Abstract
Background
Competition of probiotic bacteria with other species from the intestinal microbiota involves different mechanisms that occur regardless of probiotics’ viability. The objective of this paper was to assess the cytokine serum levels in holoxenic mice after oral administration of non-viable components (NVC) of Enterococcus faecium probiotic culture stimulated with heat-inactivated Escherichia coli and Bacillus cereus in comparison to NVC of unstimulated E. faecium probiotic culture.
Methods
Probiotic E. faecium CMGb 16 culture, grown in the presence of heat-inactivated cultures of E. coli and B. cereus CMGB 102, was subsequently separated into supernatant (SN) and heat-inactivated cellular sediment (CS) fractions by centrifugation. Each NVC was orally administered to holoxenic mice (balb C mouse strain), in three doses, given at 24 hours. Blood samples were collected from the retinal artery, at 7, 14, and 21 days after the first administration of the NVC. The serum concentrations of IL-12 and tumor necrosis factor-alpha (TNF-α) interleukins were assessed by ELISA method.
Results
After the oral administration of SN component obtained from the probiotic culture stimulated with heat-inactivated cultures of B. cereus CMGB 102 and E. coli O28, the serum concentrations of IL-12 were maintained higher in the samples collected at 7 and 14 days post-administration. No specific TNF-α profile could be established, depending on stimulated or non-stimulated probiotic culture, NVC fraction, or harvesting time.
Conclusion
The obtained results demonstrate that non-viable fractions of probiotic bacteria, stimulated by other bacterial species, could induce immunostimulatory effects mediated by cytokines and act, therefore, as immunological adjuvants.
Dual action of probiotics is manifested both on the microbiota and the host organism. The interaction of viable probiotic bacteria with other members of intestinal microbiota, achieved by competitive exclusion (competition for nutrients and adhesion sites) and the synthesis of inhibitory molecules is very well documented (Citation1). The interaction of non-viable probiotics fractions with the host organism is less studied (Citation2). However, dead probiotic cells could still adhere to specific epithelial cell receptors and stimulate MALT (mucosal lymphoid associated tissue) structures, triggering a local immune response (Citation1).
Therefore, both live and dead probiotic cells and sub-cellular fractions can generate beneficial biological responses, raising the necessity to elucidate their intimate mechanisms of action and, respectively, the benefits and the potential side effects in each case.
It has been shown that the probiotic cells are producing antimicrobial substances, but also quorum sensing inhibitors (QSIs) which are interfering with the pathogens QS mechanisms and with their virulence genes expression (Citation3). For example, lactic acid bacteria synthesize bacteriocins depending on the cellular density, using a QS regulatory mechanism, which requires an extracellular accumulation of autoinductor peptides (AI2) that function as chemical messengers (Citation4, Citation5). There are experimental evidences that the probiotic supernatants (SN), which contain antimicrobial molecules and QS signal molecules, can influence the growth and virulence features expression of some EAggEC (Enteroaggregative Escherichia coli) clinical strains (Citation6, Citation7).
It has been widely demonstrated that probiotics represent a potential effective alternative in the treatment of inflammatory and auto-immune gastrointestinal diseases due to their beneficial effects in modulating the immune response (Citation8–Citation10). However, the administration of viable bacterial cells to individuals with weaker immune systems, enhanced inflammatory responses, and/or compromised mucosal barrier functions could turn from ‘generally recognized as safe’ harmless probiotic bacteria into detrimental ones (Citation11).
There are also some results showing that gut microbiota and even some bacterial probiotics could be involved in human obesity, for example, when microbiota is enriched in M. smithii (Citation12, Citation13) or depleted in this archaeal species; in this last case, some Bifidobacterium or Lactobacillus species were associated with normal weight (B. animalis) while others (L. reuteri) were associated with obesity (Citation14).
Probiotic cell components associated with in vitro immunomodulatory properties include entire cell wall (Citation15) or some components as lypoteichoic acids (Citation16) and S-layer proteins (Citation17), or even bacterial DNA (Citation18, Citation19). Some clinical studies have also suggested that non-viable probiotic fractions can modulate the human immune system, for example, by enhancing secretory IgA production (Citation20) and by modulating host T-cell responses (Citation21) and gene expression (Citation22). In a limited number of in vitro and animal studies that have directly compared the effects of viable and inactivated probiotics on innate immunity, these have been found to be equally effective (Citation23–Citation25).
