Abstract
Microbial ecology studies have provided convincing evidence for the crosstalk between members of the autochthonous microbiota and the host immune system. The tight interrelationship between microbiota and host mucosal cells are mediated by microbial metabolites that are responsible for bacterial cell-to-cell communication by quorum-sensing mechanisms and also through the activation of eukaryotic cells following secretion of host defensins and modulation of cytokine expression profiles. All these host functions can be positively influenced by probiotic bacteria of human origin. However, few requirements for evaluating these strains for use in humans have been set according to their composition and metabolic activity. In this article, we have reported the scientific data published to date on the advantages of either mono- or multispecies probiotic products based on the outcome of the most significant clinical trials. According to published clinical trials, the efficacy of probiotic intervention for infectious or antimicrobial treatment-induced diarrhea, caused by different opportunistic bacterial or viral pathogens, was 48%. The probiotic preparations’ efficacy for silencing the clinical symptoms of IBS was 75% and for attenuating the inflammatory response during IBD was 83% whereas multistrain probiotics appear to have better efficacy.
This article has been commented on by Tore Midtvedt, Editor-in-Chief. Please follow this link http://www.microbecolhealthdis.net/index.php/mehd/article/view/11620 to read his Editorial Remarks
At the turn of the last century, a probiotic preparation was defined as ‘a live non-pathogenic microbial feed or food supplement that beneficially affects the host by improving the intestinal microbial balance’ Citation1 Citation2. Later, according to the 2002 report of the Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food (ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf), a new definition of a probiotic was adopted that stated ‘a probiotic is a viable microbial food component which has a demonstrated benefit on human health when given in specific amounts’ Citation3.
A mono-strain probiotic is defined as a product containing one strain of a well-defined microbial species, whereas multistrain probiotics contain more than one strain of the same species or genus. However, the term multispecies probiotic is often used for products containing microbial strains belonging to one or more genera as well Citation4.
Evaluation of probiotic efficacy
Currently, a probiotic product is a strain-specific preparation targeting different human metabolic functions to improve health by either supporting host physiologic activity or by reducing the risk of disease. It has been generally accepted that the probiotic potential of different strains of the same species may have different probiotic effects Citation5 Citation6 Citation7 . The project ‘Process for the Assessment of Scientific Support for Claims on Foods,’ Citation8 Citation9 several years ago proposed scientific tools for substantiating health claims made for probiotic products. Specifically, human clinical trial data based on objective measurements and defined probiotic culture characteristics needed to be evaluated to support a specific claim. Today, there are emerging technologies for the discovery and measurement of the efficacy of a probiotic preparation based on changes in specific biomarkers. In February 2009, the International Life Sciences Institute Europe workshop, ‘Emerging Technologies for Efficacy Demonstration Task Force’ reviewed the advancements of these new technologies and discussed their role in food product development and in substantiating claims by relying on measurements of functional biomarkers of the host (http://www.ilsi.org/Europe/Pages).
In contrast, the European Food Safety Authority (EFSA) requirements for authorization of a specific health claim (Regulation (EC) No. 1924/2006) have considered appropriate human clinical trials and experimental interventions (randomized control trials (RCT); non-controlled RTs), quasi-experimental interventions (non-randomized either controlled or not), observational (cohort, case control, cross-sectional) and other studies (case reports). Additional studies using experimental animal models dealing with mechanisms by which the probiotic might be responsible for the claimed effect in a causal relationship were also proposed. Furthermore, ex vivo or in vitro studies based on either human or animal samples (case description) were also suggested as useful for these evaluations.
The confirmation of many of these effects on host physiologic function has been documented in a number of clinical trials in healthy volunteers or patients with specific diseases, despite the fact that the underlying causal relationships have yet to be established Citation10 Citation11 Citation12 .
Selection of probiotics on the basis of the present knowledge on intestinal microbial ecology
The intestinal microbiota is a complex and dynamic mixture of microbes consisting of bacteria, archaea, protozoa, fungi, bacteriophages and other viruses. Based on molecular methods, current estimates indicate that intestinal microbiota in aggregate consists of 10 14 viable microbes belonging to over 1,000 species, among which anaerobic bacteria predominate Citation13 Citation14 Citation15 Citation16 Citation17 Citation18 . There are also a wide variety of host, dietary and environmental factors that affect bacterial colonization of the gastrointestinal (GI) tract.
It has been demonstrated over several decades using culture-based methods that the major groups of fecal bacteria belong to the genera Bacteroides, Eubacterium, Clostridium, Ruminococcus, Fusobacterium, Bifidobacterium and Peptostreptococcus Citation19 Citation20. Recent molecular studies have revealed that the gut microbiota is largely dominated in adults by one member of the archaea, Methanobrevibacter smithii Citation21 and by members of two other bacterial phyla, the Bacteroidetes and the Firmicutes, that include the genera listed above. More specifically, three bacterial groups predominate within these phyla: the Bacteroides-Prevotella group, the Clostridium coccoides group, and the Clostridium leptum group Citation14. Although there may be some confusion over the taxonomic designations of specific species, the recent molecular analyses largely confirm the results of previous culture-based research.
Beneficial properties of microbiota
Different biologic functions such as digestion of essential nutrients, maturation of intestinal epithelial cells, and impact on baseline physiologic parameters, including systemic effects on blood lipids, inhibition of harmful bacteria, and stimulation of the immune system have been attributed to the microbiota through careful scientific evaluations over many decades Citation22 Citation23. There is substantial evidence that modulation of pro- and antiinflammatory responses, as revealed by cytokine profiles, is an important mechanism by which probiotics provide health benefits. It has been shown that cell surface molecules of Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2 Citation24. In addition, the antimicrobial defensins, cathelicidins, eosinophil-derived neurotoxin, and AI-2 signaling compounds of Gram-positive and Gram-negative bacteria play important roles in both intra- and interspecies communication Citation25 Citation26.
Most beneficial microbes isolated from human microbiota and proposed as probiotics belong to the group of lactic acid-producing bacteria such as Lactobacillus spp., Bifidobacterium spp., Enterococcus spp. Citation3 Citation6 as well as strains of Escherichia coli, Bacillus spp., and Streptococcus spp. Research suggests that the close interrelation between host mucosal epithelial cells and microbiota Citation6 Citation27 Citation28 Citation29 is of utmost importance for good health.
Putative impact on health
In wealthy societies, the stress of living in a highly competitive environment, the increasing number of elderly people, and the concomitant reduction in physical activity and a diet consisting of high levels of fat, carbohydrate, and salt are considered risk factors for the increase in certain chronic diseases such as atherosclerosis, hypertension, type 2 diabetes, peptic ulcer disease, and neurodegenerative disorders. Furthermore, the large amount of disinfectants and antibiotics routinely used by Western societies represent significant risk factors associated with an increased number of humans suffering from GI tract-associated health problems. More specifically, during medical interventions that interrupt the balance of human resident microbiota, the sporadic numeric dominance of opportunistic pathogens may lead to translocation of these organisms into lymph nodes and systemic circulation through mucosal membranes of GI, respiratory, and urogenital tracts Citation30 Citation31. One example of this phenomenon is the parenteral administration of ceftriaxone to treat upper respiratory tract infections of children, which has been shown to induce a dysbiosis, that is an imbalance in intestinal microbial flora characterized by a shift in the viable cell density of different bacterial species and a concomitant change in host physiology Citation32. This shift has a negative impact on host colonization resistance, which can result in the overgrowth of antibiotic-resistant strains of opportunistic pathogens (such as multidrug-resistant E. coli or vancomycin-resistant Enterococcus sp.) Citation33 Citation34 Citation35 .
To correct the imbalance of microbiota composition caused by the use of antibiotics particularly in critically ill patients, the administration of probiotics has been suggested as a therapeutic intervention. In fact, several probiotic strains are intrinsically resistant to antimicrobials and can be used jointly with specific antibacterial treatments. Lactobacillus rhamnosus GG, a commercially available probiotic, is intrinsically resistant to metronidazole and vancomycin (both MIC >256) often used in the treatment of antibiotic-associated diarrhea and pseudomembranous colitis Citation36. Lactobacillus fermentum ME-3 (DSM14241) is suggested as a suitable probiotic additive in conjunction with ofloxacin (MIC 8 µg/ml) for the treatment of Salmonella enterica serovar Typhimurium infections Citation37.
