Abstract
Background : During the recent years, a substantial amount of new data has underlined the importance of the gut as a triggering place for autism. Temporary improvements in clinical status following dietary alterations and the same that may occur after an antibiotic therapy are reported. Additionally, increasing numbers of bacteria belonging to certain groups, such as clostridia, desulfovibrios, and sutterella, have been reported. So far, however, presence of any bacterial group has never been causatively linked to autism, and every time a new candidate organism is introduced the same questions have to be asked: What is the cause? What is the consequence? What is the confounder? The possibilities of answering these questions are hampered by difficulties in obtaining adequate samples. Therefore, more efforts have been made to those biochemical methods that probe possible functional alterations in the gastrointestinal (GI) microbiota in autistic children.
Conclusion : Autism is a disorder involving many organs and their functions, including the GI microbiota. More knowledge about the GI microbiota and its cross-talks with the host creates possibilities for future diagnostic and therapeutic improvements.
During the several decades after the first description of autism Citation1 in the early 1940s, autism was looked upon as a rare, non-treatable, degenerative disorder of the brain of unknown etiology. Most attention was paid to characterize the behavioral and genetic aspects in the child and psycho-sociological aspects in the family of the child.
However, an increasing number of reports, mostly from parents, of temporary improvements following dietary alterations and/or exposure – for other reasons – to antibiotics started to appear in the 1990s, and these reports served as an eye-opener for a broader view on the etiological and genetic aspects of autism and more systematic, therapeutic approaches Citation2. Very soon, alteration in the composition of the GI microbiota in autistic children compared with controls started to appear.
In the initial studies, attempts were made to define and isolate the possible, specific intestinal bacterial species responsible for the disorder. Various species in the clostridial groups, such as Clostridium tetani, Clostridium perfringens, Clostridium bolteae novo sp., etc. Citation3 Citation5, were reported to occur more frequently in the fecal samples of autistic children than in those of controls. However, despite a substantial amount of efforts, it has not been possible to clarify whether these findings represent the presence of a causative agent, reflect a consequence of previous treatment of the child, or are nothing but confounders. Recently, the focus has been on the presence of two other genera, Desulfovibrio Citation6 and Sutterella Citation7, Citation8. Again, it has not been possible to establish any causative relationship between the presence of any of these groups and the development of autism.
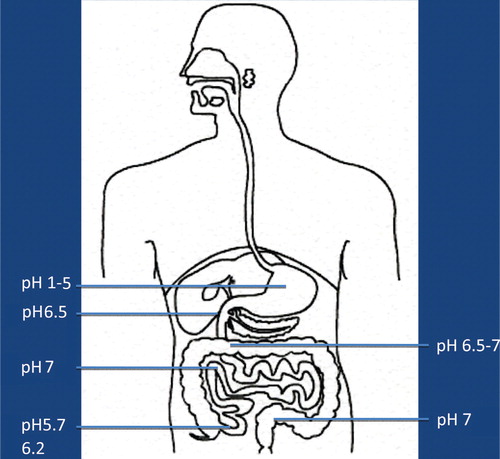
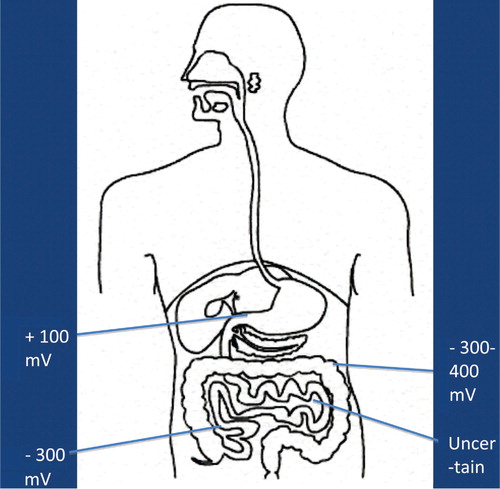
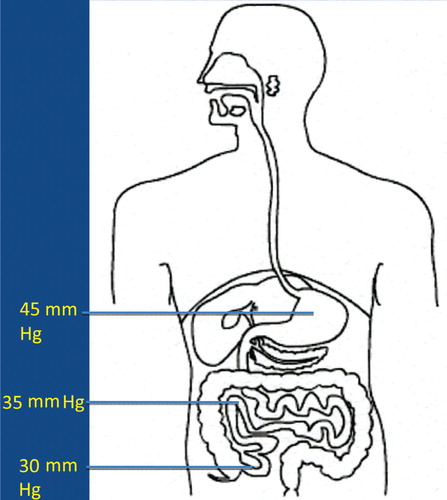