There are some evidences that modulation of inflammatory response, as revealed by cytokine profiles, is an important mechanism by which probiotics provide health benefits (Citation26). For instance, it has been shown that cell surface molecules of Lactobacillus sp. strains could elicit strong tumor necrosis factor-alpha (TNF-α) inducing activities in macrophages through Toll-like receptor 2 (Citation16). In addition, the antimicrobial defensins, cathelicidins, eosinophil-derived neurotoxin, and AI-2 signaling compounds of Gram-positive and Gram-negative bacteria play important roles in intra- and interspecies communication (Citation5, Citation27).
For some bacterial species, the viability is not necessary to induce cytokine production (Citation28), the components of dead cells being also able to induce an anti-inflammatory response in the gastrointestinal tract (Citation2). Dead bifidobacteria induce significant increases in TNF-α production (Citation29). It was also demonstrated that heat-killed cells of E. faecalis stimulate the gastrointestinal immune system in chickens (Citation30), while administration in healthy dogs increases neutrophil phagocytes number (Citation31). Based on these observations, it could be stated that probiotic bacteria (as viable cells or non-viable fractions) can act as immunomodulators.
In this context, the objective of this paper was to assess the cytokine serum levels in holoxenic mice after oral administration of non-viable components (NVC) of Enterococcus faecium probiotic culture stimulated with heat-inactivated E. coli and Bacillus cereus in comparison to NVC of unstimulated E. faecium culture.
Material and methods
Bacterial strains and growth conditions
Probiotic strain E. faecium CMGB16 (isolated from infant faeces) was cultivated in anaerobic conditions, in MRS broth. Enteropathogenic E. coli O28 (provided by National Institute of Research and Development for Microbiology and Immunology ‘Cantacuzino’, Bucharest) and B. cereus CMGB 102 (Microbial culture collection of Faculty of Biology, Microbiology Department, University of Bucharest) strains were grown in nutrient broth.
NVC obtaining
E. faecium CMGB16 was co-cultivated in the presence of heat-inactivated cultures of E. coli O28 and B. cereus, for 24 hours in MRS broth (Man Rogosa Sharp), in anaerobic conditions, at 37°C. The co-cultivation method was performed by inoculating 4 ml MRS broth with 40 µl of fresh E. faecium CMGB16 culture corresponding to standard McFarland 1 (3×108 CFU/ml) and 400 µl of heat-inactivated cultures of E. coli O28/B. cereus corresponding to standard McFarland 1 (3×108 CFU/ml), before inactivation. The heat-inactivation was performed by autoclaving the bacterial cultures at 121°C for 15 min. After co-cultivation, the NVC components (SNs and cellular fraction) were separated by centrifugation (6,000 rpm, 10 min). The cellular sediment was adjusted to McFarland 1 (3×108 CFU/ml) density in PBS (Phosphate Buffer Saline) and along with integral cultures (IC) was heat-inactivated for 15 min at 121°C. Heat-inactivated cellular suspensions obtained from the cellular sediments (CS), the IC, and the SN were further used for the immunomodulatory activity assay.
Immunomodulatory properties of the obtained NVCs were tested on holoxenic mice (BALB/c mouse strain). Each NVC was orally administered (by using a gradate pipette) in three doses of 0.2 ml/dose to three animals/sample, at 24 hours. Blood samples were collected from the retinal artery, by the non-traumatizing retro-orbital puncture method in the inner corner of the eye. Blood samples were collected after 7, 14, and 21 days from the first administration of the heat-inactivated probiotic fractions.
The serum concentrations of IL-12 and TNF-α interleukins were assessed by ELISA method, using Mouse ELISA Kit Thermo Scientific (Pierce Biotechnology, Rockford, UAS), following the manufacturers’ instructions. All experiments were performed in triplicates and absorbance was measured on Apollo LB 911 ELISA plate reader set at 450 and 550 nm. A standard curve was generated by plotting the average absorbance obtained for each Standard concentration on the vertical (Y) axis vs. the corresponding IL 12/TNF-α concentration (pg/ml) on the horizontal (X) axis. Using the standard curve, the IL-12/TNF-α amount in each sample was determined by interpolating the absorbance values (Y-axis) to IL-12/TNF-α concentration. In this way, the absorbance results were converted in pg/ml concentrations. These values were plotted on graphs presented in the Results and discussions section. Sensitivity of IL 12 Mouse ELISA kit was <12 pg/ml and the sensitivity of TNF-α Mouse ELISA kit was <9 pg/ml.