Despite these examples, there is a need for evidence-based documentation supporting probiotic treatment using properly designed clinical trials. An example of the usefulness of this approach was published by Alberda and coworkers Citation38 who showed that patients receiving a combination of viable probiotic organisms during multiple organ dysfunction syndrome demonstrated greater enhancement in immune function than patients receiving either placebo or a non-viable probiotic formulation. In well-balanced microbial ecosystems, the proportions of anaerobic, microaerobic, and facultatively anaerobic bacteria are tightly linked. Therefore, a putative danger to health may result from a decreased abundance of anaerobes, such as Bacteroides, that are inversely associated with increased fasting glucose and obesity Citation39. It seems prudent that the consumption of high doses of lactic acid bacteria should be carefully monitored, to avoid the suppression of Bacteroidetes, particularly when given over extended periods of time.
The selection of probiotic Lactobacillus species for different age groups and the age-related shifts of some health indices, such as blood glucose content or the white blood cell (WBC) count, have recently been studied Citation40 Citation41. An example of this can be found in elderly subjects, where colonization of the gut with indigenous Lactobacillus reuteri has been shown to be associated with an increased peripheral WBC. It is possible that in individuals with systemic infections, where the WBC is already elevated as a normal part of the inflammatory process, the introduction of the probiotic species L. reuteri may further provoke the inflammatory process, an additional increase in the WBC, and concomitant increase in the risk of complications for critically ill patients. The peripheral leukocyte count has also been used as a predictor of cardiovascular events and mortality suggesting that this may be a useful biomarker to evaluate as part of probiotic clinical trials Citation42. The diversity of intestinal Lactobacillus spp. in adults and elderly people is closely associated with shifts in specific metabolic markers. An example of this is the observation that Lactobacillus acidophilus is associated with increased blood glucose level in adults, whereas L. paracasei can decrease blood glucose levels in elderly Citation43.
Composition of probiotic products
The EFSA has set strict regulations regarding the hygienic, nutritional, and gusto/olfactorial indices of food/products carrying probiotics used for specific health claims (Regulation (EC) No. 1924/2006). However, this and other regulations usually do not address the compatibility of particular metabolic properties of probiotic strains with their functional efficacy in the host. For most probiotic strains, studies have mainly dealt with their safety and colonization ability but not with the actual efficacy of the probiotic product. Clearly, the effects of different metabolic properties of the probiotic product are not universally expressed in humans after consumption and may depend on different variables that are strain and host specific.
The confirmation of efficacy relies in large part on the reduction of clinical symptoms and maintenance of remission of acute disease as measured by non-objective indices, such as the perceived clinical outcome, for which the placebo effect can be a significant confounding variable. Thus, specific diagnostic biomarkers need to be developed to objectively document the efficacy of probiotic treatments.
Mono-strain probiotics
Mono-strain probiotics are defined as probiotics containing one strain of a probiotic species Citation4 Citation44. Different species of lactic acid bacteria produce many metabolites with documented antimicrobial effects such as lactic, acetic, and succinic acids. In addition to species differences, major strain-specific differences also exist. Some strains may additionally produce butyric acid, hydrogen peroxide, and bacteriocins that act as functional tools that could be applied in humans Citation5. Recently Citation40 Citation45, Mikelsaar and co-workers listed some potentially new biomarkers produced by L. fermentum ME-3, including glutathione peroxidase and reductase. Among the activities of the soluble molecules produced by probiotic strains is the interference with pathogenic bacterial cell density by a category of soluble molecules, called quorum-sensing inhibitors (QSI). The inhibition process is dependent on the cellular density of the probiotic strain and the accumulation of soluble molecules at a specific concentration. QSI can modulate the expression of different virulence factors, such as antibiotic resistance and biofilm formation, making some pathogenic strains less virulent in a human host. This can be accomplished by inducing changes in the expression of cell surface molecules and consequently, by the shift of the adherence pattern from an aggregative to a diffuse one and the stimulation of bacterial cell endocytosis by epithelial cells, respectively Citation45.
Different testing systems have been proposed for the selection of monocultures of probiotic products belonging to Bifidobacterium breve, Enterococcus faecium, L. casei, L. paracasei, L. rhamnosus, L. plantarum, L. fermentum, L. reuteri, Lactococcus lactis of human origin with antimicrobial and immunomodulatory activity against pathogenic bacterial strains based on strain characteristics and intended use Citation46 Citation47.
For probiotic candidates, it is important to assess in vitro the presence of well-identified active compounds such as SCFAs, antimicrobial substances such as reuterine, or other newly identified compounds (antioxidants, glutathione peroxidase and reductase, NO, polyamines, diacetyl, etc.) Citation40. Second, these compounds may also be produced as the result of the industrial fermentation or metabolized end products (ex vitro) also included as part of the probiotic products such as during the long ripening of cheese manufactured with probiotic bacteria Citation48.
Multistrain probiotics
Multistrain probiotics contain more than one strain of the same species or closely related species. Timmermann and co-authors Citation44 differentiated multispecies probiotics that contain strains of different probiotic species that belong to one or more genera. We keep the multistrain definition open for both variants as basically both describe a product with a composition that includes several strains of bacteria.
On the one hand, there may be antagonistic relationships between combinations of strains, if some strains of the probiotic preparation include Lactobacillus spp. that include subclass IIb plantaricin genes that suppress the growth of other species of lactobacilli Citation49. On the other hand, preliminary results demonstrate that some combinations of different bacterial species, due to increased concentrations of QS molecules, exhibit an increased probiotic potential, resulting in interference with pathogen growth and expression of virulence and antibiotic resistance markers in a synergistic manner Citation50 Citation51. Cocultivation studies for the development of multistrain probiotics should evaluate:
the optimal associations showing the absence of cross-antagonism between species;
the probiotic effect of selected probiotic combinations on specific pathogens in vitro and on different human cell lines;
the influence of combinations of probiotic strains on biofilm development;
immuno-modulatory activity, including the cytokine profile induced in cultured epithelial and immune cells by different strains separately and in combination;
the cytotoxicity of combined probiotic culture fractions on different human cell lines; and
the influence of combined microbial culture fractions on the expression of different soluble virulence factors and resistance markers of pathogens.
Assessment of efficacy of mono- or multispecies products
Under ideal conditions, different mono or multispecies probiotics should be characterized using strain or combination-specific metabolic properties. In the prevention of disease or during supportive treatment of various disorders and improvement of metabolic stress, the rationale for the choice of a particular mono-strain probiotic or multistrain probiotic combination should be described in peer-reviewed clinical trial studies. Unfortunately, there are no regulatory requirements defining the optimal number of viable organisms in a probiotic product required for use or the daily dose (single species or multispecies in different combinations) that is necessary for the achievement of documented evidence-based health effects for specific diseases Citation51.
During the early 1990s, a group of probiotic experts concluded that the optimal prophylactic probiotic culture is a mixed one; different strains can be targeted toward different symptoms and blended into a single preparation Citation52. Their hypothesis that multiple probiotic strains may provide a more effective therapy than a single strain was supported by both in vitro and in vivo studies.
Two mono-strain Bifidobacterium longum (BB536) and Lactobacillus johnsonii (La1) probiotics in a mixture were perioperatively administered to colorectal cancer patients. The evaluated strains differed in their functional properties: La1 and not BB536 adhered to colonic mucosa and affected the intestinal pathogens Citation53. These results suggested that a more strict evaluation of the role of single components of multispecies probiotic needed to be performed depending on the application for which it is intended.
To date, there are several probiotic products composed of multiple species of lactobacilli with diverse functional properties that are documented by in vitro and animal experiments that when used during clinical trials may meet the standards for health claims acceptable for EFSA (Regulation No. 1924/2004). There are also several examples of concordance between the metabolic properties of a single probiotic strain and the specific effects on human health.
