Abstract
Background
Rotavirus infection is a major cause of childhood diarrhea in Libya. The objective of this study is to evaluate the cost-effectiveness of rotavirus vaccination in that country.
Methods
We used a published decision tree model that has been adapted to the Libyan situation to analyze a birth cohort of 160,000 children. The evaluation of diarrhea events in three public hospitals helped to estimate the rotavirus burden. The economic analysis was done from two perspectives: health care provider and societal. Univariate sensitivity analyses were conducted to assess uncertainty in some values of the variables selected.
Results
The three hospitals received 545 diarrhea patients aged≤5 with 311 (57%) rotavirus positive test results during a 9-month period. The societal cost for treatment of a case of rotavirus diarrhea was estimated at US$ 661/event. The incremental cost-effectiveness ratio with a vaccine price of US$ 27 per course was US$ 8,972 per quality-adjusted life year gained from the health care perspective. From a societal perspective, the analysis shows cost savings of around US$ 16 per child.
Conclusion
The model shows that rotavirus vaccination could be economically a very attractive intervention in Libya.
Rotavirus is an important cause of diarrhea in children, with globally more than 110 million cases, 2 million hospitalizations, 25 million outpatient visits, and 450,000 deaths annually. The incidence rate of rotavirus infection is often similar in developing and developed countries. Meanwhile, the mortality rate is much higher in the developing than in the developed world, as more than 80% of all the rotavirus deaths occur in the developing countries Citation1–(Citation6) . The disease is manifested by watery diarrhea causing severe dehydration. As the virus destroys intestinal villi, the diarrhea may stop only when the villi have regenerated. The disease may lead to hospitalization and even death. During epidemic seasons, rotavirus is responsible for about 20–40% of all hospital admissions and 20% of the diarrhea deaths (Citation6). These percentages were recently confirmed for Libya (Citation7, Citation8). Adequate rehydration is the therapeutic strategy against the disease, but it does not affect disease spread. This diarrhea poses a high economic burden on the health care system and on the families because of the high hospitalization and medical costs and because of work absenteeism of family members who normally care for the children Citation9–(Citation12) .
Two safe and effective rotavirus vaccines on the global market, available since the end of 2005, can prevent diarrhea events in young children by more than 80% Citation13–(Citation18) . These two vaccines against rotavirus infection have also been licensed in Libya. One is a two-dose vaccine, called Rotarix® (GlaxoSmithKline, Biologicals, Rixensart, Belgium), which can be given at the age of 2 and 4 months, and the other is a three-dose vaccine, called RotaTeq® (Merck, Whitehouse Station, NJ, USA), which can be given at 2, 4, and 6 months (Citation15, Citation18). Introduction of the rotavirus vaccine into the National Immunization Program (NIP) of Libya could prevent many diarrhea events in children and may offset much of the health care cost Citation19–(Citation21) . In Libya, the disease leads to a massive use of public hospital services, which provide free treatment. This study assessed the cost-effectiveness of introducing the rotavirus vaccine into the NIP in Libya. We present theoretical modeling results with best estimates to measure the cost-effectiveness of the vaccine using a Libyan cohort, age ≤5. The findings may support the decision of administering rotavirus vaccination into the NIP. We considered two different perspectives for the analysis. One is the health care system and the other is societal, including all the different cost items in the equation.
Subjects and methods
This is a theoretical modeling study that helps to define the economic value of rotavirus vaccination in Libya. We used the available country-specific data whenever possible. In the absence of country-specific data, assumptions were made. These assumptions were tested in sensitivity analysis. In the next paragraphs, we present the data collected at the national level, describe the model used, and present the assumptions put forth in the model. We also define the sensitivity analysis that was undertaken.