Results and discussions
The majority of scientific studies use the term probiotic according to the FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) (Citation32) definition, i.e. live microorganisms, which when administered in adequate amounts could confer a health benefit on the host. Therefore, this definition requires that the probiotic must be viable, with many studies supporting this idea (Citation33–Citation36).
However, there are some scientific findings supporting the positive effects of the probiotic NVCs on health due to the ability of human immune cells to recognize specific bacterial components and metabolic by-products, generating an immunomodulatory effect that involves MALT activation (Citation2). Chuang (Citation37) showed that three heat-killed strains of Lactobacillus sp. modulated the immune responses by stimulating proliferation of murine splenocytes. In addition, these heat-killed probiotic cells also induced high levels of IL-12 p70 in dendritic cells of mice and the switch to a T helper 1 (Th1) immune response. Given the complexity of the phenomena induced by viable and non-viable probiotics, Taverniti (Citation38) has proposed the term ‘paraprobiotic’ defined as non-viable fractions of probiotic origin (inactivated microbial cells or cell fractions), which have been demonstrated to positively affect human/animal health. The study of these paraprobiotics is justified by the opinion of some authors that probiotics could be involved in obesity/metabolic syndrome (Citation39); simultaneously, the specific effects of probiotic components/culture fractions and targets are important to be known in order to define appropriate clinical applications (similarly to the modern, subunitary vaccines or to immunomodulators). During this study we have used probiotic culture fractions stimulated with other inactivated bacterial cultures, to mimic the interspecies interactions established in the intestinal mucosa. In order to include both probiotic and stimulating bacterial culture components found in the final fractions, we propose the term ‘parabiotic’, defined as NVC of microbial origin that exhibit beneficial effects on the health of the human or animal host organism.
The serum samples collected after 7, 14, 21 days from the first administration of probiotic fractions were analyzed for IL-12 and TNF-α serum levels by ELISA method. The obtained values represent the average of the determinations performed for each animal batch ().
Fig. 1 Graphic representation of IL-12 concentration in the serum of holoxenic mice, collected after: I – 7 days from the first oral administration of NVCs; II – 14 days from the first oral administration of NVCs; III – 21 days from the first oral administration of NVCs. IC, integral culture; CS, cellular suspension; SN, supernatant; A, serum collected from holoxenic mice after oral administration of NVC; B, serum collected from holoxenic mice after oral administration of NVC stimulated with E. coli O28C; C, serum collected from holoxenic mice after oral administration of NVC stimulated with B. cereus; MC, media control – serum collected from holoxenic mice after oral administration of MRS; NC, negative control – serum collected from holoxenic mice control.
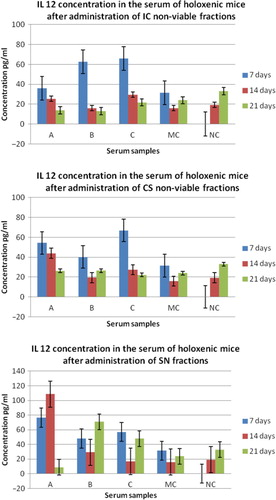
The obtained results showed that the age of the animals significantly influenced the serum concentration of IL-12. The highest levels of IL-12 were recorded at 7 days after the first administration of the IC and SN NVCs, especially those stimulated with B. cereus ().
At 14 and 21 days after the first oral administration of probiotic NVCs, the serum concentration of IL-12 generally decreased. In exchange, after the oral administration of SN component stimulated with B. cereus and E. coli, the serum concentrations of IL-12 were maintained higher in the samples collected after 21 days post-administration. These aspects suggest that B. cereus and E. coli strains stimulate the synthesis and accumulation in the probiotic cultures SN of bacterial molecules potentially involved in the activation of mucosal intestinal cells, such as macrophages or dendritic cells, to synthesize IL-12. However, it is to be noticed that the unstimulated fractions, particularly CS and SN, also induced high levels of IL-12, especially at 7 and 14 days after the first administration. IL-12 is a cytokine required for the initiation of the immune response, constituting the connecting bridge between non-specific defense reactions (it activates macrophages and NK cells) and specific immune response.