Design of clinical trials
FAO has set specific criteria for conducting safety studies for drugs that include the use of the healthy volunteers to establish product safety, followed by a second phase study to document efficacy through clinical trials with patients using a randomized double-blinded placebo-controlled (DBPC) approach. The evaluation of the impact of probiotics needs to meet the same criteria also using objective measurements to document clinical safety and efficacy according to the claimed metabolic and functional properties of the specific probiotic strain.
Perhaps the most difficult challenge for research on probiotic products is providing proof of efficacy in overtly healthy individuals (). In theory, this can be accomplished by relying on data from biomarkers (morphological and/or physiological measurements of organ function) of the individual in response to probiotic stimuli. Regardless of the subjective criteria according to which a subject feels healthy, which are skewed by the placebo effect, objective measurements are needed that are able to reveal possible changes in cellular or biochemical parameters from pretreatment levels. It is reasonable to assume that different clinical, morphologic, and biochemical indices as measured in blood, urine, and feces may be helpful for testing the impact of probiotics on the health of the individual, especially in different age groups such as newborns, infants, adults, and elderly subjects.
Fig. 1. Different stages of metabolic and inflammatory stress (modified according Fourth Interactive workshop Nutrition and Health Claims Europe: Challenges Ahead, 2009, Brussels).
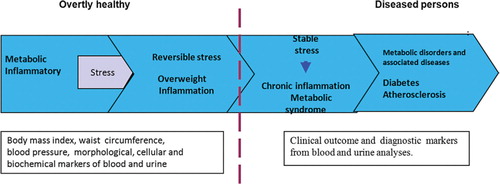
An attempt to follow this approach is shown by a research that suggests that a GI microbiome altered by probiotics influences host lipid and energy metabolism in atherosclerotic or obese patients, as documented by the decrease in the total serum lipidomic profile after treatment with the mono-strain probiotic LGG Citation54.
In clinical trials, the effect of probiotic intervention on the prevention or treatment of specific diseases has mostly relied on well-documented effects, where the degree of shift in the metabolic and inflammatory processes of gut is associated with objectively measured symptoms. However, in irritable bowel syndrome (IBS) and Crohn's disease, where the pathogenesis is not completely understood, the efficacy of probiotic treatment is often evaluated according to the perceived improvement of clinical symptoms without measurement of specific inflammatory markers.
One promising approach for assessing clinical efficacy is to evaluate the effect of probiotic interventions using meta-analyses according to the reported evidence of well-designed randomized clinical trials (RCT). This method, when applied in an appropriate manner, may aid in determining whether single or multiple probiotic strains are more effective as therapeutic interventions. Unfortunately, many papers conclude with a statement announcing that more data are required and the study will be continued. Moreover, most of the papers are case reports, uncontrolled studies in humans, or studies containing insufficient documentation on beneficial effects of the tested probiotics Citation55.
Comparison of effects of mono-strain versus multistrain probiotics
Probiotics have been documented to have activity in treating a variety of clinical conditions – ranging from infantile diarrhea, necrotizing enterocolitis, antibiotic-associated diarrhea, relapsing Clostridium difficile colitis, Helicobacter pylori infections, inflammatory bowel disease, female urogenital infections, and surgical infections Citation55. We have differentiated the efficacy of single and multiple strains of probiotic intervention for patients with various GI diseases mainly relying on the alleviation of clinical symptoms as assessed during well-designed randomized double blinded placebo controlled (DBPC clinical trials. Most of the data are derived from comprehensive meta-analyses Citation56 Citation57 Citation58 or recent references included in databases. Only studies reported between the years 2000 and 2010 were included, where clinical efficacy was documented through DBPC studies, randomized or not. The efficacy in prevention or treatment of acute watery diarrhea, antibiotic-induced intestinal microbiota imbalance, H. pylori infection, IBS, inflammatory bowel disease (IBD), and pouchitis was analyzed ().
Table 1. Comparative summary of DBPC trials on mono- versus multistrain probiotics
Infectious diarrhea
Diarrhea is defined by the World Health Organization as having three or more loose or liquid stools per day. Viral, bacterial, and parasitic agents are recognized as the causative agents of infectious diarrheal illnesses. A meta-analysis of randomized placebo-controlled trials published in the year 2006 Citation59 suggested that probiotics significantly reduced antibiotic-associated diarrhea by 52%, the risk of travelers’ diarrhea by 8%, and that of acute diarrhea due to diverse causes by 34%. Probiotics reduced the associated risk of acute diarrhea among children by 57% and by 26% among adults.
The most commonly used single bacterial strains for probiotic treatment are L. rhamnosus GG ATCC 53103(LGG), Bifidobacterium animalis ssp lactis ATCC 35624, and L. reuteri ATCC 55730 (). It is thought that the effect of these single bacteria rely on their immunomodulating efficacy for GI infections. There is convincing evidence that the immunomodulatory effect of the probiotic is strain and dose dependent, whereas the target infectious agent is also an important factor as recently reported by Guandalini Citation60. The protective effect varied significantly on the basis of the studied probiotic strains. When administered alone Citation61, Lactobacillus reuteri was providing better results as compared to B. lactis BB12. In hospitalized patients, the duration of acute rotavirus diarrhea was reduced by LGG due to the stabilization of indigenous microbiota, reduction of gut permeability caused by rotavirus infection, together with a significant increase of cells secreting IgA antibody directed at rotavirus Citation62 Citation63 Citation64 . Beside the aforementioned properties, LGG stimulates local release of interferon and facilitates antigen transport to underlying lymphoid cells, thus increasing antigen uptake and localization via Peyer's patches. Two potent inhibitory peptides, active against both Gram-positive and Gram-negative bacteria released from LGG in culture media, have been recently characterized by liquid chromatography and mass spectrometry Citation65. However, in four different studies, no prophylactic or treatment effect with LGG was found Citation66 Citation67 Citation68 Citation69 . In contrast, evidence of a significant protective effect consisting of a 14% reduction in the incidence of diarrhea in nearly 4,000 children aged 1–5 years has been reported after their treatment with the mono-strain probiotic L. casei Shirota Citation70.
Table 2. Mono or multispecies probiotic products and functional targets in the host
Dubey and coworkers Citation71 reported that VSL-#3, a multistrain probiotic, administration in patients suffering from acute rotavirus diarrhea resulted in earlier recovery and reduced need for oral rehydration solutions as compared to untreated controls, indicating that the decreased stool volume losses during diarrhea were beneficial in patient recovery. Recently, the effect of the mono-strain probiotic Saccharomyces boulardii was compared with a mixed preparation containing S. boulardii, L. acidophilus, L. rhamnosus, and B. longum strains in the treatment of rotavirus diarrhea in children less than 2 years of age; a significant reduction in diarrhea and fever duration was found only when the mono-strain probiotic was used Citation72.
The differences in host response to probiotic intervention need to be carefully evaluated. The commensal human microbiota has developed sophisticated mechanisms to counteract the inflammatory pathways and to protect host from pathogens that may provide predictions about the effect of probiotics, that are difficult in the absence of actual clinical trial information and data on improved biomarkers.
Antibiotic therapy and Clostridium difficile infection (CDI)
One of the main targets of probiotic intervention studies is the restoration of GI microbiota composition and the correction of dysbiosis-associated markers of GI function following antibiotic therapy, when the active agent is secreted via the hepatobiliary circulation. Arvola et al. Citation73 have demonstrated that a probiotic strain, Lactobacillus GG, has been effective in the prevention of diarrhea in children receiving antimicrobial treatment for respiratory infections with a single dose.It has also been reported that third generation cephalosporins, particularly ceftriaxone, induce a decrease in Escherichia coli and Lactobacillus counts and an increase in Gram-positive cocci and Clostridium counts Citation74. The same authors found that microbial shifts were associated with a reduction in the activities of enzymes such as ß-galactosidase and ß-glucosidase, and with an increase in the activity of ß-glucuronidase, an enzyme involved in the formation of toxic and carcinogenic compounds. It was concluded that parenterally administered ceftriaxone caused a significant dysbiosis that may be corrected by appropriate probiotic intervention.
