Abstract
In the human gut, millions of bacteria contribute to the microbiota, whose composition is specific for every individual. Although we are just at the very beginning of understanding the microbiota concept, we already know that the composition of the microbiota has a profound impact on human health. A key factor in determining gut microbiota composition is diet. Preliminary evidence suggests that dietary patterns are associated with distinct combinations of bacteria in the intestine, also called enterotypes. Western diets result in significantly different microbiota compositions than traditional diets. It is currently unknown which food constituents specifically promote growth and functionality of beneficial bacteria in the intestine. The aim of this review is to summarize the recently published evidence from human in vivo studies on the gut microbiota-modulating effects of diet. It includes sections on dietary patterns (e.g. Western diet), whole foods, food constituents, as wells as food-associated microbes and their influence on the composition of human gut microbiota. The conclusions highlight the problems faced by scientists in this fast-developing field of research, and the need for high-quality, large-scale human dietary intervention studies.
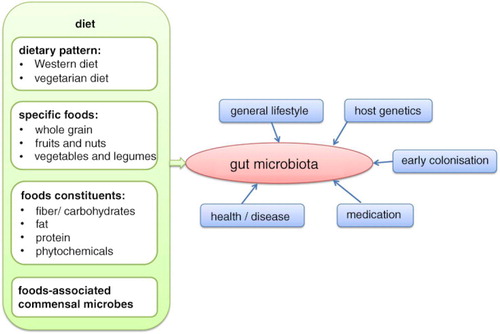
The human gut microbiota is influenced by various factors, with diet being a very important one (). Food components, which are indigestible for human enzymes (e.g. fiber), provide substrates for the intestinal microbial metabolism. As bacteria are specialized in the fermentation of different substrates, complex diets can provide a range of growth-promoting and growth-inhibiting factors for specific phylotypes (Citation1). Furthermore, the end products of bacterial metabolism, especially vitamins and short-chain fatty acids (SCFA), are vital for human health. For details of microbial metabolism and production of SCFA, the reader is referred to another review (Citation1). As many of these products of intestinal bacterial metabolism have health-promoting effects, research seeks to identify dietary patterns increasing bacterial diversity and promoting the growth of beneficial bacteria. The aim of this review is to summarize the evidence from recently published human studies regarding the role of diet on intestinal microbiota composition, and to identify further research needs. Only studies that examined a broad range of microbes were included. Studies using targeted methods and concentrating on merely a few phylotypes were excluded.
Basics of gut microbiology
Microbial colonization occurs throughout the length of the human gut from the oral cavity to the rectum. The density of colonization and the composition of the resident microbial communities, however, differ markedly between anatomical sites and depend on transit rates, host secretions, environmental conditions, substrate availability, and the organization of the gut wall. Thus, the stomach and proximal small intestine support relatively low numbers of microorganisms that can tolerate the pH conditions, oxygen exposure, and the relatively rapid transit rates that prevail in these regions. In contrast, conditions in the large intestine generally favor the establishment of an extremely dense microbial community that is dominated by obligate anaerobic bacteria. These organisms gain energy mainly through the fermentation of non-digested dietary components and of host secretions, notably mucin, forming the SCFA acetate, propionate, and butyrate as major products together with the gases H2, CO2, CH4, and H2S.
Information on the composition of the large intestinal microbiota stems mainly from the analysis of fecal samples. Based on molecular analyses, the majority of bacteria belong to two phyla, Bacteroidetes and Firmicutes (Citation2). The Gram-negative Bacteroidetes phylum includes the genera Bacteroides and Prevotella; these organisms possess the capability to use a very wide range of substrates and are major producers of propionate (Citation3, Citation4). Firmicutes include several species identified as the dominant producers of butyrate (Citation5) and specialist degraders of indigestible polysaccharides (Citation6). Actinobacteria (that include Bifidobacterium spp.), Proteobacteria (including Escherichia coli), and Verrucomicrobia (including Akkermansia mucinophila) are typically present in smaller numbers in the healthy gut microbiota, but these organisms have considerable potential to influence health outcomes. While only around 30% of the human intestinal species are currently represented by cultured isolates, the most abundant species appear to be well represented (Citation7) and most of the remaining organisms are probably capable of being cultured (Citation8, Citation9).
In addition to diet, another important external factor affecting the microbiota is medication, especially the use of antibiotics. Different antibiotics have different antimicrobial spectrums and their effect on the microbiota varies from drastic to fairly mild (Citation10, Citation11). In young children, whose microbiota is still developing and not very stable, the use of antibiotics can potentially have permanent negative effects on the microbiota. Studies performed in adults indicate that the predominant fecal microbiota is restored in about 1 month after the antibiotic treatment ends. However, not all bacterial groups recover, even after several years (Citation12–Citation14), which may have a long-term impact on human health.
