Abstract
Objective : Skin and ear infections, primarily caused by Pseudomonas aeruginosa (P. aeruginosa), are recurrent problems for saturation divers, whereas infections caused by P. aeruginosa are seldom observed in healthy people outside saturation chambers. Cystic fibrosis (CF) patients suffer from pulmonary infections by P. aeruginosa, and it has been demonstrated that CF patients have high levels of autoantibodies against Heat shock protein 60 (HSP60) compared to controls, probably due to cross-reacting antibodies induced by P. aeruginosa. The present study investigated whether rats immunised with P. aeruginosa produced autoantibodies against their own HSP60 and whether diving influenced the level of circulating anti-HSP60 antibodies.
Methods : A total of 24 rats were randomly assigned to one of three groups (‘immunised’, ‘dived’ and ‘immunised and dived’). The rats in group 1 and 3 were immunised with the bacteria P. aeruginosa, every other week. Groups 2 and 3 were exposed to simulated air dives to 400 kPa (4 ata) with 45 min bottom time, every week for 7 weeks. Immediately after surfacing, the rats were anaesthetised and blood was collected from the saphenous vein. The amount of anti-HSP60 rat antibodies in the serum was analysed by enzyme linked immunosorbent assay.
Results : The immunised rats (group 1) showed a significant increase in the level of autoantibodies against HSP60, whereas no autoantibodies were detected in the dived rats (group 2). The rats both immunised and dived (group 3) show no significant increase in circulating autoantibodies against HSP60. A possible explanation may be that HSP60 is expressed during diving and that cross-reacting antibodies are bound.
Saturation diving is widely used in the North Sea for maintenance and inspection of off-shore sub-sea petroleum production systems. Exposure to hyperbaric environments is associated with a risk of developing decompression sickness (DCS), arterial gas embolism, neurological symptoms and pulmonary dysfunctions Citation1 Citation2 Citation3 . Divers are usually compressed in their working and living environment for 12–24 days and are exposed to an ambient pressure of 0.6–2.1 MPa (50–200 msw). The hyperbaric working and living environment is warm and humid and so maintains a rich microbial flora Citation4.
Health problems, such as skin and ear infections, in divers working on the Norwegian Continental Shelf have been systematically registered since 1985 Citation4. Within the period from 1990 to 2010, four incidents of DCS and 201 incidents of outer ear infection were reported to the Petroleum Safety Authority Norway Citation5.
Skin and ear infections in saturation diving are usually caused by Pseudomonas aeruginosa (P. aeruginosa) Citation6. Such infections have been common since the beginning of saturation diving, and infectious outbreaks have caused costly breaks in operations Citation7 Citation8. The P. aeruginosa bacterium is commonly found all over the world, occurring in both fresh- and seawater, soil and on plants, and the species is noted for its metabolic versatility and its exceptional ability to adapt to and colonise various ecological niches.
Infections caused by P. aeruginosa are seldom observed in healthy people outside a saturation chamber, but the bacterium is well known as an opportunistic pathogen. Patients with cystic fibrosis (CF) suffer recurrently from pulmonary infections due to P. aeruginosa Citation9. Insulin-dependent diabetes is the most prevalent co-morbidity condition in CF Citation10, and it has been suggested that destruction of the insulin-producing beta-cells in the pancreas is caused by autoantibodies that act against heat shock protein 60 (HSP60) Citation11. HSP60 molecules are highly phylogenetically conserved with about 50% sequence homology between human HSP60s and those of P. aeruginosa Citation12. Thus, the presence of autoantibodies against HSP60 in patients with CF may be due to human antibodies cross-reacting in a process induced by the presence of bacterial HSP60.