In , different trials performed using six commercial probiotics tested for their ability to counteract the side effects associated with ceftriaxone therapy are reported and the results suggest the following:
Three mono-strain products containing Saccharomyces boulardii, Enterococcus faecium SF 68, or L. rhamnosus GG respectively; having no effect on stool frequency;
Two multistrain preparations containing Bifidobacterium bifidum and L.acidophilus, or a multispecies preparation containing a combination of B.breve, B. infantis, B.longum, L. acidophilus, L. casei, L. delbrueckii subsp. bulgaricus, L. plantarum, S. faecium, S. salivarius subsp. thermophilus decreased stool frequency.
The conclusion of this comparison was that probiotics containing multiple species of lactobacilli and bifidobacteria at high concentration are more effective in preventing dysbiosis induced by ceftriaxone treatment than mono-strain probiotic preparations Citation74. The reduction of stool frequency associated with ceftriaxone treatment, accompanied with a decreased fecal pH, clearly supports the efficacy of multistrain probiotics in antibiotic-associated diarrhea and the concomitant dysbiosis associated with antibiotic use. However, due to some strain variability in antibiotic sensitivity of Lactobacillus sp., the reduction of antibiotic-associated diarrhea with mono-strain probiotics cannot be excluded because only a limited number of strains have been evaluated in clinical trials.
Antibiotic therapy is often associated with Clostridium difficile infection (CDI). Probiotics that have been proposed for prevention and treatment of antibiotic-associated diarrhea and CDI include different bacterial species (Bifidobacterium, LGG, L. rhamnosus, L. casei, L. plantarum 299v, E. faecium [SF68]), and yeasts (S. boulardii, S. cerevisiae). These commercially available probiotic formulations are commonly available as lyophilized capsules or in the form of a fermented drink. A well-designed study by Guandalini Citation60 compared the efficacy of probiotic intervention by mono-strain probiotics such as L. rhamnosus GG, B. lactis, and L. reuteri, reporting only a modest clinical benefit after antibiotic treatment. Thomas and co-authors Citation75 performed a prospective, randomized double-blind, placebo-controlled trial to assess the clinical efficacy of LGG in prevention of C. difficile diarrhea in patients taking antibiotics. No differences in the rate of occurrence of diarrhea were found between patients treated with the probiotic formulation and those receiving a placebo.
In 2003, Wullt and colleagues Citation76 performed a double-blind, placebo-controlled trial to analyze the ability of Lactobacillus plantarum 299v to prevent recurrent episodes of Clostridium difficile-associated diarrhea. The lactobacilli treatment had no side effects, but the small sample size did not allow any conclusions to be drawn concerning the efficacy of L. plantarum in patients with recurrences.
Overall, none of the published papers on this topic Citation77 Citation78 Citation79 unequivocally prove that the use of probiotics for the prevention and/or therapy of CDI is able to reconstitute the gut microflora and to prevent recurrences. At the present time, proof of probiotic efficacy for CDI is inconclusive and a significant number of prospective randomized trials are urgently needed.
Helicobacter pylori infection
It has been reported that Lactobacillus sp., Bifidobacterium sp., Saccharomyces sp., Clostridium sp. strains, etc. have inhibitory effects on H. pylori infection in vitro and in vivo studies Citation5 Citation47 Citation80. Cremonini et al. Citation81 analyzed three randomized, double-blind, placebo-controlled studies documenting a decrease in the side effects of triple antibiotic therapy for H. pylori colonization both with single and double probiotics, but no effect on the clearing of H. pylori was registered in all three variations. Wang et al. Citation82 observed that out of the two probiotic strains present in AB yogurt, Bifidobacterium Bb12, and Lactobacillus La5, only Bb12 exerted an inhibitory effect in vitro against H. pylori. If they were administered as yogurt (containing >107 CFU/mL of each strain), the authors reported a steeper decrease in H. pylori colonization in those individuals who were more intensely colonized with the pathogen. The study group consisted of 59 H. pylori-positive individuals receiving yogurt twice daily for 6 weeks, whereas 11 subjects were given a placebo. Besides LAB, the effect of the mono-strain probiotic Clostridium butyricum MIYAIRI588 (generally used in Japan for treatment of patients with antibiotic-associated diarrhea and C. difficile infection) has been also demonstrated using germ-free mice. The number of H. pylori in gastric mucosa was significantly reduced by coinfection with vegetative cells of C. butyricum Citation83.
Mikelsaar and coworkers studied healthy individuals without GI complaints who were persistently colonized with H. pylori. The enteric coated mix of L. paracasei 8700:2, B. longum 46, and L. fermentum ME-3 with the probiotic Raftilose p65, although having in vitro antagonistic properties did not exert any antimicrobial effect on H. pylori infection in a randomized, double-blind, placebo-controlled trial Citation40 Citation80. Seemingly, the target pathogen was not reached in the stomach.
Lee et al. Citation84 reported the use of probiotics as a non-antibacterial strategy during H. pylori infection where they serve as suppressors for proinflammatory cytokine signaling,exerting a beneficial antiinflammatory effect. Mikelsaar and coworkers found that the systematic oxidative stress (ox-S) caused by H. pylori infection in overtly healthy persons () was suppressed by the antioxidative probiotic L. fermentum ME-3 consumed over a 3-week course of therapy. Specifically, blood antioxidative indices, such as lipid peroxidation, content of reduced glutathione, and total antioxidative activity, were reduced Citation80 Citation85. These same biomarkers are also often used as predictive factors for the development of atherosclerosis Citation86. The ability of L. fermentum ME-3 strain to induce IL-10 Citation87 seems to be an important factor in suppression of systemic inflammation during any chronic infection. The evaluation of small numbers of probiotic strains may limit the conclusions on the increased IL-10 response provoked by bifidobacteria and E. coli Nissle as compared to the lack of activity for the four strains of lactobacilli that have also been evaluated Citation88.
In vitro studies and clinical trials have shown that mono-strain probiotic-supplemented regimens increased eradication rates and reduced side effects during antibiotic therapy for H. pylori. Because probiotics are effective against antibiotic-resistant H. pylori strains, their use needs to be considered as a supportive intervention during antibiotic eradication of this pathogen Citation83.
In summary, the results of clinical trials suggest that probiotic supplementation during anti-H. pylori therapy decreases adverse side effects, resulting in better compliance with antibiotic therapy and less systemic effects.
Irritable bowel syndrome
Irritable bowel syndrome is a functional bowel disorder that manifests as chronic, recurrent abdominal pain, or discomfort associated with disturbed bowel habit in the absence of structural abnormalities likely to account for these symptoms. The epigenetic model of IBS incorporates proinflammatory markers, neuroendocrine alterations, and links with both psychosocial and infectious stresses Citation89. In addition to the role of GI infections, it is suggested that IBS patients have an abnormal composition and a temporary instability of their intestinal microbiota. The altered microbiota raises the possibilities of therapeutic interventions using selective antibiotic therapy or probiotic administration.
In contrast, ulcerative colitis and Crohn's disease, the two distinct idiopathic pathologies of inflammatory bowel diseases, are characterized by documented gut epithelial lesions and alteration of intestinal microbiota, particularly Bacteroides spp. Citation90. Both diseases are spontaneously relapsing and generally accepted as immunologically mediated disorders of the GI tract. The etiology and risk factors of these complex inflammatory diseases of the bowel remain elusive. However, it should be noted that IBS and IBD patients account for 30–50% of office visits for gastroenterology services.
As stated previously, IBS is a debilitating disorder. The evidence for efficacy of most drug therapies in the treatment of IBS is weak. Recent meta-analyses confirm a role for probiotics in IBS, but also make it clear that the effects of probiotics in IBS, as elsewhere, are highly strain specific. Variability and the formulation of specific strains used as probiotic products vary dramatically depending on where they are produced. Lack of quality control for probiotics hampers the ability to make recommendations for their use.