It is now more and more realized that the development of autism is not caused by the presence/absence of one – or a few – bacterial species, but may be caused by functional alterations of the GI microbiota. In order to evaluate such possible alterations, it has to be kept in mind that the GI tract consists of several functional compartments, each harboring its own microbiota. Biochemical reactions – either microbial- or host-derived – are governed by the ‘microclimate’ in the respective compartment. Out of the many factors that may influence the microclimate, pH, reduction/oxidation (red/ox) potential, and oxygen tension are of imperative importance. The values shown in – are based on studies conducted in healthy adults. So far, pH is the easiest parameter to follow. Recent improvements in technology allow us now to investigate pH individually in the whole GI tract collectively. (). Values regarding red/ox potential () and oxygen tensions () are hampered by a considerable degree of uncertainty because of technical problems of in vivo measurements. The values shown in are related to human content, whereas the values shown in reflect oxygen tension on luminal cell surfaces. Oxygen tensions in luminal content are assumed to be close to zero in the lower part of ileum and in the colon.
The fate of any compound – dietary or environmentally derived – reaching any of these compartments will depend on the composition of the microbiota and the microclimate in that specific compartment. It should also be mentioned that neither the composition nor the microclimate is a static parameter; rather they are influenced by a wide variety of factors, such as diet, antibiotics, age, etc. Additionally, except for measurements of pH, it is at present not possible to follow alterations in red/ox potential, oxygen tension, or the composition of the microbiota in most of these compartments. Therefore, we have to rely on the evaluation of fecal microbiota and on products, derived from biochemical reactions, present in feces, urine, or serum, reflecting microbial/host metabolism of endogenous and exogenous compounds.
It may sound difficult – and it is. Out of the many attempts that have been made to evaluate the possible microbial-derived biochemical alterations, I will briefly comment on three main approaches.
Alteration in sulfur metabolism: It has been observed that autistic children have a greater urinary excretion of sulfur than the controls. The GI tract is by far the most sulfur-rich organ in the body. Sulfur is a substantial part of the huge amounts of mucus produced daily in the GI tract, and several sulfur-containing compounds (sulfate, taurin, etc.) are excreted as conjugates of bile. Parts of the GI microbiota may produce hydrogen sulfide –a compound not only more toxic than cyanide, but also a well-known neurotransmitter. It is also known that the degree of mucus sulfation and the presence of sulfate-containing bile conjugates are altered during infancy. Whether, and to what extent, such alterations can be found in autistic infants have, to the best of my knowledge, not been evaluated.
Production of some organic acids, especially propionic acid Citation9: Short chain fatty acids (acetic, propionic, butyric, valeric, and caproic acids) represent microbial anaerobic degradation of carbohydrates. Most of these acids are absorbed and may influence many functions in the human body. It has now been convincingly shown in animal models that propionic acid induces behavioral and functional alterations in the brain of exposed animals. Most likely, the presence of this acid alters basic mitochondrial functions, thus underlining the importance of alterations in mitochondrial functions in autistic children, hypothesized already in the late 1990s Citation10.