Methods for analyzing the composition of microbial communities have progressed rapidly over the past 10 years mainly due to developments in DNA sequencing technology and bioinformatics. There is now a huge database of sequences for the 16S rRNA gene, which occurs universally among bacteria and archaea, that allows phylogenetic assignment of sequences amplified by polymerase chain reaction (PCR) from gut or stool samples (Citation15). Analysis of amplified 16S rRNA genes has been made far more efficient by the ability to use signature-tagged primers in conjunction with high throughput sequencing, thus obviating the need for cloning and allowing in-depth descriptions of the bacterial diversity present within multiple samples. The 16S rRNA gene also provides the basis for a number of useful targeted approaches for enumerating bacterial groups, including qPCR and fluorescent in situ hybridization (FISH) and microarrays; these allow detection of less abundant groups and in some cases absolute quantification of bacterial cell numbers (especially with FISH). By contrast, approaches that are based only on non-targeted sequence analysis produce information on relative, rather than absolute, abundance. In general, it should be recognized that biases in DNA recovery and PCR amplification can affect the recovery of different groups of bacteria, a problem that has been encountered particularly with bifidobacteria. This can make it worthwhile to use both targeted and non-targeted approaches on the same sample set (Citation7). Also of interest is the targeted detection of functionally relevant genes other than 16S rRNA, for example butyryl-CoA: acetate CoA transferase that is involved in butyrate synthesis (Citation5, Citation16). Most recently, however, the development of high throughput sequencing has allowed analysis of all DNA present in a sample. This removes the problem of PCR bias (although not biases in DNA extraction) and provides information on all genes and non-coding sequences whether of viral, bacterial, archaeal or eukaryotic origin. Despite considerable bioinformatic challenges, such metagenome information has been used to describe the diversity of the bacterial community of the human fecal samples in terms of ‘metagenomic species’ – revealing the dominance of a number of cultured species whose genomes have been determined, but also sequence information on many so far uncultured species (Citation16, Citation17).
Based on metagenomic analysis, it has been proposed that the human large intestinal community exists in different states or ‘enterotypes’ in different individuals with three enterotypes suggested to be dominated by Prevotella, Bacteroides, and Ruminococcus spp., respectively (Citation18). On the contrary, other studies (Citation19, Citation20) have shown that individuals whose fecal microbiota are high in Prevotella tend to consume more fiber, while those high in Bacteroides tend to consume more protein and fat, indicating that there is a strong influence of long-term dietary intake upon gut microbiota composition. More recently, the interpretation of metagenomic analysis of the microbiota has moved toward a subdivision in terms of diversity, with the discovery of a bimodal distribution involving low gene count (LGC) and high gene count (HGC) individuals within the general population (Citation21). The microbiota of LGC individuals tends to be Bacteroides-dominated (perhaps corresponding to the Bacteroides-dominated ‘enterotype’) and this group of individuals was reported to show a higher incidence of obesity and metabolic syndrome (Citation21, Citation22). Putting obese LGC volunteers onto a controlled weight loss diet increased microbiota diversity toward that of the HGC individuals and to improvement in the symptoms of metabolic syndrome, suggesting that diet may have led to the LGC state and to the development of metabolic syndrome (Citation22). The possibility exists that the altered microbiota composition in the LGC state also contributes to the disease state by influencing metabolic outputs and inflammation.
Dietary impact
During the past years, the interest in ‘optimizing’ the intestinal microbiota composition by dietary means has literally exploded. However, several challenges and limitations regarding design and interpretation of human studies have to be kept in mind.
Human studies normally provide fecal samples reflecting the microbiota of the distal colon, but do not allow access to the microbiota of the actual site of food fermentation (caecum and proximal colon). Further, human studies in this field usually have low numbers of participants and participants differ severely in their dietary behavior and life-styles. Mostly fecal samples are collected at a single point in time reflecting the short-term rather than the long-term impact of diet, and the total number of bacteria is usually not assessed. To improve the validity of human studies, it is important to determine the habitual diet of the study participants and to control the diet during the intervention period. Further difficulties arise with the interpretation of the results, as our knowledge of the health effects of microbiota is still very limited. A great advantage of recent studies is that it is now possible to screen the whole community of bacteria to assess the impact of diet on gut microbiota composition.
With all these aspects in mind, this review intended to evaluate the available human data on the influence of diet on human gut microbiota. A summary of the studies selected for this review is provided in the Supplementary Tables 1 and 2.
Dietary patterns
Vegetarian diets
Studies on vegetarian/vegan diets often lack clear definitions regarding the dietary pattern of such specific diets. Unless food intake has been assessed by appropriate and validated methods, the only fundamental difference between vegetarian and omnivorous diets is the lack or presence of meat and products thereof. Only a few observational studies investigated differences in fecal microbiota composition between vegetarians and omnivores (Citation23–Citation25). The results of these studies indicate that the microbiota of omnivores is enriched with bacteria of the Clostridium cluster XIVa, which are butyrate-producing bacteria (Citation23, Citation25). Furthermore, Kabeerdoss et al. (Citation23) reported an increased gene level of butyryl-CoA CoA-transferase in the omnivore group. This indicates an enhanced capacity for the production of butyrate, which has been associated with health benefits. However, this study was conducted in rural southern India and the dietary habits of Indian omnivores are very different to those of Western country omnivores. Therefore, it is questionable whether these results can be translated to the typical Western vegetarian/omnivorous diet.
Matijasic et al. (Citation25) also reported increased proportions of Bacteroides/Prevotella group, Bacteroides thetaiotaomicron, Clostridium clostridioforme, and Faecalibacterium prausnitzii in vegetarians.
In contrast to these studies Liszt et al. (Citation24) reported no significant differences in the microbiota composition of vegetarians and omnivores. Nonetheless, they observed a tendency for a higher abundance of Bacteroides and a decreased abundance of Clostridium cluster IV in vegetarians. The lack of statistical significance might be due to the small sample size (n=15 vegetarians/14 omnivores) and large inter-individual variations. Only one of these studies (Citation23) assessed food consumption and quantified dietary intake of macronutrients, which is mandatory for further interpretation of results.
Due to major limitations in experimental design concerning medication and recruitment of volunteers, further studies (Citation26, Citation27) have not been included in this review.