A wide variety of probiotic trials have been conducted with mono- or multistrain probiotic interventions. However, a literature search has revealed a few comparative studies on mono strains of different probiotic genera or species under the same clinical trial conditions. A well conducted study by O'Mahony et al. Citation91 showed that if two strains, Bifidobacterium infantis ATCC 35624 or Lactobacillus salivarius UCC4331, were administered to separate groups of patients with IBS, only B. infantis 35624 alleviated the symptoms of disease (). The symptomatic response was associated with normalization of the ratio of the antiinflammatory to proinflammatory cytokines, suggesting an immune-modulating role for this strain of B. infantis. A possibile confounding variable for this study was the use of a milk product for delivery of the probiotic and not a capsule. At the same time, there are several failures of clinical efficacy for IBS treatment employing other single-strain probiotics. Clinical trials of L. plantarum MF1298 Citation92 did not document relief of the IBS symptoms.
Hoveyda et al. Citation57 have published a systematic review and meta-analysis of randomized trials performed up to 2007 to evaluate efficacy of probiotics for alleviating symptoms in IBS patients. They identified 14 randomized placebo controlled trials that report modest improvement in overall symptoms after several weeks of treatment, with mono-strain probiotics generally demonstrating better results. In five trials, a combination probiotic containing different numbers and strains of bacteria were used as intervention while nine clinical trials evaluated single strains alone or combined with a prebiotic substrate. The mono-strains included a variety of species, including B. infantis Citation93 and L. rhamnosus GG Citation94 Citation95 used as single agents. Only L. plantarum 299v was evaluated in two trials Citation96 Citation97 with the results showing a reduction of pain, bloating, and flatulence. Brenner and coauthors Citation98 have evaluated several clinical studies of IBS on the basis of the following approved criteria: Citation1 Random Controlled Trials (RCTs), Citation2 adults with IBS as defined by Manning or Rome II criteria, Citation3 single or combination probiotics versus placebo, and Citation4 improvement in IBS symptoms and/or decrease in frequency of adverse events reported. Only B. infantis 35624 Citation91 Citation93 could be shown to provide a significant improvement in the composite score for abdominal pain/discomfort, bloating/distention, and/or bowel movement difficulty compared with placebo in two appropriately designed studies. No other probiotic in their studies demonstrated significant ability to improve IBS symptoms. In 2009, Agrawal and coworkers Citation99 reported that the probiotic B. lactis is able to improve abdominal distension and GI transit in IBS patient with constipation. These results support the acceleration of transit as a useful strategy for treating distension.
Several multispecies mixtures have been reported to have efficacy for the treatment of IBS. These studies Citation100 have combined L. plantarum LP 01 with B. breve BR 03 or L. plantarum LP 01 with L. acidophilus LA 02, B. lactis DN-173 010 mixed with yoghurt as a delivery system Citation101. Kajander et al. Citation102 evaluated L. rhamnosus GG, L. rhamnosus Lc705, Propionibacterium freudenreichii spp. shermanii JS, and B. breve Bb99 and documented a decrease in bowel symptoms, abdominal pain, and bloating. Later, the same group. Citation103 found that Ruminococcus torques phylotype was decreased in the probiotic group during the intervention at 6 months. In addition, the clostridial phylotype, Clostridium thermosuccinogenes, was stably elevated. The bacterial alterations detected were in accordance with previously observed alleviation of symptoms in IBS. No alleviation of IBS symptoms was found when evaluating a Lactobacillus multispecies probiotic Citation104 in French children. However, the probiotic combination VSL#3, composed of eight different strains, has been observed to decrease flatulence scores Citation105 and alleviate abdominal bloating Citation106 but did not bring relief for stool frequency-related symptoms, abdominal pain scores, and colonic transit time. Guandalini et al. Citation107 in a more recent randomized, double-blind, placebo-controlled crossover study reported that VLS#3 was effective in ameliorating symptoms including abdominal pain/discomfort in patients with IBS.
The World Gastroenterology Organization Global Guideline Citation108 has suggested both B. lactis DN-173 strain and VSL#3 for the treatment of IBS.
Inflammatory bowel disease
Over 1 million people suffer from IBD in the United States, whereas in the UK one-quarter million people are afflicted with IBD Citation109 Citation110. Current understanding of IBD pathogenesis includes the adherence of bacteria to the intestinal mucosa and bacterial invasion into mucosal epithelial cells with a concomitant inflammatory response. This chronic bowel inflammation cannot subside as long as the mucus barrier remains defective. The inflammatory response interferes with epithelial cell tolerance to intestinal bacteria and leads to characteristic changes in the composition of the fecal microbiota Citation109. In biopsies and stool samples of 58 adolescents with inflammatory bowel disease, an increase in the total populations of aerobic bacteria but not of anaerobes was demonstrated Citation111. This corresponds with a significant decrease in the concentrations of propionic and butyric acids in the feces of patients with IBD. The authors postulated that different components of Enterobacteriaceae, especially their lipopolysaccharides, may also contribute to perpetuation of chronic colon inflammation. However, most of the microbiota adhering to the colonic mucosa surrounding the mucus layer comprises Clostridium coccoides and Bifidobacterium spp. These findings suggest that IBD is not caused by a specific intestinal bacterial cluster or species and that a disordered intestinal microflora may be involved in the pathogenesis of IBD. A role for hydrogen peroxide-producing colonic bacteria as causative agents of the inflammatory process in young adults suffering from IBD has been hypothesized Citation112.
Selected mono-strain probiotics have been proven to be clinically effective in maintaining remission in patients with ulcerative colitis. E. coli strain Nissle 1917 has been used as a probiotic for the treatment of inflammatory bowel disease, chronic constipation, and acute protracted diarrhea Citation113. The strain Nissle 1917 expresses a K5 capsule that mediates interactions with intestinal epithelial cells. Additionally, this strain exhibits a particular lipopolysaccaride with immunomodulating properties without showing immunotoxic effects associated with endotoxin. The induction of chemokines by the Nissle 1917 strain was observed in vitro following the interaction with the basolateral surface of Caco-2 cells, suggesting that this strain is effective in repairing the epithelial barrier Citation114 Citation115. The main result of the Nissle 1917 strain has been the maintenance of remission in patients with ulcerative colitis equal to the effect of mesalazine Citation115. Campieri and Gionchetti Citation116 Citation117 Citation118 and Guslandi Citation119 have provided convincing evidence supporting the role of intestinal bacteria as a cause of ulcerative colitis. Guslandi et al. Citation120 have reported the efficacy of mono-strain probiotics (S. boulardii) together with mesalazine in the prevention of recurrences in Crohn's disease and in the prolongation of remission that were superior to mesalazine alone. In addition, it has been shown that Bifidobacterium spp. and Lactobacillus spp. fermented milk reduced the exacerbation of ulcerative colitis symptoms Citation121.
In seven different trials using VSL#3, it has been demonstrated that a remarkable induction of remission for mild or moderate ulcerative colitis occurs, along with maintenance of remission in children Citation122 Citation123 and in adults Citation124 Citation125 Citation126 . Ishikawa and coworkers Citation127, using a mixture of B. breve, B. bifidum, and L. acidophilus YIT 0168 in fermented milk, reported reduced exacerbation of ulcerative colitis symptoms in 21 adult patients with maintenance of remission. The prevention of flare-ups in chronic pouchitis has been also demonstrated with VSL#3 treatment Citation117 Citation128 Citation129 Citation130 .
Based on these trials, it appears that the VSL#3 multispecies probiotic mixture is effective in the maintenance of remission in IBS, IBD, and pouchitis. However, Haller and Autenrieth Citation51 have raised the question as to which of the eight single bacterial strains from the VSL#3 mixture play a major role in these effects and whether the whole mixture is necessary to obtain the reported probiotic effects. In most studies with VSL#3, a significant increase in bifidobacteria has been observed in patient's feces during treatment. High concentrations of some Bifidobacterium strains of the combination, such as B. infantis Y1 and B. breve Y 8, were detected in feces by Brigidi and coworkers Citation131 Citation132 suggesting their putative role in beneficial shifts of specific biochemical markers such as β-galactosidase and urease. The induction of the antiinflammatory cytokine IL-10 by Bifidobacterium genomic DNA in peripheral blood mononuclear cells Citation133 and by the probiotic cocktail of VSL#3 in dendritic cells Citation134 has also been assessed. VSL#3 DNA Citation133 attenuated the release of systemic TNF-α and colonic IFN-γ in experimental models Citation135 providing in vitro evidence for the limitation of the epithelial proinflammatory responses. In addition, it is possible that maintaining tight junction protein expression may prevent epithelial cell permeability Citation136 and serve as a clue to probiotic intervention in the successful treatment of IBD using VSL#3.