Heat shock proteins are involved in folding and unfolding of other proteins Citation13and are expressed in response to various stressors such as hyperoxia, hypoxia, heat, cold, exercise, some heavy metals and drugs, and many of these factors are involved in diving Citation14. HSP60, a member of this family, is highly expressed in vitro in endothelial cells. It is normally an intracellular protein, but in response to various stresses it is expressed on the surface Citation15. Binding of anti-HSP60 antibodies to HSP60 has been suggested to be present in the development of atherosclerosis Citation16. Furthermore, immunisation of mice with human sera containing high levels of anti-HSP60 induces atherosclerosis Citation17. They even found a marked induction of atherosclerotic lesions after a single injection of purified anti-HSP antibodiesCitation17.
Hence, a relevant question is whether P. aeruginosa infections amongst saturation divers may induce production of autoantibodies that might cross-react and bind to human HSP60.
In the present study, we investigated whether rats immunised with P. aeruginosa produced autoantibodies against rat HSP60 and whether the autoantibody level was affected by diving.
Material and methods
A total of 24 young female Sprague–Dawley albino rats (Scanbur, Denmark), weighing 0.262±0.013 kg, were used in the experiment. All animals used in the experiment were bought at the same time, from the same supplier and had equal amount of time for acclimatisation. All experimental procedures and the care of experimental animals conformed to the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, and the protocol was approved by the Norwegian Council for Animal Research.
Following 1 week of acclimatisation, the rats were randomly assigned to one of three groups, ‘Immunised’, ‘Dived’ and ‘Immunised and dived’ (n=8 for each group). There was no significant difference in weight between the groups.
One strain of P. aeruginosa (genotype E) isolated from an infected saturation diver was used in this study Citation4. The isolated bacterium was inactivated by 65°C for 30 min. Cultures were solved in sterile and filtered phosphate buffered saline (PBS) buffer (pH 7.2) and diluted to fit optical density (OD) of 600 nm To ensure that the vaccine is sterile, growth was examined by coating 100 µl of the vaccine on Blood Agar and incubating at 37°C for 2 days. The vaccine was preserved in aliquots at –80°C until vaccinations.
The rats in groups 1 and 3 were immunised with P. aeruginosa crude antigen, 0.2 ml every other week, from week 1 to week 9, in total five times. Two weeks after the first immunisation, groups 2 and 3 were exposed to simulated air dives. The rats only subjected to pressure exposure had injections of saline solution (0.9% NaCl, B. Braun, Melsungen, Germany), at the same time as the other ones had immunisations. The compressions were performed in a 20-L hyperbaric chamber with continuous air supply. Both dive groups were compressed (200 kPa/min) to 400 kPa (4 ata) with 45 min bottom time. The decompression rate was 50 kPa/min. The dive protocol in both groups was repeated every seventh day for 7 weeks, in total seven times.
Immediately after surfacing, the rats were anaesthetised with a subcutaneous injection of a mixture of Haldol 0.33 mg, Fentanyl 0.05 mg and Midazolan 0.5 mg at a dose of 2.5 ml/kg of body weight. Blood was collected from the saphenous vein. After blood sampling, the rats were moved to their housing facilities where they were allowed to recover.
Two weeks after the last test protocol was performed, the rats received a 1.2-ml subcutaneous injection of the same anaesthetic mixture as described previously and were sacrificed by heart puncture.
Serological analyses: Enzyme linked immunosorbent assay (ELISA) for detecting antibodies against rat HSP60.