In conclusion, the analysis of the available data does not allow any conclusion to be drawn about the effect of vegetarian diets on the composition of the intestinal microbiota. It demonstrates the need for well-conducted studies, with appropriate sample sizes and detailed assessment of food consumption over appropriate time periods.
Western diet
The diet of people living in Western countries is usually rather low in fiber and provides a high amount of fat and refined carbohydrates compared with the diet of people living in rural countries. Several studies compared the gut microbiota of US Americans or Europeans with those of Africans or South Americans (Citation20, Citation28–Citation31).
Differences in gut microbiota composition were already present in 6-month-old infants from Malawi in comparison to age-matched Finnish infants (Citation31). This is in line with the results from Yatsunenko et al. (Citation29), who determined the microbiota composition of volunteers (0–70 years of age) from Venezuela, Malawi, and the United States. They reported that irrespective of age, the microbiota composition clustered according to country. Malawian and Venezuelan samples were rather similar, compared with the US American samples. The least microbial diversity in this study was observed for adult Americans (Citation29). Furthermore, the genus Prevotella was underrepresented in Americans leading the authors to the conclusion that this might be a ‘discriminatory taxon’. This hypothesis is affirmed by the results of De Filippo et al. (Citation20), who observed increased amounts of Prevotella in African children compared with European children, the study of Ou et al. (Citation28), who report enrichment in Prevotella in Africans compared with African Americans, and Schnorr et al. (Citation30), with similar observations in the Hadza hunter-gatherers (from Tanzania) compared with Italian people. In addition, enrichment in Succinivibrio and Treponema in several African populations has been reported (Citation20, Citation28, Citation30). These bacteria possess a high-fiber-degrading potential, which is important as the typical diet of the rural African populations is high in fiber and complex carbohydrates (Citation20, Citation28, Citation30). Interestingly, Schnorr et al. (Citation30) reported that no bifidobacteria were detected in the Hadza samples. The authors hypothesize that the occurrence of bifidobacteria in the gut of adult humans is associated with the consumption of agro-pastoral-derived foods. So far bifidobacteria have been assumed to be an important part of a healthy human gut microbiota, but this observation raises the question whether there is anything like a healthy gut microbiota per se.
Specific foods
Whole grain products
Whole grain (WG) products are, amongst other things, characterized by a high amount of dietary fiber. The human body does not have any enzymes, which digest these structures; therefore fibers reach the colon, where they are metabolized by the microbiota, affecting the growth of different bacterial groups. Several studies investigated the influence of WG breakfast cereals or flakes on gut microbiota composition (Citation32–Citation34). A controlled study showed, that after 3 weeks of maize-based WG breakfast cereal consumption, the bifidobacteria levels were increased in feces of the volunteers (Citation32). Furthermore, the proportion of Lactobacillus/Enterococcus group was increased during both intervention periods (WG rich cereals and placebo cereals). Similar results were observed by Costabile et al. (Citation33), who compared a WG wheat breakfast cereal with a wheat-bran-based breakfast cereal in a controlled study. After consumption of the WG wheat breakfast, the abundance of Bifidobacterium spp. and the Lactobacillus/Enterococcus group were significantly increased in comparison to the intervention with wheat-bran-based breakfast cereal. However, neither study detected effects on SCFA concentrations (Citation32, Citation33).
Martinez et al. (Citation34) determined the influence of WG barley flakes, WG brown rice flakes, or a mixture of both on gut microbiota composition. One limitation of this study is that a non-WG control group was lacking. Nonetheless, compared to baseline values, all WG interventions led to an increased microbial diversity, as well as a rise in the proportion of Firmicutes and a reduction of the Bacteroidetes phylum. The changes at phylum level during the WG barley intervention were primarily due to three genera: a higher abundance of Blautia and Roseburia and a lower abundance of Bacteroides. Furthermore, the results are in line with the studies of Carvalho-Wells et al. and Costabile et al. (Citation32, Citation33), who also detected an increase in bifidobacteria reaching statistical significance only during the WG barley intervention.
One further study (Citation35) assessed the impact of WG rye bread on the microbiota composition in comparison to refined wheat bread. Although no differences were observed between both dietary groups, the microbiota composition within the white wheat bread group changed during the 12 weeks of intervention. In this group, the numbers of Bacteroidetes decreased, whereas levels of Clostridium cluster IV, Collinsella, and Atopobium spp. increased. No significant differences were observed within the WG rye bread group for any bacterial taxa. The authors assume that the lack of effects of the WG rye bread is due to the high amount of WG rye bread in the habitual Finnish diet (Citation35).
Taken together the results of these studies indicate a possible bifidogenic effect of WG products. To confirm this hypothesis long-term, placebo-controlled studies with a variety of WG products are needed.
Fruits and nuts
Several studies investigated the influence of fruit and nut consumption on the composition of the gut microbiota. Red berries containing anthocyanins have been investigated in several experimental studies, with only a few human studies available. Vendrame et al. (Citation36) investigated the influence of a daily consumption of a wild blueberry drink (containing freeze-dried and powdered wild blueberries and water) for 6 weeks in a placebo-controlled crossover study. They detected an increased amount of Bifidobacterium spp. in the feces of volunteers after blueberry drink consumption. Furthermore, abundance of Lactobacillus acidophilus was higher after both blueberry and placebo treatment, whereas no effects were observed on Bacteroides spp., Prevotella spp., Enterococcus spp., and Clostridium coccoides (current designation Blautia coccoides). In addition, the authors quantified specific Bifidobacterium species (Citation37). B. longum subspecies longum and B. adolescentis were the most abundant species, but the only phylotype, which was significantly increased after the intervention with blueberry drink, was B. longum subspecies infantis.