Additional studies indicate that probiotic therapy with VSL#3 increased Citation137 the richness and total number of microbiota, especially obligate anaerobes. The number of mucosal regulatory cells was expanded in patients with ileal pouch-anal anastomosis for ulcerative colitis Citation138, resulting in an antiinflammatory cytokine response. Appropriately, powered studies with different (combinations of) probiotics show positive results for reduction of symptoms, although a considerable placebo effect is also found. The attenuation of the proinflammatory immune response using probiotic bacteria through TLR signaling, IL-10 upregulation, and expression of COX-2 appear to provide a theoretical explanation for a pathophysiological mechanism for probiotic impact in inflammatory bowel diseases Citation139 Citation140.
None of the probiotics such as LGG Citation141 Citation142 or L. johnsonii La1 Citation143 tested to date have been shown to be effective in induction or in maintenance of remission in patients with Crohn's disease.
A summary of comparisons between the efficacy of probiotic preparations in different diseases and the preferences for mono- or multistrain probiotics are provided in . In , there are depicted 25 trials with probiotic prevention or treatment concerning infectious or antimicrobial treatment-induced diarrhea, caused by different opportunistic bacterial or viral pathogens with 48% efficacy are depicted. The monostrain probiotic preparations (17 trials) were effective in 41% of cases, whereas the multistrain ones (8 trials) expressed somewhat higher (63%) efficacy. In IBS (16 trials), the total efficacy was 75% while by applying monostrain preparations, the efficacy was 67% and multistrain probiotic preparations 86%. In 18 trials with patients of IBD, the high preference for multistrain probiotic efficacy (40% vs. 100%) was registered. The reasons for better efficacy of multistrain probiotic preparations seemingly derive from the large individual interrelations between microbiota and health markers of humans apparent also in case of GI diseases.
Health risks and beneficial effects associated with probiotics
Probiotics are live microorganisms, so it is possible that their administration may result in host infection under certain circumstances such as profound immunosuppression of the host. The risk of sepsis due to probiotic bacteria should be weighed against the potential for sepsis due to more pathogenic bacteria and the morbidity for diseases in which probiotic bacteria are used as therapeutic agents Citation36 Citation55. No experimental or clinical data concerning increased risk of infection by either mono- or multistrain probiotics have been published to date, although anecdotal cases of infection have been reported.
Well-designed placebo controlled studies of the infection rates during use of probiotic products for a specific condition in a particular target population are needed to address this issue Citation144. Results based on objective criteria are required for ascertaining the real health benefits and risks for the use of probiotics. In this regard, a careful selection of the probiotic agent, a dose standardization, and a thorough knowledge of the beneficial functional effects are the most important issues for clearly defining appropriate use of probiotics as interventions in a variety of clinical syndromes.
Conclusions
1. Probiotic strains of specific species, either in mono- or multiculture, should have specific and well-defined metabolic and functional properties measureable by objective criteria. The probiotic effect should target a particular host function that has been altered through environmental stress, antibiotic utilization, or during specific clinical diseases that result in the alteration of the normal microbiota.
2. According to published clinical trials, the efficacy of probiotic intervention for infectious or antimicrobial treatment-induced diarrhea, caused by different opportunistic bacterial or viral pathogens, was 48%, whereas the mono-strain probiotics have expressed somewhat lower efficacy than the combinations of different species.
3. The probiotic preparations efficacy for silencing the clinical symptoms of IBS was 75% and for attenuating the inflammatory response during IBD was 83%, whereas multi-strain probiotics appear to have better efficacy.
Conflict of interest and funding
Authors are President (M.M.), Secretary (V.L.), President- Elect (A.B.O.) and Past-President (G.D.) of the Society for Microbial Ecology and Disease (SOMED), respectively. No other conflicts reported. This study was supported by grant from the Estonian Ministry of Higher Education and Research (grant no. SF0180132s08).
Acknowledgements
This review article largely reflects the opinions expressed by authors during the consensus conference held in the framework of the International Workshop on Probiotics organized by Professor Claudio De Simone and Dr. Florence Pryen in Rome February 25–27, 2010. Authors are indebted to Professor Franco Dellaglio from the University of Verona for the useful suggestions provided during the Consensus Conference and the critical reading of this article.
References
- Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989; 66: 365–78.
- Elmer GW, Surawicz CM, McFarland LV. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996; 275: 870–6.
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003; 16: 658–72.
- Timmerman HM, Niers LE, Ridwan BU, Koning CJ, Mulder L, Akkermans LM, et al. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr. 2007; 26: 450–9.
- Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002; 82: 279–89.
- Ljungh A, Wadstrom T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol. 2006; 7: 73–89.
- van Hemert S, Meijerink M, Molenaar D, Bron PA, de Vos P, Kleerebezem M, et al. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010; 10: 293.
- Contor L, Asp NG. Process for the assessment of scientific support for claims on foods (passclaim) phase two: moving forward. Eur J Nutr. 2004; 43(Suppl 2): I3–6.
- Aggett PJ, Antoine JM, Asp NG, Bellisle F, Contor L, Cummings JH, et al. Passclaim: consensus on criteria. Eur J Nutr. 2005; 44(Suppl 1): 5–30.
- Floch MH, Walker WA, Guandalini S, Hibberd P, Gorbach S, Surawicz C, et al. Recommendations for probiotic use–2008. J Clin Gastroenterol. 2008; 42(Suppl 2): S104–8.
- Mercenier A, Lenoir-Wijnkoop I, Sanders ME. Physiological and functional properties of probiotics. Bul Int Dairy Federation. 2008; 429: 2–6.
- Sanders ME, Marco ML. Food formats for effective delivery of probiotics. Ann Rev Food Sci Technol. 2010; 1: 65–85.
- Zoetendal EG, Cheng B, Koike S, Mackie RI. Molecular microbial ecology of the gastrointestinal tract: from phylogeny to function. Curr Issues Intest Microbiol. 2004; 5: 31–47.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005; 308: 1635–8.
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005; 307: 1915–20.
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006; 124: 837–48.
- Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends Ecol Evol. 2006; 21: 517–23.
- Ventura M, Turroni F, Canchaya C, Vaughan EE, O'Toole PW, van Sinderen D. Microbial diversity in the human intestine and novel insights from metagenomics. Front Biosci. 2009; 14: 3214–21.
- Moore WE, Holdeman LV. Special problems associated with the isolation and identification of intestinal bacteria in fecal flora studies. Am J Clin Nutr. 1974; 27: 1450–5.
- Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984; 86: 174–93.
- Huys G, Vanhoutte T, Joossens M, Mahious AS, De Brandt E, Vermeire S, et al. Coamplification of eukaryotic DNA with 16s rrna gene-based pcr primers: possible consequences for population fingerprinting of complex microbial communities. Curr Microbiol. 2008; 56: 553–7.
- McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis. 2000; 12: 193–218.
- Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within?. Infect Immun. 2008; 76: 3360–73.
- Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic acids from lactobacillus strains elicit strong Tumor Necrosis Factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol. 2003; 10: 259–66.
- Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004; 22: 181–215.
- Moslehi-Jenabian S, Gori K, Jespersen L. Ai-2 signalling is induced by acidic shock in probiotic strains of Lactobacillus spp. Int J Food Microbiol. 2009; 135: 295–302.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009; 457: 480–4.
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998; 62: 1157–70.
- Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009; 136: 65–80.
- Leibovici L, Arav-Boger R, Danon YL. A clinical model for diagnosis of urinary tract infection in young women. Arch Intern Med. 1993; 153: 1011–1015.
- Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011; 15: R183.
- Welling GW, Meijer-Severs GJ, Helmus G, van Santen E, Tonk RH, de Vries-Hospers HG, et al. The effect of ceftriaxone on the anaerobic bacterial flora and the bacterial enzymatic activity in the intestinal tract. Infection. 1991; 19: 313–6.
- Wlodarska M, Finlay BB. Host immune response to antibiotic perturbation of the microbiota. Mucosal Immunol. 2010; 3: 100–3.
- Sekirov I, Finlay BB. The role of the intestinal microbiota in enteric infection. J Physiol. 2009; 587: 4159–67.
- Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric salmonella infection. Infect Immun. 2009; 77: 2741–53.
- Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, et al. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis. 2006; 42: e35–44.
- Truusalu K, Mikelsaar RH, Naaber P, Karki T, Kullisaar T, Zilmer M, et al. Eradication of Salmonella typhimurium infection in a murine model of typhoid fever with the combination of probiotic Lactobacillus fermentum ME-3 and ofloxacin. BMC Microbiol. 2008; 8: 132.
- Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007; 85: 816–23.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444: 1027–31.
- Mikelsaar M, Zilmer M. Lactobacillus fermentum ME-3-an antimicrobial and antioxidative probiotic. Micr Ecol Health Dis. 2009; 1: 1–27.
- Mikelsaar M, Stsepetova J, Hutt P, Kolk H, Sepp E, Loivukene K, et al. Intestinal Lactobacillus sp. is associated with some cellular and metabolic characteristics of blood in elderly people. Anaerobe. 2010; 16: 240–6.
- Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the women's health initiative observational study. Arch Intern Med. 2005; 165: 500–8.
- Stsepetova J, Sepp E, Kolk H, Loivukene K, Songisepp E, Mikelsaar M. Diversity and metabolic impact of intestinal Lactobacillus species in healthy adults and the elderly. Br J Nutr. 2011; 105: 1235–44.
- Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics–a comparison of functionality and efficacy. Int J Food Microbiol. 2004; 96: 219–33.
- Lazar V, Balotescu C, Vassu T, Barbu V, Smarandache D, Sasarman E, et al. Experimental study on rats of the probiotic effect of some lactic acid bacteria previously selected for their in vitro capacity to interfere with salmonella enteritidis infection. Roumanian Biotech Lett. 2005; 10: 2123–2133.
- Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Moller PL, Michaelsen KF, Paerregaard A, et al. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. By in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999; 65: 4949–56.
- Hutt P, Shchepetova J, Loivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006; 100: 1324–32.
- Songisepp E, Kullisaar T, Hutt P, Elias P, Brilene T, Zilmer M, et al. A new probiotic cheese with antioxidative and antimicrobial activity. J Dairy Sci. 2004; 87: 2017–23.
- Maldonado A, Ruiz-Barba JL, Floriano B, Jimenez-Diaz R. The locus responsible for production of plantaricin s, a class IIb bacteriocin produced by Lactobacillus plantarum LPCO10, is widely distributed among wild-type Lact. plantarum strains isolated from olive fermentations. Int J Food Microbiol. 2002; 77: 117–24.
- Lazar V. Miyazaki Y. Hanawa T. Chifiriuc M. Ditu L. Marutescu L, et al. The influence of some probiotic cultures supernatants on the growth rate and virulence expression of several selected enteroaggregative E. coli (EAGGEC) clinical strains. Rom Arch Microbiol Immunol. 2009; 68(4): 207–14.
- Haller D, Autenrieth IB. Microbe-host interaction in chronic diseases. Int J Med Microbiol. 2010; 300: 1–2.
- Sanders ME. Summary of conclusions from a consensus panel of experts on health attributes of lactic cultures: significance to fluid milk products containing cultures. J Dairy Sci. 1993; 76: 1819–28.
- Gianotti L, Morelli L, Galbiati F, Rocchetti S, Coppola S, Beneduce A, et al. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol. 2010; 16: 167–75.
- Kekkonen RA, Sysi-Aho M, Seppanen-Laakso T, Julkunen I, Vapaatalo H, Oresic M, et al. Effect of probiotic Lactobacillus rhamnosus GG intervention on global serum lipidomic profiles in healthy adults. World J Gastroenterol. 2008; 14: 3188–94.
- Gupta V, Garg R. Probiotics. Indian J Med Microbiol. 2009; 27: 202–9.
- Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006; 12: 5941–50.
- Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009; 9: 15.
- Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I. Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J Nutr. 2010; 140: 690S–7S.
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006; 6: 374–82.
- Guandalini S. Probiotics for children with diarrhea: an update. J Clin Gastroenterol. 2008; 42(Suppl 2): S53–7.
- Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005; 115: 5–9.
- Isolauri E, Rautava S, Kalliomaki M, Kirjavainen P, Salminen S. Probiotic research: learn from the evidence. Allergy. 2002; 57: 1076–7; author reply 1078.
- Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Efficacy of Lactobacillus rhamnosus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001; 138: 361–5.
- Szajewska H, Skorka A, Ruszczynski M, Gieruszczak-Bialek D. Meta-analysis: lactobacillus rhamnosus GG for treating acute diarrhoea in children. Aliment Pharmacol Ther. 2007; 25: 871–81.
- Lu R, Fasano S, Madayiputhiya N, Morin NP, Nataro J, Fasano A. Isolation, identification, and characterization of small bioactive peptides from Lactobacillus rhamnosus GG conditional media that exert both anti-gram-negative and gram-positive bactericidal activity. J Pediatr Gastroenterol Nutr. 2009; 49: 23–30.
- Basu S, Chatterjee M, Ganguly S, Chandra PK. Efficacy of Lactobacillus rhamnosus GG in acute watery diarrhoea of indian children: a randomised controlled trial. J Paediatr Child Health. 2007; 43: 837–42.
- Hojsak I, Snovak N, Abdovic S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2010; 29: 312–6.
- Mastretta E, Longo P, Laccisaglia A, Balbo L, Russo R, Mazzaccara A, et al. Effect of Lactobacillus GGand breast-feeding in the prevention of rotavirus nosocomial infection. J Pediatr Gastroenterol Nutr. 2002; 35: 527–31.
- Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001; 322: 1327.
- Sur D, Manna B, Niyogi SK, Ramamurthy T, Palit A, Nomoto K, et al. Role of probiotic in preventing acute diarrhoea in children: a community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiol Infect. 2010: 1–8.
- Dubey AP, Rajeshwari K, Chakravarty A, Famularo G. Use of VSL#3 in the treatment of rotavirus diarrhea in children: preliminary results. J Clin Gastroenterol. 2008; 42((Suppl 3, Pt 1)): S126–9.
- Grandy G, Medina M, Soria R, Teran CG, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in bolivian children. BMC Infect Dis. 2010; 10: 253.
- Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999; 104: e64.
- Zoppi G, Cinquetti M, Benini A, Bonamini E, Minelli EB. Modulation of the intestinal ecosystem by probiotics and lactulose in children during treatment with ceftriaxone. Curr Ther Res. 2001; 62: 418–435.
- Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001; 76: 883–9.
- Wullt M, Hagslatt ML, Odenholt I. Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: a double-blind, placebo-controlled trial. Scand J Infect Dis. 2003; 35: 365–7.
- Miller M. The fascination with probiotics for Clostridium difficile infection: lack of evidence for prophylactic or therapeutic efficacy. Anaerobe. 2009; 15: 281–4.
- McFarland LV. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe. 2009; 15: 274–80.
- Bauer MP, van Dissel JT. Alternative strategies for Clostridium difficile infection. Int J Antimicrob Agents. 2009; 33(Suppl 1): S51–6.
- Mikelsaar M, Hütt P, Kullisaar T, Zilmer K, Zilmer M. Double benefit claims for antimicrobial and antioxidative probiotic. Micr Ecol Health Dis. 2008; 20: 184–188.
- Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, et al. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002; 97: 2744–9.