Sera were analysed by indirect ELISA. Recombinant rat HSP60 (Stressgen, Victoria, Canada) was diluted at a ratio of 1:1,000 in coating buffer, which contained 1.59 g of Na2CO3, 2.93 g of NaHCO3 and 1 L of distilled water (pH 9.6), and 100 µl/well were incubated in microplates (Sterilin) overnight at room temperature. Plates were then washed three times with PBS containing 0.05% Tween 20 (PBS-T). Plates were pre-incubated at room temperature with 300 ml/well of blocking buffer, containing 1% skimmed milk powder (Molico, Nestlè) in PBS, for 30 min. After washing three times with PBS-T, wells were incubated in duplicate with rat serum in a dilution of 1:10 ml/well, in blocking buffer, for 2 h. Plates were washed three times with PBS-T and incubated with polyclonal rabbit anti-rat IgG/ HRP (DakoCytomation, Denmark), diluted at a ratio of 1:1,000 in blocking buffer, for 1 h at room temperature. After washing four times with PBS-T, 100 ml of freshly made Sigmafast OPD (containing 0.4 mg/ml o-phenylenediamine, 0.4 mg/ml urea hydrogen peroxide and 0.05 M phosphate-citrate in 20 ml distilled water, pH 5.0) (Sigma, USA) was added to each well. After 30 min, the reaction was stopped with 50 ml of 2 M H2SO4 and plates were read at 490 nm. OD values were derived from duplicate determinations.
Statistics
The results are presented as mean±S.D. Mann–Whitney and the Wilcoxon signed-rank test were used to analyse the ELISA results. The level of statistical significance was set at P<0.05. All statistical analyses were performed using SPSS 14.0 (SPSS Inc., Chicago, Illinois, USA).
Results
The results from the ELISA measurements of anti-HSP60 antibodies in serum are shown in Figs. . In the immunised group (group 1), there was a significant increase in anti-HSP60 level from week 1 to week 11 (p ≤ 0.01) (). No significant increase in the concentration of free anti-HSP60 antibodies was detected in the diving group (group 2, ) or in the immunised and dived group (group 3, ). There are significantly higher levels of anti-HSP60 at week 9 (p=0.011) and 11 (p=0.006) in group 1, compared to group 3.
Fig. 1. Longitudinal study of anti-HSP60 level in serum in the immunised group (group 1). Bars represent level of anti-HSP60 analysed by ELISA in blood samples taken every other week, before each immunisation. Notes: **There is a significant increase in anti-HSP60 level from week 1 to week 11 (p < 0.01). Bars represented by * are significantly different from same week in group 3 (). Results are presented as mean optical density (OD) values measured at 490 nm. The vertical lines represent the standard deviation.
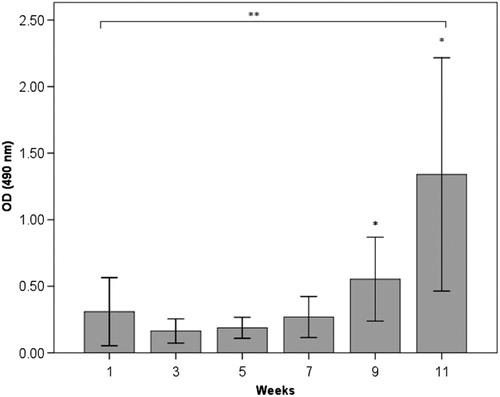
Fig. 2. Longitudinal study of anti-HSP60 level in serum in the dived group (group 2). Notes: Bars represent level of anti-HSP60 analysed by ELISA in blood samples taken every other week/dive, immediately after surfacing/decompression. There is no change in circulating anti-HSP60. Results are presented as mean OD values measured at 490 nm. The vertical lines represent the standard deviation.
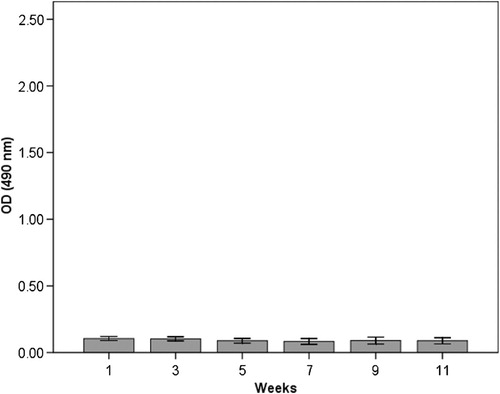
Fig. 3. Longitudinal study of anti-HSP60 level in serum in the immunised and dived group (group 3). Notes: Bars represent level of anti-HSP60 analysed by ELISA in blood samples taken every other week/dive immediately after surfacing/decompression. In contrast to the immunised rats (group 1), there is no significant change in level of circulating anti-HSP60.Bars represented by * are significantly different from same week in group 1 (Fig. 1).Results are presented as mean OD values measured at 490 nm. The vertical lines represent the standard deviation.