The increase of the Bifidobacterium genus after consumption of red berries was also observed in another study (Citation38). After consumption of red wine and de-alcoholized red wine the abundance of Bifidobacterium, Enterococcus, Eggerthella lenta, and the phylum Fusobacteria were increased compared to baseline values and control (gin). Interestingly, most effects were more pronounced after red wine consumption in comparison to de-alcoholized red wine consumption, although baseline values did not differ from the gin intervention. These results indicate that alcohol might have a synergistic effect in combination with other red wine constituents.
Furthermore, the influence of almonds and pistachios on human gut microbiota composition has been investigated in two randomized, controlled, cross-over studies (Citation39). The participants consumed 0, 1.5, and 3 servings of nuts per day, with each intervention period lasting for 18 days. The authors reported that the consumption of pistachios had a stronger impact on microbiota composition than the consumption of almonds. Interestingly, operational taxonomic units (OTU), which were increased during the intervention period, showed a higher capacity for the production of butyrate. Other studies investigated the influence of apples (Citation40), bananas (Citation41), and almonds (Citation42) on human gut bacteria. But as these studies investigated only targeted bacteria they are not considered in this review.
Vegetables and legumes
Hardly any study investigated the association between the consumption of vegetables or legumes and the composition of gut microbiota. The influence of chickpeas on gut microbiota was investigated by Fernando et al. (Citation43). After 3 weeks of chickpea consumption, less volunteers were positive for Clostridium cluster XI (approximately 30%) and Clostridium cluster I/II (approximately 40%) than after the control diet or the control diet supplemented with raffinose. However, samples did not cluster according to diet in UPGMA dendrograms (unweighted pair group method with arithmetic mean) and no differences were observed in the Shannon diversity index. There was also no effect on SCFA concentrations.
Another study investigated the impact of conventional soymilk on gut microbiota compared with low glycinin soymilk and bovine milk (Citation44). Both soymilk groups showed a decreased Firmicutes to Bacteroidetes ratio after the intervention period compared to baseline values, whereas no differences were observed in the bovine milk group.
Due to the small amount of data available, it is not possible to conclude as to what extent fruits, nuts, vegetables and legumes influence the gut microbiota composition.
Food constituents
The following section presents studies investigating the influence of specific food constituents on gut microbiota composition. A summary of the available studies is given in Supplementary Table 2.
Dietary fiber
The definition of dietary fiber is still being discussed, but according to the Codex Alimentarius, dietary fiber is defined as carbohydrate polymers with three or more monomeric units, which are neither digested nor absorbed in the human small intestine. This definition includes lignin and components associated with dietary fiber (Citation45).
Resistant starch
Starch may escape digestion in the small intestine and reach the colon for fermentation. This resistant starch (RS) is usually referred to as physically inaccessible starch (RS1), native granules (RS2), retrograded starch (RS3), or chemically modified starch (RS4). A randomized cross-over study including 14 overweight men investigated the effects of consuming RS3 for 10 weeks (Citation7). The bacterial profile of an individual was constant over time for the specific diet. The abundance of Ruminococcus bromii increased in most subjects on a RS diet and 17% of total bacteria could be ascribed to this species compared with 3.8% on a non-starch polysaccharide (NSP) diet containing wheat bran. Levels of uncultured Oscillibacter and Eubacterium rectale also increased with the diet containing RS (Citation7).
In another study on RS, 10 subjects were given RS2, RS4, or native starch as crackers for 3 weeks (Citation46). RS4 led to higher numbers of Actinobacteria and Bacteroidetes, and reduced those of Firmicutes. At species level, the proportions of Bifidobacterium adolescentis and Parabacteroides distasonis increased with RS4, while RS2 raised the proportion of R. bromii and E. rectale as compared with RS4. There was a large individual variation and the changes were reversible and correlated to the RS consumed (Citation46). Taken together, the results of both studies indicate that RS might have a growth-promoting effect on R. bromii and E. rectale, but this effect probably depends on the type of RS used.
Inulin
Several studies investigated the influence of inulin on human gut microbiota composition, often in combination with other fibers. One study examined the effect of a mixture of inulin and partially hydrolyzed guar gum (I-PHGG) or maltodextrin on gut microbiota in 60 constipated women (Citation47). Bacteria of the genera Bifidobacterium and Lactobacillus were analyzed as well as Bacteroides, Clostridium, and the species Escherichia coli. Total numbers of Clostridium spp. (some species are associated with diarrhea) decreased in the fiber group. No other differences could be seen in bacterial composition or in SCFA concentrations (Citation47).
In another study very-long-chain inulin extracted from globe artichoke (Cynara scolymus) was given to healthy volunteers (Citation48). The study lasted for 3 weeks, and after a 3-week washout period the subjects consumed maltodextrin (placebo) for another 3 weeks. Total bacterial levels remained unaffected by the intervention. However, there was a significant increase in the numbers of Bifidobacterium after inulin consumption, both compared with baseline and after intake of maltodextrin. Also, the numbers of lactobacilli/enterococci were higher after inulin consumption, whereas it decreased after maltodextrin consumption. Furthermore, there was an increase in the abundance of the Atopobium group and a reduction in the Bacteroides/Prevotella group. No differences in SCFA concentrations could be seen (Citation48).