Data collection
We collected data during the period from August 2012 to April 2013 (9 months) to determine the proportion of rotavirus cases among children with diarrhea aged ≤5 in three public hospitals of two cities, Zliten and Khoms, located in the north-western part of Libya. Stool samples were collected from suspected cases in hospital care and in outpatient clinics to test for rotavirus. A suspected case was defined as a child aged ≤5 with episodes of diarrheal illness (loose stools more than three times a day) (Citation18, Citation22). Once the sample was taken by the nurse, it was stored in a fridge at the hospital, and collected samples were transported once a week to the National Laboratory by car in fridge boxes. The samples were analyzed at the National Laboratory because the relevant tests were not routinely performed at the study hospitals. To identify rotavirus infections (group A), we used an immunoassay kit ProSpect Rotavirus Test (Oxoid Ltd, UK). The kit was provided by the World Health Organization, which also provided training for the laboratory staff on how to use the test.
Cost items
The confirmed rotavirus cases in the hospital setting were used to estimate the hospitalization cost and the indirect cost paid by parents. Since public hospitals provide health services free of charge, the overall cost for treating diarrhea is poorly documented because there are no bills of payment to refer to. We estimated the hospital cost by including the bed-day cost, the drug cost, and the laboratory test cost during hospitalization. The bed-day cost was estimated from staff salary, furniture, equipment, food, laundry, disposal, cleaning, utilities, and maintenance. Details of drugs and laboratory tests administered to the patients were retrieved from the patient files, and their costs were obtained from the central pharmacy and the main laboratory at the hospital, respectively. Details of the expenditure involved were also collected from the pediatric ward in the year 2012. Furthermore, the total number of bed-days at the pediatric ward in 2012 was considered to determine the cost per bed-day. Cost per consultation of the general practitioner and that of the pediatrician in the outpatient clinic was determined according to expert opinion (Citation23). Rate of inflation in Libya was obtained from the World Bank [4.7%, (Citation24)]. It was used to estimate the current cost of treatment of a rotavirus patient.
Details of indirect cost was collected from interviews with the parents (n=130). When a child was discharged from hospital care or from an outpatient clinic, each parent was asked to participate in an interview about all the costs they incurred due to the disease. The interview was voluntary and involved no payment. Each interview took about 15 min, and the same person interviewed all the participants. The information collected consisted of transportation cost from home to the medical or hospital and back, household cost, which included the extra cost families have when relatives and friends come to visit in order to provide support. These visits necessitate provision of food and beverages. Moreover, there is loss of income due to absence from work during the illness.
The model
A simple disease-specific decision tree model was used to assess the cost-effectiveness of rotavirus vaccination for a birth cohort estimated at 160,000 children. The model was developed in MS Excel and has already been published for the economic assessment of rotavirus vaccination in other countries, including Turkey (Citation25). The model compares two theoretical situations of non-vaccinated and vaccinated children during the risk period from birth to the age of 5. It subdivides the disease condition into four health states: mild (staying home), moderate (seeking medical advice), severe (being hospitalized), and death. The model was selected because it is easy to use, transparent, and has a limited number of variables to work with (n=20). The model also allows performing sensitivity analysis and budget impact estimates. The outcome is close to the more extended Markov cohort models that are more precise in some aspects of the vaccine impact related to vaccine compliance and completion (Citation25).
Cost-effectiveness calculation
The cost for the rotavirus vaccine is presented as a condition for being cost-effective. It included the cost of the vaccine with the cost of administration per child and per full course. Vaccine administration cost was estimated considering the cost for staff, training, and transportation related to the rotavirus vaccine. Cold chain cost was excluded since rotavirus vaccine could utilize the same storage used for other vaccines. The vaccine coverage rate is assumed to be the same as for the DPT vaccine in 2013, which was estimated at 98% for a three-dose vaccine because rotavirus vaccination can be given at the same age together with the DPT vaccine (Citation26).