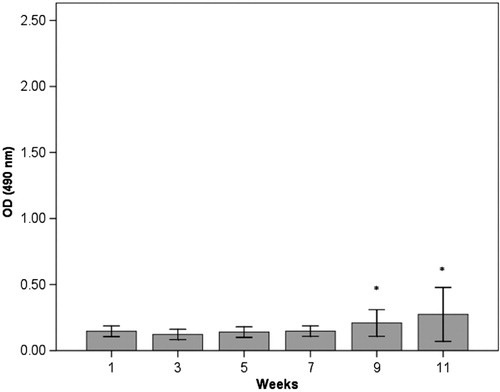
Discussion
This study demonstrates that the level of circulating autoantibodies against HSP60 significantly increases over time in rats with repeated immunisations with P. aeruginosa (group 1).The dive rats (group 2) did not show any increase in the level of circulating antibodies during the experimental period, but more surprisingly, we were not able to detect circulating autoantibodies against HSP60 in immunised rats when the blood sample was taken immediately after diving (group 3).
The increased level of autoantibodies against HSP60 in the immunised rats in the current study is in accordance with the situation seen in patients infected by the bacteria P. aeruginosa Citation18. High levels of HSP60 autoantibodies are associated with an increased risk of coronary heart disease Citation19 Citation20 and have been associated with the development of type-1 diabetes in CF patients Citation11.
The animals only subjected to pressure did not show any increased level of circulating autoantibodies against HSP60. Hence, the pressure exposures did not themselves produce circulating autoantibodies.
When rats immunised with P. aeruginosa were exposed to dives, they were not observed to have the same rise in the level of circulating antibodies against HSP60 compared to the immunised, non-diving rats. One possible explanation for this may be that diving suppresses the immune response. An alternative explanation may be that HSP60 molecules are expressed on endothelial cells in such a way that the cross-reacting epitopes are exposed to the surface and, thus, enable binding between circulating anti-HSP antibodies and exposed HSP60 epitopes.
There is considerable research on cross-reacting autoantibodies in relation to development of atherosclerosis and in relation to several pathological reactions in CF patients. Bindings between anti-HSP60 antibodies to HSP60 have been demonstrated Citation16, and such bindings may in turn give rise to inflammatory reactions Citation11 Citation18 Citation19 Citation20 Citation21 Citation22 Citation23 Citation24 .
We are not aware of any studies evaluating level of autoantibodies against HSP60 in saturation divers. Observations in the present study of higher autoantibody level after combining bacterial exposure and diving point in the direction of having cross-reacting autoantibodies. Hence, it seems likely that the exposure to P. aeruginosa in the diving environment and recurrent skin infections Citation6 may give rise to production of autoantibodies against HSP60.
Conflict of interest and funding
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. This study was supported by Petromaks/The Research Council of Norway and the Norwegian Petroleum Directorate, Norsk Hydro, Esso Norge and Statoil under the ‘Dive Contingency Contract’ (No 4600002328) with Norwegian Underwater Intervention (NUI).
References
- Mollerlokken A, Gaustad SE, Havnes MB, Gutvik CR, Hjelde A, Wisloff U, et al. Venous gas embolism as a predictive tool for improving CNS decompression safety. Eur J Appl Physiol. 2012, 112((2)):, 401–409.
- Francis TJR, Mitchell SJ. Pathophysiology of decompression sickness. In: Brubakk AO, Neuman TS, Bennett and Elliott's physiology and medicine of diving. 5th ed. Saunders., Philadelphia; 2003, pp. 530–57.
