23: 339–44.