The production of immunomodulatory molecules by probiotic bacteria is thus influenced by the presence of other bacterial species, both Gram-positive and Gram-negative, which inhabit the gut (autochthonous and allochthonous microbiota), by complex and incompletely understood cross-talk mechanisms. The induction of a late immunostimulatory response by these soluble molecules could suggest that they are resistant to digestive enzymes degradation, and could slowly and gradually diffuse in the submucosa, inducing the activation of MALT. This effect was not evident after the administration of CS and IC from the unstimulated NVCs, supporting the hypothesis that the late immunomodulatory effect is due to small, heat-stable, soluble molecules. Studies on peptide fractions (with molecular weight between 2 and 10 Da) isolated from Lactobacillus helveticus culture (using HPLC method) showed their immunomodulatory effects after oral administration in holoxenic mice infected with E. coli O157:H7 (Citation40). Cytokine profile of these mice revealed a stimulation of Th2 lymphocyte-mediated response and an increase of the B cells number in the intestine (lamina propria), and thus the concentration of secretory IgA.
The scientific researches states that probiotics (either viable cells or cellular fragments with antigenic potential) must interact with MALT structures (dendritic cells, M cells from the Peyer patches) to generate an immunostimulatory effect, the route of antigen internalization being essential for the initiation of an immune response in the gastrointestinal mucosa (Citation11, Citation28, Citation41). These observations may explain why the early stimulation of IL-12 synthesis at 7 days after the first administration was induced mainly by the IC stimulated fraction, containing strong particulate antigens.
Being the dominant cytokine involved in the maturation of CD4+ T cells, IL-12 plays an important role in immunomodulation, its synthesis being an essential process in triggering early cytokine cascade activation as a response to adjuvants (Citation42).
In the case of TNF synthesis, the obtained results were variable, no specific cytokine profile dependent on probiotic NVCs, stimulating species, or serum harvesting time being identified. However, at all three harvesting intervals, the highest levels of TNF were induced after the administration of the IC fractions stimulated with E. coli (). After 7 days, the most intensive stimulating effect was obtained for SN fraction stimulated with B. cereus, while the un-stimulated CS fraction induced the highest levels of TNF at 14 and 21 days after administration.
Fig. 2 Graphic representation of TNF α concentration in the serum of holoxenic mice, collected after: I – 7 days from the first oral administration of NVCs; II – 14 days from the first oral administration of NVCs; III – 21 days from the first oral administration of NVCs. IC, integral culture, CS, cellular suspension; SN, supernatant; A, serum collected from holoxenic mice after oral administration of NVC; B, serum collected from holoxenic mice after oral administration of NVC stimulated with E. coli O28C; C, serum collected from holoxenic mice after oral administration of NVC stimulated with B. cereus; MC, media control – serum collected from holoxenic mice after oral administration of MRS; NC, negative control – serum collected from holoxenic mice control.
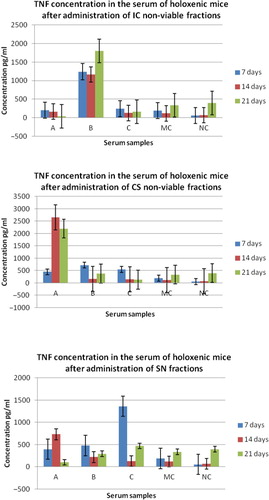
This effect suggests again that the cellular fragments with strong antigenic properties could induce the activation of macrophage cells from the sub-endothelial compartment (the main TNF producing cells). It has been shown that both crude extracts and purified lipoteichoic acids from L. casei and L. fermentum could significantly induce TNF secretion by mouse splenic mononuclear cells (Citation16). This suggests that purified lipoteichoic acids may be a better candidate for clinical use than whole bacteria since they do not contain other bacterial components which might cause side effects (Citation2).
The induction of TNF-α synthesis by probiotic bacteria, observed in many other studies (Citation28) seems to be necessary in order to establish functional connections between intestinal epithelial cells and the immune cells (B cells, dendritic cells, macrophages) from the lamina propria.
Conclusions
Our results, similar to other scientific studies, demonstrate that inactivated soluble and cellular fractions of probiotic cultures, whose composition can be influenced by the stimulation with other microbial species, could induce immunomodulatory effects mediated by cytokines. Further studies are required in order to identify the chemical nature of these so-called parabiotic fractions.