The model is used to evaluate under baseline conditions the effect of the vaccine on any rotavirus diarrhea event, medical visits, hospitalizations, and deaths. In addition, the model allows calculation of the cost-effectiveness of rotavirus vaccination when introduced into the NIP by comparing non-vaccinated and vaccinated situations. The model does this from different perspectives. One perspective is the health care system, where only the cost incurred by that institution is considered, and the other is the societal situation, which includes all costs from whatever source. The rate of rotavirus diarrhea in the birth cohort of ≤5 years old is based on the definition of diarrhea as set by the WHO criteria. The rates of medical visits and hospitalizations of the birth cohort were assumed based on literature review as well as on the deaths reported due to rotavirus in the birth cohort (Citation7). Finally, we introduced a quality-adjusted life years (QALY) assessment per health state in the model equivalent to the one used in the Turkish model (Citation25). The QALYs gained from avoiding cause-specific deaths is based on the life expectancy at birth for Libya (Citation27). QALYs were selected here as we could easily refer to the reported literature on how QALYs were estimated for each health state in the model. The incremental cost-effectiveness ratio (ICER) was calculated per QALY gained, as well as per diarrhea event avoided, death avoided, hospitalization avoided, and medical visit avoided.
Sensitivity analysis
An extended univariate sensitivity analysis was performed on the key variables in the model assuming a range testing of 20% change (above and under) in order to identify the robustness of the ICER result. A 20% change should be able to identify a more meaningful value change for some of the variables selected. This is certainly the case when the baseline value such as vaccine efficacy has a high starting value. The results are presented as a tornado diagram of the 15 most influential variables considered separately from a health provider and from a societal perspective.
Approval
Approval for the study was given by the University of Malaya Medical Ethics Committee (IRP 908.6). Health authorities in the places of the study and National Center for Disease Control (NCDC) provided the permissions to carry out the study. Parents signed a consent form for the interview.
Results
Data collection
During the 9 months of data collection at the three hospitals, there were 545 cases of diarrhea in children aged ≤5, of which 311 were due to rotavirus (57%). Most of the rotavirus cases were inpatient (77%). No deaths due to rotavirus diarrhea occurred at the hospitals during that period. The proportion of rotavirus diarrhea in each hospital and for inpatient and outpatient is presented in .
Table 1 Proportion of rotavirus diarrhea in children aged≤5
Costs
Total treatment cost for a rotavirus patient was estimated from hospitalization and patient data (). The average treatment cost for a rotavirus case was estimated at US$ 661. The provider cost was US$ 475, representing 72% of all the cost, while the contribution of patient cost was US$ 186 (28%). The per diem (bed-day cost) was the highest cost item (50%), while the lowest cost item (5%) was the laboratory test. The analysis also indicates that all the cost data are much skewed, with big outliers to the right when we look in particular at the upper range values in . The average duration for hospital stay is around 3.02 days.
Table 2 Cost of rotavirus treatment among children aged≤5
The household cost includes the cost of extra diapers and specific hygienic items for the child, as well as the hospitality cost for relatives or friends visiting due the child's illness.
Overall data input
The required variables with their baseline values for measuring the effect and the cost-effectiveness of rotavirus vaccination in children using the model are shown in .
Table 3 Input variables for estimating the cost-effectiveness of rotavirus vaccination with baseline, minimum (Min), and maximum (Max) values
For 25 values (highlighted in the table), a minimum and a maximum estimate were calculated based on a 20% value change. These data were used in the sensitivity analysis.
Outcome and offsets
The effect of rotavirus vaccination and the cost analysis measured by the model for one cohort aged≤5 is reported in . Introducing the vaccine would avoid 47,000 diarrhea events with a decrease of 3,400 hospitalizations, and 20,100 outpatient visits. Deaths related to rotavirus infection would be reduced by nine cases.
Table 4 Health outcomes and cost of treatment of rotavirus patients in no vaccination and vaccination scenarios
The model predicts that the universal rotavirus vaccination could offset more than 2 million US$ in direct medical costs. The highest cost-offset is in hospitalizations avoided (1.6 million US$) followed by the offset in medical visits (380,000 US$).
ICER of rotavirus vaccine
The ICER in the base cases is US$ 8,972 per QALY gained. This is below the Libyan per capita GDP of US$ 10,132 in 2012 (Citation24). Being under that threshold identifies the new intervention as being highly cost-effective according to the WHO's definition of cost-effectiveness (Citation31, Citation32). Similarly, the ICER result per hospitalization avoided is US$ 642, per outpatient visit avoided is US$ 110, and per diarrhea event avoided is US$ 47. For each death avoided the vaccine results in an extra cost of US$ 245,734 ().