- Neuman TS. Arterial gas embolism and pulmonary barotrauma. In: Brubakk AO, Neuman TS, Bennett and Elliott's physiology and medicine of diving. 5th ed. Saunders., Philadelphia; 2003, pp. 557–77.
- Ahlen C, Mandal LH, Iversen OJ. Identification of infectious Pseudomonas aeruginosa strains in an occupational saturation diving environment. Occup Environ Med. 1998; 55(7): 480–84.
- Report from the DSYS database. Stavanger: the petroleum safety authority. Norway., 2010.
- Ahlen C, Mandal LH, Iversen OJ. An in-field demonstration of the true relationship between skin infections and their sources in occupational diving systems in the North Sea. Ann Occup Hyg. 2003; 47(3): 227–33.
- Thalman E. A prophylactic program for the prevention of otitis externa in saturation divers, research report. WashingtonDC: US Navy Experimental Diving Unit, Washington Navy Yard. 1974.
- Alcock SR. Acute otitis externa in divers working in the North Sea: a microbiological survey of seven saturation dives. J Hyg (Lond). 1977; 78(3): 395–409.
- Govan JR, Nelson JW. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992; 48(4): 912–30.
- Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract. 1999; 45(1): 61–73.
- Jensen P, Johansen HK, Carmi P, Hoiby N, Cohen IR. Autoantibodies to pancreatic hsp60 precede the development of glucose intolerance in patients with cystic fibrosis. J Autoimmun. 2001; 17(2): 165–72.
- Jensen P, Fomsgaard A, Hoiby N, Hindersson P. Cloning and nucleotide sequence comparison of the groE operon of Pseudomonas aeruginosa and Burkholderia cepacia. APMIS. 1995; 103(2): 113–23.
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986; 55: 1151–91.
- Brubakk AO, Eftedal OS, Wisløff U. Endothelium and diving. In: Aird W, Endothelial Biomedicine. Cambridge University Pres., Cambridge, ; 2007. pp. 497–505.
- Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994; 75(6): 1078–85.
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, et al. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995; 96(6): 2569–77.
- Foteinos G, Afzal AR, Mandal K, Jahangiri M, Xu Q. Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation. 2005; 112(8): 1206–13.
- de Graeff-Meeder ER, Rijkers GT, Voorhorst-Ogink MM, Kuis W, van der Zee R, van Eden W, et al. Antibodies to human HSP60 in patients with juvenile chronic arthritis, diabetes mellitus, and cystic fibrosis. Pediatr Res. 1993; 34(4): 424–8.
- Prohaszka Z, Duba J, Horvath L, Csaszar A, Karadi I, Szebeni A, et al. Comparative study on antibodies to human and bacterial 60 kDa heat shock proteins in a large cohort of patients with coronary heart disease and healthy subjects. Eur J Clin Invest. 2001; 31(4): 285–92.
- Prohaszka Z, Duba J, Lakos G, Kiss E, Varga L, Janoskuti L, et al. Antibodies against human heat-shock protein (hsp) 60 and mycobacterial hsp65 differ in their antigen specificity and complement-activating ability. Int Immunol. 1999; 11(9): 1363–70.
- Alard JE, Dueymes M, Youinou P, Jamin C. Modulation of endothelial cell damages by anti-Hsp60 autoantibodies in systemic autoimmune diseases. Autoimmun Rev 2007. 6(7): 438–43.
- Jamin C, Dugue C, Alard JE, Jousse S, Saraux A, Guillevin L, et al. Induction of endothelial cell apoptosis by the binding of anti-endothelial cell antibodies to Hsp60 in vasculitis-associated systemic autoimmune diseases. Arthritis Rheum. 2005; 52(12): 4028–38.
- Lamb JR, Bal V, Mendez-Samperio P, Mehlert A, So A, Rothbard J, et al. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989; 1(2): 191–6.
- Wick G, Schett G, Amberger A, Kleindienst R, Xu Q. Is atherosclerosis an immunologically mediated disease?. Immunol Today. 1995; 16(1): 27–33.