Conflict of interest and funding
The authors have no potential conflicts of interest with any companies/organizations whose products or services may be discussed in this article.
Acknowledgements
This work has been supported by the national research projects PCCA 77/2012.
References
- Adams CA . The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev. 2010; 23: 37–46. [PubMed Abstract].
- Cocconier MH , Bernet MF , Kernéis S , Chauviére G , Fourniat J , Servin A . Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett. 1993; 110: 299–306.
- Lazar V . Quorum sensing in biofilms – how to destroy the bacterial citadels or their cohesion/power?. Anaerobe. 2011; 17: 280–5. [PubMed Abstract].
- Quadri LEN . Regulation of class II bacteriocin production by cell-cell signaling. J Microbiol. 2003; 41: 175–82.
- Moslehi Jenabian S , Gori K , Jespersen L . AI-2 signalling is induced by acidic shock in probiotic strains of Lactobacillus spp. Int J Food Microbiol. 2009; 135: 295–302. [PubMed Abstract].
- Lazar V , Miyazaki Y , Hanawa T , Chifiriuc MC , Diţu LM , Măruţescu L , etal. The influence of some probiotic supernatants on the growth and virulence features expression of several selected enteroaggregative E. coli clinical strains. Roum Arch Microbiol Immunol. 2009; 68: 207–14. [PubMed Abstract].
- Ditu LM , Chifiriuc MC , Bezirtzoglou E , Voltsi C , Bleotu C , Pelinescu D , etal. Modulation of virulence and antibiotic susceptibility of enteropathogenic Escherichia coli strains by Enterococcus faecium probiotic strain culture fractions. Anaerobe. 2011; 17: 448–51. [PubMed Abstract].
- Lazar V , Bezirtzoglou E , Vezyraki P , Voidarou C , Tsiotsias A , Bulai D , etal. Influence of Lactobacillus casei strains on the adhesion and invasion capacity of some opportunistic enterobacteria strains to HeLa cell line. Microecol Ther. 2002; 29: 163–72.
- Fedorak RN , Madsen KL . Probiotics and prebiotics in gastrointestinal disorders. Curr Opin Gastroenterol. 2004; 20: 146–55. [PubMed Abstract].
- Fedorak RN , Madsen KL . Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004; 10: 286–99. [PubMed Abstract].
- Besselink MG , Santvoort HC , Buskens E . Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008; 371: 651–9. [PubMed Abstract].
- Eckburg PB , Lepp WP , Relman DA . Archaea and their potential role in human disease. Infect Immun. 2003; 71: 591–6. [PubMed Abstract] [PubMed CentralFull Text].
- Raoult D . Obesity pandemics and the modification of digestive bacterial flora. Eur J Clin Microbiol Infect Dis. 2008; 27: 631–4. [PubMed Abstract].
- Million M , Maraninchi M , Henry M , Armougom F , Richet H , Carrieri P , etal. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii . Int J Obes. 2012; 36: 817–25.
- Solis Pereyra B , Lemonnier D . Induction of human cytokines by bacteria used in dairy foods. Nutr Res. 1993; 13: 1127–40.
- Matsuguchi T , Takagi A , Matsuzaki T , Nagaoka M , Ishikawa K , Yokokura T . Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol. 2003; 10: 259–66. [PubMed Abstract] [PubMed CentralFull Text].
- Konstantinov SR , Smidt H , de Vos WM , Bruijns SC , Singh SK , Valence F , etal. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci U S A. 2008; 105: 19474. [PubMed Abstract] [PubMed CentralFull Text].
- Rachmilewitz D , Karmeli F , Takabayashi K , Hayashi T , Leider-Trejo L , Lee J . Immunostimulatory. DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002; 122: 1428–41. [PubMed Abstract].
- Takahashi N , Kitazawa H , Iwabuchi N , Xiao JZ , Miyaji K , Iwatsuki K . Immunostimulatory oligodeoxynucleotide from Bifidobacterium longum suppresses Th2 immune responses in a murine model. Clin Exp Immunol. 2006; 145: 130–8. [PubMed Abstract] [PubMed CentralFull Text].