Table 5 Incremental cost-effectiveness analysis of rotavirus vaccination
Including indirect cost
Including the indirect cost in the equation will heavily impact the economic value of the vaccine when considering the patient perspective. These indirect costs include three factors: the transport cost to and from the medical care unit, the household cost, and the loss of production. reports the impact of each factor on the total cost of vaccination. As can be seen, it leads to important savings in the range of 16 US$ per child of the birth cohort, especially because of the reduction in the household cost, with more than 2.5 million US$ cost gain. Under such circumstances, there is benefit for each family unit to pay for the vaccine because the cost of transportation plus the household cost is much higher than the vaccination cost.
Table 6 Including the indirect cost (US$) in the economic evaluation of the vaccine
Sensitivity analysis
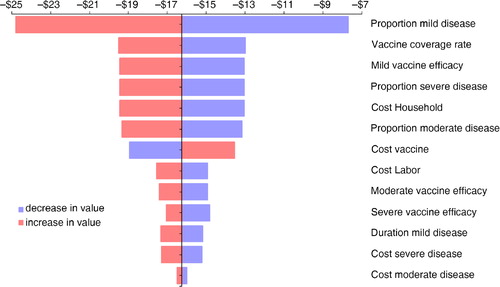
For the health care perspective, we analyzed and tested the ICER results on many different variables. shows the results presented as a tornado diagram. This diagram indicates from high to low which variable has the greatest/smallest impact on the outcome measure as indicated in the X-axis above. For instance, the highest impact on the ICER result, as expected, is the vaccine price. The discount factor that takes into account the value decrease of the QALY per year (equivalent to the inflation rate) heavily impacts the QALY result on life expectancy. It is the next impact driver on the ICER result. The blue color indicates that a lower value for the variable is selected than the baseline. The red color indicates a higher value. So, the lower the price of the vaccine, the lower will be the ICER result. In contrast is the red color of death cases (the third variable). If that value is in red color (higher), the ICER result will decrease because there are more deaths to be avoided and therefore more benefit to be expected.
Fig. 1 Sensitivity analysis (tornado diagram) of the incremental cost per QALY gained analyzed from the health provider perspective (example: the lower the cost of the vaccine, blue color, the lower the cost-effectiveness result; the color changes at the baseline value of $8,972, see ).
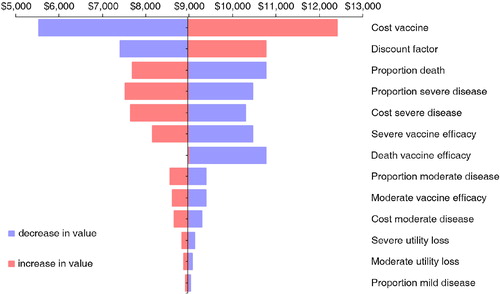
The sensitivity analysis for the societal perspective is analyzed on a different outcome measure than the ICER, because from the overall analysis in , we observed important cost savings. So the outcome measure selected is a cost difference and not an ICER result. presents the tornado diagram. Surprisingly, the cost of the vaccine is no longer the first driver of the analysis. Instead, the prevalence of the disease, the vaccine coverage rate, and the cost incurred by a household are the big influencing factors. There is a clear difference in what matters most depending on whether one considers a narrow perspective (health provider only) or a broader view (society as a whole).
Discussion
This study aimed to evaluate the economic value of rotavirus vaccination in Libya from the healthcare providers’ perspective and from a societal perspective. Given the limited available data to estimate the disease burden, interpolation of data from various sources, with several assumptions, had to be made to perform the cost-effectiveness analysis. A simple decision tree model was selected to calculate the ICER for a group of children aged≤5.