In addition, the influence of inulin and xylo-oligosaccharides was investigated in a study by Lecerf et al. (Citation49). Sixty healthy subjects were given xylo-oligosaccharides, a mixture of inulin and xylo-oligosaccharides and wheat maltodextrin for 4 weeks in a randomized cross-over study. Xylo-oligosaccharides alone increased the fecal concentration of Bifidobacterium and butyrate compared with maltodextrin. Further, the activity of α-glucosidase and β-glucuronidase was higher, while the fecal concentration of acetic acid and ρ-cresol were lower. The combination of inulin and xylo-oligosaccharides increased fecal SCFA and propionate, while lipopolysaccharide (LPS) concentrations in blood were reduced. No differences were detected between the groups regarding the Firmicutes, Bacteroidetes, Clostridium, Faecalibacterium prausnitzii, and Roseburia spp. populations (Citation49).
Further studies on inulin and fructo-oligosaccharides were excluded from this review, due to flaws in study design or as they quantified only selected bacteria (Citation50, Citation51).
Fructo-oligosaccharides and Galacto-oligosaccharides
In a prospective, double-blind, randomized, cross-over trial healthy volunteers consumed liquid formula diets for 2 weeks each. One formula diet contained dietary fiber consisting of fructo-oligosaccharides (FOS) and pea fiber whereas the other formula diet contained no added fiber (Citation52). At the beginning of the study and between the two intervention periods (washout phases of 6 weeks), the volunteers consumed their habitual diet. All targeted bacterial species decreased after both diet periods, except bifidobacterial proportions, which increased with the fiber-supplemented diet. The levels of the F. prausnitzii group and the Roseburia intestinalis group were reduced regardless of the diet’s fiber content and correlated with a diminished concentration of butyrate in feces, while occurrence of the Bacteroides group only decreased with the fiber-free diet. Fecal SCFA (acetic, propionic, and butyric acid) concentrations decreased after the fiber-free diet, while butyrate was also reduced following the fiber-supplemented diet (Citation52).
Overweight adults were fed a mixture of galactooligosaccharides (GOS) or maltodextrin (placebo) for 12 weeks in a double-blind placebo-controlled cross-over study (Citation53). After 6 and 12 weeks, the number of fecal bifidobacteria was elevated with the diet containing GOS, whereas the number of Bacteroides spp. and Clostridium histolitycum group bacteria were diminished compared with the placebo at the same time (Citation53).
Polydextrose
In a controlled study that lasted for 21 days, the influence of polydextrose (PDX) intake on gut microbiota was examined (Citation54). On a daily basis the volunteers consumed three snack bars providing PDX, soluble corn fiber, or no fiber (control). Intake of PDX and soluble corn fiber resulted in a higher concentration of Clostridiaceae and lower quantity of Eubacteriaceae compared with the control bars. The level of Faecalibacterium, Phascolarctobacterium, and Dialister was higher after both PDX and soluble corn fiber, while for Lactobacillus this effect was seen only after soluble corn fiber consumption. The number of F. prausnitzii, a butyrate producer known for its anti-inflammatory properties, was also elevated after fiber consumption. Firmicutes was the most abundant bacterial group in all samples (93%) regardless of treatment, whereas the abundance of Actinobacteria was reduced after fiber consumption (Citation54).
In another controlled study (Citation55) including healthy human subjects receiving PDX for 3 weeks, it was shown that Ruminococcus intestinalis, a known butyrate producer, increased in numbers as well as bacteria of Clostridium clusters I, II, and IV, while there was a decrease of Lactobacillus/Enterococcus compared to the placebo group, which received maltodextrin. The changes in gut microbiota composition lasted for 10 weeks (Citation55).
Resistant maltodextrin
Fifteen men were enrolled in a controlled study (Citation56), where each participant underwent three treatments with different dosages of resistant maltodextrin, lasting 24 days and separated by wash-out periods of 2 weeks. Intervention with the lower dose of resistant maltodextrin had no influence on the composition of the gut microbiota. With the higher dose only slight effects were observed, which were not consistent when using different methods of quantification.
Arabinoxylans
A controlled study was performed with healthy adults consuming bread containing arabinoxylo-oligosaccharides (AXOS) (Citation57). For the intervention, an endoxylanase preparation was added to the dough of wheat/rye bread, resulting in an average degree of polymerization (DP) of 18, whereas placebo bread contained arabinoxylans with an average DP of 174. Proportions of Bacteroides and bacteria in the E. rectale group, the Roseburia–Eubacterium subgroup and F. prausnitzii were higher following placebo treatment. Total bacteria and fecal butyrate increased after intervention with the AXOS bread, while concentrations of branched-chained SCFA were reduced, which is concurrent with a decrease in protein fermentation (Citation57).
Furthermore, a randomized, placebo-controlled cross-over study examined the effects of consuming AXOS or maltodextrin (placebo) (Citation58). The bifidobacterial levels were increased after intake of AXOS (but also following placebo after 3 weeks) compared to baseline levels. There were no changes in total numbers of bacteria, the levels of lactobacilli, Roseburia-E. rectale, or enterobacteria. Urinary ρ-cresol, a bacterial metabolite, was higher after AXOS diet (Citation58).