- Kotani Y , Shinkai S , Okamatsu H , Toba M , Ogawa K , Yoshida H , etal. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin a secretion in the elderly: a randomized, placebo-controlled, double-blind trial. Immun Ageing. 2010; 7: 11. [PubMed Abstract] [PubMed CentralFull Text].
- Hirose Y , Murosaki S , Yamamoto Y , Yoshikai Y , Tsuru T . Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J Nutr. 2006; 136: 3069–73. [PubMed Abstract].
- van Baarlen P , Troost FJ , van Hemert S , van der MC , de Vos WM , de Groot PJ . Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A. 2009; 106: 237.
- Haller D , Blum S , Bode C , Hammes WP , Schiffrin EJ . Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect Immun. 2000; 68: 752–9. [PubMed Abstract] [PubMed CentralFull Text].
- Korhonen R , Korpela R , Saxelin M , Maki M , Kankaanranta H , Moilanen E . Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation. 2001; 25: 223–32. [PubMed Abstract].
- Perdigón G , de Macias ME , Alvarez S , Oliver G , de Ruiz Holgado AA . Effect of perorally administered lactobacilli on macrophage activation in mice. Infect Immun. 1986; 53: 404–10.
- Mikelsaar M , Lazar V , Onderdonk AB , Donelli G . Do probiotic preparations for humans really have efficacy?. Microbial Ecol Health Dis. 2011; 22: 10128.
- Yang D , Biragyn A , Hoover DM , Lubkowski J , Oppenheim JJ . Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004; 22: 181–215. [PubMed Abstract].
- Maldonado Galdeano C , Moreno de Le Blanc A , Vinderola G , Bibas Bonet ME , Perdigon G . Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007; 5: 485–92.
- Tejada-Simon MV , Pestka JJ . Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. Food Prot. 1999; 62: 1435–44.
- Sakai Y , Tsukahara T , Bukawa W , Matsubara N , Ushida K . Cell preparation of Enterococcus faecalis strain EC-12 prevents vancomycin-resistant enterococci colonization in the cecum of newly hatched chicks. Poultry Sci. 2006; 85: 273–7.
- Kanasugi H , Hasegawa T , Goto Y , Ohtsuka H , Makimura S , Yamamoto T . Single administration of enterococcal preparation (FK-23) augments non-specific immune responses in healthy dogs. Int J Immunopharmacol. 1997; 19: 655–9. [PubMed Abstract].
- Food and Agriculture Organization & World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. 2002; Rome: FAO.
- Borriello SP , Hammes WP , Holzapfel W . Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003; 36: 775–80. [PubMed Abstract].
- Cross ML , Ganner A , Teilab D , Fray LM . Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol Med Microbiol. 2004; 42: 173–80. [PubMed Abstract].
- O'Toole PW , Cooney JC . Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis. 2008; 2008: 175285. [PubMed Abstract] [PubMed CentralFull Text].
- Gobbetti M , Cagno RD , De Angelis M . Functional microorganisms for functional food quality. Crit Rev Food Sci Nutr. 2010; 50: 716–27. [PubMed Abstract].
- Chuang L , Keh-Gong W , Pal C , Hsieh PS , Tsai JJ , Yen JH , etal. Heat-killed cells of lactobacilli skew the immune response toward T helper 1 polarization in mouse splenocytes and dendritic cell-treated T cells. J Agric Food Chem. 2007; 55: 11080–6. [PubMed Abstract].
- Taverniti V , Guglielmetti S . The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 2011; 6: 261–74. [PubMed Abstract] [PubMed CentralFull Text].
- Raoult D . Probiotics and obesity: a link?. Nat Rev Microbiol. 2009; 7: 616. [PubMed Abstract].
- LeBlanc J , Fliss I , Matar C . Induction of a humoral immune response following an Escherichia coli O 157:H7 infection with an immunomodulatory peptidic fraction derived from Lactobacillus helveticus – fermented milk. Clin Diagn Lab Immunol. 2004; 11: 1171–81. [PubMed Abstract] [PubMed CentralFull Text].
- Kataria J , Li N , Wynn JL , Neu J . Probiotic microbes: do they need to be alive to be beneficial?. Nutr Rev. 2009; 67: 546–50. [PubMed Abstract].
- Dima FV , Balotescu C , Dima SV . Potentiation of the activity of mucosal vaccines by immunological adjuvant. Roum Arch Microbiol Immunol. 2000; 3: 158–210.