The percentage of diarrhea cases in children in three major hospitals in Libya attributable to rotavirus infection was 57%. This is similar to many studies conducted in other countries, such as Oman (70%) and Iran (58%) (Citation7). Cost of rotavirus diarrhea treatment was collected primarily from the patients who sought treatment for rotavirus infection. These were a good approximation of the true cost incurred for the disease treatment. In this study, treatment cost for rotavirus disease was US$ 661 per patient, which is similar to that reported in Algeria (US$ 650) (Citation33). It is lower than that in Belgium (US$ 1,005), Norway (US$ 2,382), and Portugal (US$ 2,172), but much higher than that in Egypt (US$ 19), Vietnam (US$ 20), and Brazil (US$ 200) (Citation11, Citation12) (Citation33). Using the same coverage rate of DPT as for rotavirus vaccine of 98%, (Citation26, Citation34), the model showed a large reduction in rotavirus infection of 47,040 for the birth cohort. This may offset 2 million US$ in direct treatment cost. The ICER per event avoided was US$ 47, but per QALY gained it was US$ 8,972. Both values are below the Libyan per capita GDP of US$ 10,132 in 2012 (Citation24). If we accept that the GDP per capita is the appropriate threshold for being cost-effective, we can say that the vaccine is good value for money, and that rotavirus vaccination is highly cost-effective using the WHO criteria for an intervention, and below the Libyan GDP per capita in 2012 (Citation31, Citation32) (Citation35). Similarly, the rotavirus vaccine was highly cost-effective in many countries, such as Vietnam, Indonesia, and Japan Citation36–(Citation38) . In other countries, such as the Latin American countries, Thailand, and the Caribbean, rotavirus vaccination could also be cost-effective should the price of the vaccine be lower (Citation39, Citation40). Most studies showed through sensitivity analysis that the price of rotavirus vaccine is a big driver of the cost-effectiveness result (Citation39). Also, in Belgium, France, Ireland, England, and Wales, the cost-effectiveness of rotavirus vaccination was sensitive to the assumption of the vaccine price Citation41–(Citation44) .
The findings from this analysis provide evidence for the expected benefit of the introduction of the rotavirus vaccine in Libya. Generalization of the findings related to the estimate of the disease burden has to be made with caution because the study was hospital based and cases of mild rotavirus infection in the population were not captured. The vaccine will avoid the latter cases and therefore improve the economic results. Real-life conditions will see a dramatic reduction in hospitalizations within the first 2 years after the introduction of the vaccine, with a high uptake because of the herd effect it will cause. The use of the model with imputation of some data from the literature helped to provide a sensitive estimate. But the ICER of rotavirus vaccination shows that it is highly cost-effective. Hence, the decision to incorporate the vaccine into the NIP of Libya is justified, and priority should be given to continuing allocation of a budget for this preventive program.
One limitation of this study is that it does not capture the total burden of the disease in the analysis. Another is that many assumptions had to be introduced in the model to keep it running. A more complete epidemiologic analysis of the disease burden would have helped understanding the management of the disease in the whole country, including remote areas.
An additional part of the study that is not presented in other cost-effectiveness analyses reported for that region is the collection of data on money spent on visits by relatives and neighbors who come to provide support during the stressful situation. During those visits, the cost incurred on drinks and cookies negatively impact the budgets of the families, which are already reeling under the cost of treatment. Those household costs were estimated here at 71 US$ per event, which is much higher than the vaccination cost (see ). This part of household cost has been underreported in the literature and is not included in economic evaluations of the vaccine. Vaccination could help saving the family some money. Moreover, those visits are a means for spreading the disease. So, there are at least two hidden reasons of additional benefit by introducing the vaccine: an important reduction in social cost burden for the parents and the limitation of the spread of the virus.
Conclusion
Rotavirus infection represents 57% of hospital treated diarrhea in Libyan children aged ≤5. Introduction of rotavirus vaccination is expected to alleviate a significant burden of the disease and is highly cost-effective in Libya. The decision by the government to incorporate this vaccine into the NIP is justified.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
The authors greatly appreciate the National Center for Diseases Control in Libya (NCDC) and the managers and medical staff at the hospitals for the permissions. The authors are also grateful to the patients and their parents for their participation in this study. The study was supported by the University of Malaya/Ministry of Higher Education (UM/MOHE) High Impact Research Grant (E000010-20001), Malaysia, and also supported by the AADUN RP026-2012C grant. The authors thank the two anonymous reviewers for their comments.