Taken together, the studies included in this review show that dietary fibers with varying chemical composition appear to stimulate the growth and activity of butyrate-producing bacteria, such as Roseburia, E. rectale, and F. prausnitzii. Furthermore, abundance of bifidobacteria and lactobacilli increase after fiber intake and a shift from Bacteroides to Parabacteroides can often be observed. The higher concentration of butyrate in the gut is likely beneficial for health, both locally and systemically. However, the role of other bacterial species, SCFA and other bacterial metabolites for human health need to be investigated further. In addition, there is currently not enough evidence to relate specific dietary fibers, and thus their physico-chemical properties, to an increase of individual bacterial species and SCFA formation in the intestinal microbiota. A number of studies in murine models show that the amount of specific bacteria and the formation of SCFA can be correlated to the composition of dietary fiber.
Fat
The proportion of one macronutrient to overall energy intake inherently affects the share of other macronutrients to the energy intake. Therefore, biological effects induced by changes in macronutrient intake usually result from the combinatory effect of all macronutrients. As an example, high-fat diets (HFD) are normally low in carbohydrates, and this lack of complex carbohydrates contributes to the specific effects caused by a high-fat intake.
Independent of these experimental challenges, fat quantity and quality may affect intestinal microbiota composition. Preliminary data from human intervention studies suggest that dietary fat indirectly modulates intestinal microbiota composition via its impact on bile acid secretion as well as on bile acid composition. It is well known that high fat intakes stimulate the secretion of bile acids and increase fecal concentrations of secondary bile acids, such as deoxycholic acid (DCA) (Citation59). Due to their selective antimicrobial activity, bile acids, such as DCA, could mediate fat-induced intestinal microbiota alterations, as recently shown in rats (Citation60).
So far, hardly anything is known about whether the fat quality has an influence on bile acid composition and thereby on microbiota composition. Wu et al. (Citation19) reported that the Bacteroides enterotype is positively correlated with the intake of saturated fats, while the Prevotella enterotype is inversely (weakly) associated with the total intake of dietary fat. In a recent short-term intervention study, a high-fat, animal-based diet significantly increased fecal DCA concentrations and altered microbiota composition, resulting in an increase in bile-acid-tolerant bacteria (Citation61). These results corroborate a link between dietary fat, bile acid metabolism, and changes in intestinal microbiota. Clearly, more data from controlled human intervention studies are required to better understand the impact of fat quantity/quality on microbiota composition and functionality.
Protein
The effect of protein on the human microbiota composition has only been studied to a minor extent. A diet high in protein and low in carbohydrates was shown to affect the gut microbiota and fatty acid profiles in obese men. After a 4 week period, the high-protein diet resulted in an increase in branched-chain fatty acids, a decrease in butyrate, and a decrease in Roseburia/Eubacterium numbers. Furthermore, a high intake of protein also decreased fiber-derived antioxidant phenolic acids (Citation62).
Phytochemicals
There are no studies investigating the influence of isolated phytochemicals on human gut microbiota, although the influence of several foods containing phytochemicals has been studied (see previous section on ‘fruits and nuts’). As it is known that phytochemicals, for example, anthocyanins, are metabolized by gut microbiota, studies on the influence of specific phytochemicals on human gut microbiota composition are needed.
Long-term versus acute impact
The composition of the human gut microbiota is relatively stable during adulthood. Currently, it is not known whether the stability of the microbiota composition is primarily determined by acute dietary intakes or by long-term dietary behavior.
The problem in answering this question is that no information is available from studies observing dietary habits and the gut microbiota composition over a period of several years. Recently, Wu et al. (Citation19) investigated the stability of human gut microbiota composition. In their study, the results of food frequency questionnaires and dietary records indicated that habitual dietary intake of fat and fiber is associated with the occurrence of specific bacterial phyla. Furthermore, they could show that a short-term (10 days) dietary intervention with high-fat/low-fiber or low-fat/high-fiber diets led to changes in microbiota composition within 24 hours, but the magnitude of the effect did not overcome inter-subject variations in the intestinal microbiota.
These results are in line with another study, which showed that diets supplemented with RS, NSP, or low in carbohydrates influenced the composition of gut microbiota within a couple of days (Citation7). But even after 3 weeks of interventions, stool samples still clustered by individual and no changes on bacterial phylum level were observed.
Davenport et al. (Citation63) investigated whether microbiota composition shows seasonal variations. They observed increased ratios of Bacteroidetes, but decreased ratios of Actinobacteria and Firmicutes in summer in comparison to winter in samples of 60 Hutterites, a communal branch of Anabaptists. Furthermore, the Shannon diversity index was increased in winter. The authors hypothesized that one reason for the seasonal differences might be the higher consumption of fresh fruits and vegetables in summer in comparison to winter. One flaw of this study is that only one fecal sample was taken in summer and one sample in winter. However, it is important to keep in mind, that there might be seasonal variations in gut microbiota when interpreting data of long-term studies.
Dietary interventions probably have a greater impact on the functionality of the microbiota than on the composition of the microbiota per se. A cross-over study by David et al. (Citation61) showed that already after 3 or 4 days of an exclusively plant- or animal-based diet the microbial gene expression profile already clustered according to diet rather than individual. Interestingly, the changes in gene expression affected, amongst other things, pathways for amino acid metabolism, indicating an adaptation to the nutrient intake. This functional adaptation to the diet was also detected as an enrichment of bile-acid-tolerant bacteria during the animal-based diet.
In conclusion, these results indicate that the composition of the human gut microbiota is rather stable and short-term dietary interventions do not profoundly change the microbiota composition. Nonetheless, the microbial gene expression and therefore the functional profiles seem to adapt to changes in diet rapidly.