The presence of bioactive peptides in urine Citation11: It is a well-known fact that many autistic infants improve on a casein- and gluten-free diet. Biochemical analysis of urine samples collected from children with autism shows an altered urinary peptide profile compared with those from the age-matched controls, and that this discrepancy decreased significantly after changing to a casein- and gluten-free diet in the cohort of children with autism. Additionally, there are several reports describing peculiar peptides in urine and/or serum from autistic children Citation12, Citation13 and animal models Citation14. Taken together, these reports support a view that the host/microbe metabolism of protein is altered in patients with autism. However, the mechanism(s) behind and the specific use of such alterations as biomarkers in diagnosing autism is (are) not sufficiently evaluated.
Since autism was first described in the 1940s, it has been looked upon as a rare disorder, occurring at a rate of 4–5 per 10,000 children. In the 1970s, the incidences started to rise and have continued to do so in nearly all countries reported Citation15. Now autism is generally accepted to be a grand challenge to global mental health. Data from the Unites States in 2010 indicate an incidence rate of 1/188 and data from South Korea indicate an incidence as high as 1/38. The mechanism(s) behind this dramatic increase is of course a matter of great concern Citation16. A genetic aspect is well accepted in some cases of autism. The key question is whether this increased incidence is genotypically or phenotypically based, i.e. reflects an increased mutation rate (chromosomal or mitochondrial) induced by factors not yet identified or is caused by environmental factors acting on already existing phenotypes. The geographical variations, especially the extremely high rate of incidences reported from South Korea, indicate that the latter possibility might be the most plausible one. A glance through the historical window might strengthen this assumption. Clioquinol was introduced in the 1930s as an amebicide drug, but was soon used worldwide as an anti-diarrheal drug – although its use was questioned very early Citation17. In the 1960s a peculiar neurological disease became prevalent in the Far East, especially in Japan. It was named subacute myelo-optic neuropathy (SMON), characterized by sensory and motor disturbances and visual changes, often leading to blindness. After some 10,000 cases worldwide, but especially in the Far East, including Japan, SMON was associated to the use of clioquinol, and the drug was withdrawn from the market Citation18. It has been known for years that there is an ethnic peculiarity in some mitochondrial function in people from Far East, and this has been assumed to be involved in the high incidence of SMON in Japan. It is now generally accepted that clioquinol is a very potent zinc and copper chelator, and that it may – under specific circumstances – act as a potent mitochondrial toxin Citation18, Citation19. As for a personal speculation, initially it was the presence of green urine in some patients with SMON that triggered some chemical analyses, thereby unmasking the fact that ‘this disease was caused by the intoxication of the administered Clioquinol’ Citation20. Green urine is sometimes found in autistic children. To the best of my knowledge, neither the mechanism(s) behind nor the presence of possible toxicant(s) has been satisfactorily evaluated.
The burning question in autism research today is: how to unmask the patho-physiological mechanisms behind the development of autism in a child? Citation21. It is more and more realized that a major key in this aspect is increased knowledge of the many complicated biochemical interactions established between a child and its GI microbiota in the first few years of its life, i.e. the period of life for autism to occur. A way forward might be to closely follow a large cohort of newborns – microbiologically as well as biochemically – from birth up to 2–3 years of age, in order to unmask the possible early deviations in these interactions in individuals, later developing autism. It is widely acknowledged that early identification and intervention is of imperative importance in several disorders included in the group of ASD (especially phenylketonuria and Rett syndrome Citation22 and the same may hold true also for autism). Additionally, the mere fact that dietary interventions change behavior in an autistic child Citation2 clearly underlines the functional, more than the degenerative, aspect in the pathogenesis of autism. The world-wide rapid increase in the incidence of autism is a challenge, as it indicates that it is human-made, and consequently it has to be solved by humans.
Conflict of interest and funding
The author has not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943; 2: 237–50.
- Walker HB. A true case story. Microb Ecol Health Dis. 2012. doi: 10.3402/mehd.v23i0.18982.
- Bolte ER. Autism and Clostridium tetani. Med Hypotheses. 1998; 51: 133–44. 10.3402/mehd.v23i0.18982.