References
- Berry SA, Johns B, Shih C, Berry AA, Walker DG. The cost-effectiveness of rotavirus vaccination in Malawi. J Infect Dis. 2010; 202: S108–15. [PubMed Abstract] [PubMed CentralFull Text].
- Cortese MM, Parashar UD, Centers for Disease Control and Prevention . Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009; 58: 1–25. [PubMed Abstract].
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, etal. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009; 301: 2243. [PubMed Abstract].
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011; 30: S1. [PubMed Abstract].
- WHO. Generic protocol for monitoring impact of rotavirus vaccination on gastroenteritis disease burden and viral strains. 2008. Switzerland: Department of Immunization, Vaccines and Biologicals.
- Valencia-Mendoza A, Bertozzi S, Gutierrez JP, Itzler R. Cost-effectiveness of introducing a rotavirus vaccine in developing countries: the case of Mexico. BMC Infect Dis. 2008; 8: 103. [PubMed Abstract] [PubMed CentralFull Text].
- Khoury H, Ogilvie I, El Khoury AC, Duan Y, Goetghebeur MM. Burden of rotavirus gastroenteritis in the Middle Eastern and North African pediatric population. BMC Infect Dis. 2011; 11: 9. [PubMed Abstract] [PubMed CentralFull Text].
- NCIRS. Rotavirus vaccines for Australian children. 2009; Australia: National Centre for Immunization Research & Surveillance.
- Gray J, Vesikari T, Damme V. Economic burden of rotavirus disease. J Pediatr Gastroenterol Nutr. 2008; 46: S24–S31. [PubMed Abstract].
- Bilcke J, Van Damme P, De Smet F, Hanquet G, Van Ranst M, Beutels P. The health and economic burden of rotavirus disease in Belgium. Eur J Pediatr. 2008; 167: 1409–19. [PubMed Abstract].
- Constenla DO, Linhares AC, Rheingans RD, Antil LR, Waldman EA, da Silva LJ. Economic impact of a rotavirus vaccine in Brazil. J Health Popul Nutr. 2008; 26: 388. [PubMed Abstract] [PubMed CentralFull Text].
- Standaert B, Harlin O, Desselberger U. The financial burden of rotavirus disease in four countries of the European Union. Pediatr Infect Dis J. 2008; 27: S20.
- Masters J. Gastroenteritis and the rotavirus vaccine. 2007; UK: Health University of Bristol.
- Catherine T-R. Rotavirus live, oral, pentavalent vaccine. Clin Therapeut. 2007; 29: 2724–37.
- CDC. Rotavirus vaccine. 2010; Atlanta, GA: National Immunization Program, US Department of Health and Human Service.
- NNII. Rotavirus. Available from: http://www.immunizationinfo.org/vaccines/rotavirus [cited 2 December 2010]..
- Payne J, Elliott E. Gastroenteritis in children. Clin Evid. 2009; 2009: 0314.
- Tate JE, Rheingans RD, O'Reilly CE, Obonyo B, Burton DC, Tornheim JA, etal. Rotavirus disease burden and impact and cost-effectiveness of a rotavirus vaccination program in Kenya. J Infect Dis. 2009; 200: S76–84. [PubMed Abstract].
- Constenla D, Velázquez FR, Rheingans RD, Antil L, Cervantes Y. Economic impact of a rotavirus vaccination program in Mexico. Rev Panam Salud Pública. 2009; 25: 481–90.
- Soárez PC, Valentim J, Sartori AMC, Novaes HMD. Cost-effectiveness analysis of routine rotavirus vaccination in Brazil. Rev Panam Salud Pública. 2008; 23: 221–30.
- Widdowson MA, Meltzer MI, Zhang X, Bresee JS, Parashar UD, Glass RI. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007; 119: 684. [PubMed Abstract].
- WHO. Generic protocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children. 2002. Switzerland: Vaccines and Biologicals, World Health Organization.
- CDC. Rotavirus surveillance – Worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep. 2008; 57: 1255–7.