Food-associated microbes
Raw and unprocessed foods harbor autochthonous, well adapted, and diverse bacterial communities. As an example, plants, providing a variety of nutrients, are attractive hosts for microbes that colonize their surfaces (epiphytes) and tissues (endophytes). Factors such as plant type, farming practices, availability and concentration of substrates for microbial growth, potential presence of competing microorganisms, and natural plant antagonists all provide a unique environment for a specific and stable food-associated microbiota (Citation64, Citation65). The level of microbial populations of raw vegetables and fruits fluctuates between 5.0 and 7.0 log CFU g−1(Citation66). The plant microbiota may contain spoilage (e.g. Erwinia carotovora), pathogenic (e.g. Listeria monocytogenes) and beneficial (e.g. lactic acid bacteria) microbes. As shown by deep sequencing approaches, the microbiota of fruits is dominated by yeasts and fungi, and that of vegetables mainly consists of bacteria, especially aerobes (e.g. pseudomonads, enterobacteria and coryneforms) (Citation65, Citation66). Modern food production typically involves very intensive processing, including hulling, extrusion, heating, and the use of preservatives, which affect the microbes associated with foods. Processes such as heating and the application of preservatives aim to decrease the abundance of pathogenic and spoilage bacteria guaranteeing save foods and prolonging shelf-life. Because these measures are not specific for pathogenic or spoilage bacteria, beneficial bacteria are decreased, too. Therefore, the frequent consumption of highly processed and preserved foods reduces the intake of commensal, food-associated microbes.
In some cases microbes are used for fermentation in food processing. During fermentation, the composition of food-associated microbes changes, for example, in case of sauerkraut or kimchi, the lactic acid bacteria increase (Citation66). Data from controlled human intervention studies on this issue are lacking. So far, only one intervention study based on short-term consumption of diets that consisted entirely of animal or plant foods showed that bacteria used as starters to ferment food transiently colonized the gut. For example Lactococcus lactis and Pediococcus acidilactici, used for making cheese and cured meat, were prevalent in fecal samples from subjects on animal-based diets (Citation61). Therefore, the intake of raw as well as fermented foods might be another possibility how diet affects gut microbiota composition.
Conclusions
It is obvious that diet has an important influence on the composition of human gut microbiota. But as shown in this review, there is a need for more studies investigating the prebiotic effect of foods and food constituents, especially fruits, vegetables, and phytochemicals. Several aspects should be kept in mind when studies in this field are planned. First of all, it is mandatory to control the diets during the intervention periods, in order to detect their short-term effects. To investigate the influence of long-term dietary impact on gut microbiota, it is important to carefully assess the habitual food intake via state of the art food frequency questionnaires and 24-hour dietary recalls.
Concerning methodology, the collection of intestinal samples should be improved. Taking only single ‘snap-shots’ is prone to generate biased data, therefore multiple collections are recommended. Furthermore, collections along the length of the intestinal tract and across the mucosa-lumen gradient would expand our knowledge regarding diet-induced changes in the intestinal microbiota. However, this type of sampling requires invasive sampling and is not usually possible in human intervention studies.
For the interpretation of results, we need more basic data from well-designed intervention studies to understand inter- and intra-subject variability in the microbiota composition. The present focus on phyla shifts neglects low-abundance species which may be more relevant than previously thought. In addition, the use of metabolomics applied to fecal water will expand our knowledge about metabolic differences between bacterial groups.
Finally, a common agreement is needed on whether a ‘healthy’ composition of the intestinal microbiota per se exists and, if so, how this composition can be achieved. The study of Schnorr et al. (Citation30) indicates that the optimal microbiota composition for one individual might depend on the lifestyle of that particular person. As bacteria can rapidly adapt their metabolic properties to different conditions, it seems that not only the microbiota composition is a crucial factor, but gene expression profile and functionality could be even more important. Therefore, it is not sufficient to assess only the microbiota composition, as similar combinations of bacterial phylotypes may have different functional properties.
All these aspects emphasize that we are still at the very beginning of fully understanding these complex issues, and that more well-controlled human intervention studies are needed.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this review.
Supplemental Material
Download PDF (334.1 KB)Acknowledgements
The authors acknowledge the support of the European Science Foundation (ESF), in the framework of the Research Networking Programe, The European Network for Gastrointestinal Health Research.
References
- Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012; 9: 577–89.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science 2005; 308: 1635–8.
- El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 2013; 11: 497–504.
- Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam LC, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 2014; 8: 1323–35.
- Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: acetate CoA-transferase gene. Environ Microbiol 2010; 12: 304–14.
- Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 2012; 6: 1535–43.
- Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2011; 5: 220–30.
- Lagier JC, Armougom F, Million M, Hugon P, Pagnier I, Robert C, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect 2012; 18: 1185–93.
- Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA 2011; 108: 6252–7.
- Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001; 1: 101–14.
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 2010; 8: 423–35.
- De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol 2005; 43: 5588–92.
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6: e280.
- Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 2010; 5: e9836.
- Tringe SG, Hugenholtz P. A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol 2008; 11: 442–6.
- Walker AW, Duncan SH, Louis P, Flint HJ. Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends Microbiol 2014; 22: 267–74.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–65.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature 2011; 473: 174–80.
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334: 105–8.
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691–6.
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500: 541–6.
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500: 585–8.
- Kabeerdoss J, Devi RS, Mary RR, Ramakrishna BS. Faecal microbiota composition in vegetarians: comparison with omnivores in a cohort of young women in southern India. Br J Nutr 2012; 108: 953–7.