- Finegold SM, Molitoris D, Song Y, Vaisanen ML, Bolte E, McTeague M, et al. Gastrointestinal microflora studied in late-onset autism. Clin Infect Dis. 2002; 35: S6–16. 10.3402/mehd.v23i0.18982.
- Song Y, Molitoris DR, Tomzynski TJ, Lawson PA, Collins ND, Finegold SM. Clostridium bolteae sp. Nov., isolated from human sources. Syst Appl Microbiol. 2003; 26: 84–9. 10.3402/mehd.v23i0.18982.
- Finegold SM, Downwa J, Sumanen PH. Microbiology of regressive autism. Anaerobe. 2012; 2: 260–262. 10.3402/mehd.v23i0.18982.
- Williams BL, Homig M, Parekh T, Lipkin VM. Application of novel PR-based methods for detection, quantitation and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012; 3: 1–11. 10.3402/mehd.v23i0.18982.
- Benach JL, Li E, McGovern MM. A microbial association with autism. MBio. 2012; Feb 14 3(1). pii: e00019–12. doi: 10.3402/mehd.v23i0.18982.
- MacFabe DF. The role of enteric bacterial metabolites in mitochondrial dysfunction in autism – from animal models to human population. Microb Ecol Health Dis. doi: 10.3402/mehd.v23i0.18982.
- Pons R, Andru AL, Checcarelli N, Vila MR, Engelstad K, Sue SM, et al. Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr. 2004; 144: 81–5. 10.3402/mehd.v23i0.18982.
- Reichelt KL. Dietary derived, bioactive peptides in urine from autistic children. Microb Ecol Health Dis. 2012. doi: 10.3402/mehd.v23i0.18982.
- Ratajczak HV. Theoretical aspects of autism: biomarkers – a review. J Immunotoxicol. 2011; 8: 80–94. 10.3402/mehd.v23i0.18982.
- Wang L, Angley MT, Gerber JP, Sorich MJ. A review of candidate urinary biomarkers for autism spectrum disorder. Biomarkers. 2011; 16: 537–552. 10.3402/mehd.v23i0.18982.
- Clayton TA. Metabolic differences underlying two distinct rat urinary phenotypes, a putative role for gut microbial metabolism of phenylalanine and a possible connection to autism. FEBS Lett. 2012; 586: 956–961.
- Ratajczak HV. Teoretical aspects of autism. Causes – a review. J Immunotoxicol. 2011; 8: 68–79. 10.3402/mehd.v23i0.18982.
- Christophersen OA. Should autism be considered a canary bird telling that Homo sapiens may be on its way to extinction?. Microb Ecol Health Dis. 2012. doi: 10.3402/mehd.v23i0.18982.
- Midtvedt T. Oxyquinoline derivatives – is one more dangerous than the others?. J Norw Med Assoc. 1971; 91: 1470–1480.
- Arbiser JL, Kraeft SK, van Leeuwen R, Hurwitz S, Selig M, Dickersin GR, et al. Clioquinol-zinc chelate: a candidate causative agent of subacute myelo-optic neuropathy. Mol Med. 1998; 10: 665–70.
- Bareggi SR, Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurogenerative disorders. CNS Neurosci Ther. 2012; 1: 41–6. 10.3402/mehd.v23i0.18982.
- Igata A. Clinical studies on rising and re-rising neurological diseases in Japan – a personal contribution. Proc Jpn Acad Sci Ser B Phys Biol Sci. 2010; 86: 366–77. 10.3402/mehd.v23i0.18982.
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012; 17: 389–401. 10.3402/mehd.v23i0.18982.
- De Fellice C, Signorini C, Durand T, Siccoli L, Leoncini S, D'Exposito M, et al. Partial rescue of Rett syndrome by omega-3 polyunsaturated fatty acids (PUFAs) oil. Genes Nutr. 2012; 7: 447–458. 10.3402/mehd.v23i0.18982.