- Wang KY, Li SN, Liu CS, Perng DS, Su YC, Wu DC, et al. Effects of ingesting lactobacillus- and bifidobacterium-containing yogurt in subjects with colonized helicobacter pylori. Am J Clin Nutr. 2004; 80: 737–41.
- Kamiya S, Takahashi M, Manzoku T, Oka K, Osaki T, Hanawa T, et al. Probiotics and Helicobacter pylori. Micr Ecol Health Dis. 2006; 18: 177–180.
- Lee JS, Paek NS, Kwon OS, Hahm KB. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J Gastroenterol Hepatol. 2010; 25: 194–202.
- Hutt P, Andreson H, Kullisaar T, Vihalemm T, Unt E, Kals J, et al. Effects of a synbiotic product on blood antioxidative activity in subjects colonized with Helicobacter pylori. Lett Appl Microbiol. 2009; 48: 797–800.
- Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004; 84: 1381–478.
- Truusalu K, Kullisaar T, Hutt P, Mahlapuu R, Aunapuu M, Arend A, et al. Immunological, antioxidative, and morphological response in combined treatment of ofloxacin and Lactobacillus fermentum ME-3 probiotic in Salmonella typhimurium murine model. Apmis. 2010; 118: 864–72.
- Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, Caramelli E, et al. Lactobacilli, bifidobacteria and E. coli Nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol. 2006; 12: 5978–86.
- Dinan TG, Cryan J, Shanahan F, Keeling PW, Quigley EM. Ibs: an epigenetic perspective. Nat Rev Gastroenterol Hepatol. 2010; 7: 465–71.
- Noor SO, Ridgway K, Scovell L, Kemsley EK, Lund EK, Crawford J, et al. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010; 10: 134.
- O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005; 128: 541–51.
- Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010; 10: 16.
- Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006; 101: 1581–90.
- Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007; 25: 177–84.
- Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005; 147: 197–201.
- Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299v in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001; 13: 1143–7.
- Nobaek S, Johansson ML, Molin G, Ahrne S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000; 95: 1231–8.
- Brenner DM, Chey WD. Bifidobacterium infantis 35624: a novel probiotic for the treatment of irritable bowel syndrome. Rev Gastroenterol Disord. 2009; 9: 7–15.
- Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol. 2009; 104: 1998–2004.
- Saggioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol. 2004; 38: S104–6.
- Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007; 26: 475–86.
- Kajander K, Myllyluoma E, Rajilic-Stojanovic M, Kyronpalo S, Rasmussen M, Jarvenpaa S, et al. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008; 27: 48–57.
- Lyra A, Krogius-Kurikka L, Nikkila J, Malinen E, Kajander K, Kurikka K, et al. Effect of a multispecies probiotic supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol. 2010; 10: 110.
- Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008; 32: 147–52.
- Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003; 17: 895–904.
- Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005; 17: 687–96.
- Guandalini S, Magazzu G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010; 51: 24–30.
- World Gastroenterology Organisation. (2009). WGO Practice Guideline. Irritable bowel syndrome: a global perspective. MilwaukeeUSA: WGO.pp. 1–20.
- McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008; 14: 2650–61.
- McFarland LV. State-of-the-art of irritable bowel syndrome and inflammatory bowel disease research in 2008. World J Gastroenterol. 2008; 14: 2625–9.
- Swidsinski A, Loening-Baucke V, Herber A. Mucosal flora in Crohn's disease and ulcerative colitis-an overview. J Physiol Pharmacol. 2009; 60(Suppl 6): 61–71.
- Strus M, Gosiewski T, Fyderek K, Wedrychowicz A, Kowalska-Duplaga K, Kochan P, et al. A role of hydrogen peroxide producing commensal bacteria present in colon of adolescents with inflammatory bowel disease in perpetuation of the inflammatory process. J Physiol Pharmacol. 2009; 60(Suppl 6): 49–54.
- Sonnenborn U, Schulze J. The non-pathogenic Escherichia coli strain Nissle 1917-features of a versatile probiotic. Microb Ecol Health Dis. 2009; 21: 122–158.
- Hafez M, Hayes K, Goldrick M, Warhurst G, Grencis R, Roberts IS. The K5 capsule of Escherichia coli strain Nissle 1917 is important in mediating interactions with intestinal epithelial cells and chemokine induction. Infect Immun. 2009; 77: 2995–3003.
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004; 53: 1617–23.
- Campieri M, Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut. 2001; 48: 132–5.
- Gionchetti P, Amadini C, Rizzello F, Venturi A, Poggioli G, Campieri M. Diagnosis and treatment of pouchitis. Best Pract Res Clin Gastroenterol. 2003; 17: 75–87.
- Gionchetti P, Rizzello F, Poggioli G, Pierangeli F, Laureti S, Morselli C, et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther. 2007; 25: 1231–6.
- Guslandi M. Of germs in inflammatory bowel disease and of how to fight them. J Gastroenterol Hepatol. 2003; 18: 115–6.
- Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of crohn's disease. Dig Dis Sci. 2000; 45: 1462–4.
- Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004; 20: 1133–41.
- Huynh HQ, deBruyn J, Guan L, Diaz H, Li M, Girgis S, et al. Probiotic preparation VSL#3induces remission in children with mild to moderate acute ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2009; 15: 760–8.
- Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009; 104: 437–443.
- Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004; 10: PI126–31.
- Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, et al. VSL#3probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005; 100: 1539–46.
- Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009; 7: 1202–9–1209 e1.
- Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003; 22: 56–63.
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000; 119: 305–9.
- Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum 2007; 50: 2075–82, discussion 2082–4.
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004; 53: 108–14.
- Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001; 152: 735–41.
- Brigidi P, Vitali B, Swennen E, Altomare L, Rossi M, Matteuzzi D. Specific detection of bifidobacterium strains in a pharmaceutical probiotic product and in human feces by polymerase chain reaction. Syst Appl Microbiol. 2000; 23: 391–9.
- Lammers KM, Brigidi P, Vitali B, Gionchetti P, Rizzello F, Caramelli E, et al. Immunomodulatory effects of probiotic bacteria DNA: Il-1 and il-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2003; 38: 165–72.
- Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004; 53: 1602–9.
- Bibiloni R, Simon MA, Albright C, Sartor B, Tannock GW. Analysis of the large bowel microbiota of colitic mice using pcr/dgge. Lett Appl Microbiol. 2005; 41: 45–51.
- Menningen R, Nolte K, Rijcken E. Probiotic mixture vsl#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009; 296: G1140–9.
- Kuhbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006; 55: 833–41.
- Pronio A, Montesani C, Butteroni C, Vecchione S, Mumolo G, Vestri A, et al. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis. 2008; 14: 662–8.
- Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005; 143: 895–904.
- Otte JM, Mahjurian-Namari R, Brand S, Werner I, Schmidt WE, Schmitz F. Probiotics regulate the expression of cox-2 in intestinal epithelial cells. Nutr Cancer. 2009; 61: 103–13.
- Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, et al. A randomized, double-blind trial of lactobacillus gg versus placebo in addition to standard maintenance therapy for children with crohn's disease. Inflamm Bowel Dis. 2005; 11: 833–9.
- Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol. 2004; 4: 5.
- Marteau P, Lemann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, et al. Ineffectiveness of Lactobacillus johnsonii La1 for prophylaxis of postoperative recurrence in crohn's disease: a randomised, double blind, placebo controlled getaid trial. Gut. 2006; 55: 842–7.
- Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, et al. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr. 2010; 140: 671S–6S.
- Chouraqui JP, Van Egroo LD, Fichot MC. Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004; 38: 288–92.
- Narayanappa D. Randomized double blinded controlled trial to evaluate the efficacy and safety of bifilac in patients with acute viral diarrhea. Indian J Pediatr. 2008; 75: 709–13.
- Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine. 2009; 27: 1073–9.
- Tursi A. Balsalazide plus high-potency probiotic preparation (VSL#3) in the treatment of acute mild-to-moderate ulcerative colitis and uncomplicated diverticulitis of the colon. J Clin Gastroenterol. 2008; 42((Suppl 3, Pt 1)): S119–22.
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003; 124: 1202–9.