- World Bank. Countries and economies. 2012; Libya. Available from: http://data.worldbank.org/country/libya [cited 13 May 2014]..
- Bakir M, Standaert B, Turel O, Bilge ZE, Postma M. Estimating and comparing the clinical and economic impact of paediatric rotavirus vaccination in Turkey using a simple versus an advanced model. Vaccine. 2013; 31: 979–86. [PubMed Abstract].
- WHO. WHO vaccine-preventable diseases: monitoring system. 2013 global summary. 2013. Available from: http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria[country][]=LBY&commit=OK [cited 26 March 2014]..
- WHO. Statistics. 2012. Available from: http://www.who.int/countries/lby/en/ [cited 9 November 2014]..
- General Authority for Information. 2009. Statistical book. Libya Government.
- National Statistics. Full report – women in the labour market. 2013. Available from: http://www.ons.gov.uk/ons/dcp171776_328352.pdf [cited 13 May 2014]..
- Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, etal. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009; 124: 465. [PubMed Abstract].
- Guilbert J. The world health report 2002-reducing risks, promoting healthy life. Educ Health (Abingdon). 2003; 16: 230.
- WHO. WHO guide for standardization of economic evaluations of immunization programmes. 2008; Switzerland: WHO. 58–68.
- Standaert B, Ethgen O, Emerson R, Postma M, Mauskopf J. Comparing cost-effectiveness results for a vaccine across different countries worldwide: what can we learn?. Adv Ther. 2014; 31: 1095–108. [PubMed Abstract] [PubMed CentralFull Text].
- NCDC. Annual report for infectious disease in Libya. 2009. Libya: Surveillance Department, National center for Diseases Control.
- WHO. Tables of costs and prices used in WHO-CHOICE analysis. 2007. Available from: http://www.who.int/choice/costs/CER_thresholds/en/ [cited 22 March 2014]..
- Kim S-Y, Goldie SJ, Salomon JA. Cost-effectiveness of rotavirus vaccination in Vietnam. BMC Public Health. 2009; 9: 29. [PubMed Abstract] [PubMed CentralFull Text].
- Sato T, Nakagomi T, Nakagomi O. Cost-effectiveness analysis of a universal rotavirus immunization program in Japan. Jpn J Infect Dis. 2011; 64: 277–83. [PubMed Abstract].
- Suwantika AA, Tu HAT, Postma MJ. Cost-effectiveness of rotavirus immunization in Indonesia: taking breastfeeding patterns into account. Vaccine. 2013; 31: 3300–7. [PubMed Abstract].
- Rheingans RD, Constenla D, Antil L, Innis BL, Breuer T. Potential cost-effectiveness of vaccination for rotavirus gastroenteritis in eight Latin American and Caribbean countries. Rev Panam Salud Pública. 2007; 21: 205–16.
- Chotivitayatarakorn P, Chotivitayatarakorn P, Poovorawan Y. Cost-effectiveness of rotavirus vaccination part of the National Immunization Program for Thai children. Southeast Asian J Trop Med Publ Health. 2010; 41: 114.
- Bilcke J, Van Damme P, Beutels P. Cost-effectiveness of rotavirus vaccination: exploring caregiver (s) and “no medical care” disease impact in Belgium. Med Decis Making. 2009; 29: 33–50. [PubMed Abstract].
- Jit M, Edmunds W. Evaluating rotavirus vaccination in England and Wales: part II. The potential cost-effectiveness of vaccination. Vaccine. 2007; 25: 3971–9. [PubMed Abstract].
- Melliez H, Levybruhl D, Boelle P, Dervaux B, Baron S, Yazdanpanah Y. Cost and cost-effectiveness of childhood vaccination against rotavirus in France. Vaccine. 2008; 26: 706–15. [PubMed Abstract].
- Tilson L, Jit M, Schmitz S, Walsh C, Garvey P, McKeown P, etal. Cost-effectiveness of universal rotavirus vaccination in reducing rotavirus gastroenteritis in Ireland. Vaccine. 2011; 29: 7463–73. [PubMed Abstract].