- Liszt K, Zwielehner J, Handschur M, Hippe B, Thaler R, Haslberger AG. Characterization of bacteria, clostridia and Bacteroides in faeces of vegetarians using qPCR and PCR-DGGE fingerprinting. Ann Nutr Metab 2009; 54: 253–7.
- Matijasic BB, Obermajer T, Lipoglavsek L, Grabnar I, Avgustin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr 2014; 53: 1051–64.
- Kim MS, Hwang SS, Park EJ, Bae JW. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep 2013; 5: 765–75.
- Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr 2012; 66: 53–60.
- Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013; 98: 111–20.
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–7.
- Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014; 5: 3654.
- Grzeskowiak L, Collado MC, Mangani C, Maleta K, Laitinen K, Ashorn P, et al. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr 2012; 54: 812–16.
- Carvalho-Wells AL, Helmolz K, Nodet C, Molzer C, Leonard C, McKevith B, et al. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: a human feeding study. Br J Nutr 2010; 104: 1353–6.
- Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr 2008; 99: 110–20.
- Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 2013; 7: 269–80.
- Lappi J, Salojarvi J, Kolehmainen M, Mykkanen H, Poutanen K, de Vos WM, et al. Intake of whole-grain and fiber-rich rye bread versus refined wheat bread does not differentiate intestinal microbiota composition in Finnish adults with metabolic syndrome. J Nutr 2013; 143: 648–55.
- Vendrame S, Guglielmetti S, Riso P, Arioli S, Klimis-Zacas D, Porrini M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem 2011; 59: 12815–20.
- Guglielmetti S, Fracassetti D, Taverniti V, Del Bo C, Vendrame S, Klimis-Zacas D, et al. Differential modulation of human intestinal bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. J Agric Food Chem 2013; 61: 8134–40.
- Queipo-Ortuno MI, Boto-Ordonez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 2012; 95: 1323–34.
- Ukhanova M, Wang X, Baer DJ, Novotny JA, Fredborg M, Mai V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br J Nutr 2014; 111: 2146–52.
- Shinohara K, Ohashi Y, Kawasumi K, Terada A, Fujisawa T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010; 16: 510–15.
- Mitsou EK, Kougia E, Nomikos T, Yannakoulia M, Mountzouris KC, Kyriacou A. Effect of banana consumption on faecal microbiota: a randomised, controlled trial. Anaerobe 2011; 17: 384–7.
- Liu Z, Lin X, Huang G, Zhang W, Rao P, Ni L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014; 26: 1–6.
- Fernando WM, Hill JE, Zello GA, Tyler RT, Dahl WJ, Van Kessel AG. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef Microbes 2010; 1: 197–207.
- Fernandez-Raudales D, Hoeflinger JL, Bringe NA, Cox SB, Dowd SE, Miller MJ, et al. Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microbes 2012; 3: 490–500.
- Jones JM. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr J 2014; 13: 34.
- Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 2010; 5: e15046.
- Linetzky WD, Alves Pereira CC, Logullo L, Manzoni JT, Almeida D, Teixeira da Silva ML, et al. Microbiota benefits after inulin and partially hydrolized guar gum supplementation: a randomized clinical trial in constipated women. Nutr Hosp 2012; 27: 123–9.
- Costabile A, Kolida S, Klinder A, Gietl E, Bauerlein M, Frohberg C, et al. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br J Nutr 2010; 104: 1007–17.
- Lecerf JM, Depeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 2012; 108: 1847–58.
- Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013; 62: 1112–21.
- Garcia-Peris P, Velasco C, Lozano MA, Moreno Y, Paron L, de la Cuerda C, et al. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: a randomised, double-blind, placebo-controlled trial. Nutr Hosp 2012; 27: 1908–15.
- Benus RF, van der Werf TS, Welling GW, Judd PA, Taylor MA, Harmsen HJ, et al. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br J Nutr 2010; 104: 693–700.
- Vulevic J, Juric A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr 2013; 143: 324–31.
- Hooda S, Boler BM, Serao MC, Brulc JM, Staeger MA, Boileau TW, et al. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr 2012; 142: 1259–65.
- Costabile A, Fava F, Roytio H, Forssten SD, Olli K, Klievink J, et al. Impact of polydextrose on the faecal microbiota: a double-blind, crossover, placebo-controlled feeding study in healthy human subjects. Br J Nutr 2012; 108: 471–81.
- Baer DJ, Stote KS, Henderson T, Paul DR, Okuma K, Tagami H, et al. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J Nutr 2014; 144: 1023–9.
- Walton GE, Lu C, Trogh I, Arnaut F, Gibson GR. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr J 2012; 11: 36.
- Cloetens L, Broekaert WF, Delaedt Y, Ollevier F, Courtin CM, Delcour JA, et al. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: a randomised, placebo-controlled cross-over study. Br J Nutr 2010; 103: 703–13.
- Rafter JJ, Child P, Anderson AM, Alder R, Eng V, Bruce WR. Cellular toxicity of fecal water depends on diet. Am J Clin Nutr 1987; 45: 559–63.
- Islam SKBM, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141: 1773–81.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–63.
- Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 2011; 93: 1062–72.
- Davenport ER, Mizrahi-Man O, Michelini K, Barreiro LB, Ober C, Gilad Y. Seasonal variation in human gut microbiome composition. PLoS One 2014; 9: e90731.
- Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol 2012; 10: 828–40.
- Leff JW, Fierer N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS One 2013; 8: e59310.
- Di Cagno R, Coda R, De Angelis M, Gobbetti M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol 2013; 33: 1–10.