Abstract
Background
The field of autism research is currently divided based on a fundamental question regarding the nature of autism: Some are convinced that autism is a pandemic of modern culture, with environmental factors at the roots. Others are convinced that the disease is not pandemic in nature, but rather that it has been with humanity for millennia, with its biological and neurological underpinnings just now being understood.
Objective
In this review, two lines of reasoning are examined which suggest that autism is indeed a pandemic of modern culture. First, given the widely appreciated derailment of immune function by modern culture, evidence that autism is strongly associated with aberrant immune function is examined. Second, evidence is reviewed indicating that autism is associated with ‘triggers’ that are, for the most part, a construct of modern culture. In light of this reasoning, current epidemiological evidence regarding the incidence of autism, including the role of changing awareness and diagnostic criteria, is examined. Finally, the potential role of the microbial flora (the microbiome) in the pathogenesis of autism is discussed, with the view that the microbial flora is a subset of the life associated with the human body, and that the entire human biome, including both the microbial flora and the fauna, has been radically destabilized by modern culture.
Conclusions
It is suggested that the unequivocal way to resolve the debate regarding the pandemic nature of autism is to perform an experiment: monitor the prevalence of autism after normalizing immune function in a Western population using readily available approaches that address the well-known factors underlying the immune dysfunction in that population.
This paper is part of the Supplement: The Microbiome in Autism Spectrum Disorder. More papers from this supplement can be found at http://www.microbecolhealthdis.net
In this review, evidence is examined regarding the fundamental nature of autism: is it a pandemic of modern culture, or has it plagued humans for millennia despite the fact that detailed descriptions of the disease emerged only decades ago? The view is taken that autism fits the basic profile of non-infectious immune diseases, both allergic and autoimmune, which have emerged in human societies only since the industrial revolution of the mid-1800s. This view, first proposed by Kevin Becker at the NIH (Citation1), posits a central role of the ‘human biome’ in the development of autism. This biome is composed of all life associated with the ecosystem of the human body, and normally consists of diverse microbial communities (the microbiome) and abundant fauna (animals such as helminths). In Becker's model, autism has emerged in large part due to profound dysregulation of immune function as a result of equally profound alterations in the human biome. In this review, this perspective of autism as a result of a profound destabilization of the ecosystem of the human body by modern culture is examined, and the potential roles of various components of the biome, including helminths and the microbiome, in the pathogenesis of disease are discussed.
Non-epidemiological evidence that autism is a modern pandemic: the role of immune dysfunction in autism
The most compelling argument that autism is a modern pandemic is based on a simple deduction: since modern culture has led to immune system dysfunction, and since immune dysfunction is a hallmark of autism, then autism is a result of modern culture. With as much as 40% of the population affected by allergies or autoimmune conditions, no debate exists regarding the well-documented rise of immune dysfunction in modern society. However, the association between autism and immune dysfunction merits review.
Autism has been strongly associated with a variety of immune system abnormalities. These abnormalities include the presence of autoantibodies, some of which are brain-specific, in many individuals with autism. These autoantibodies have been documented in a number of studies Citation2–(Citation5) , and may be directly involved in the pathogenesis of many cases of autism. Autism is also associated with altered T, B, and NK cell responses, as well as altered cytokine production Citation6–(Citation13) . Further, autism is associated with an increased incidence of allergies and some other autoimmune disorders Citation14–(Citation16) . For example, infantile autism is associated with ulcerative colitis in mothers and type 1 diabetes in fathers (Citation17). In addition, functional changes in brain glial cells (microglia and astrocytes), the primary immunocompetent cells of the central nervous system, have been observed in patients with autism (Citation18). Glial cells are critical for several aspects of normal brain development, including synapse formation and the refinement and phagocytosis of apoptotic debris Citation19–(Citation23) . It has been proposed that microglia prune inappropriate or weak synapses while sparing appropriate or strong connections (Citation22, Citation23). Autism has been well described as a disease of synaptic dysfunction. Whereas hundreds of novel rare mutations and gene number variations have been linked to autism, functional network analyses have nearly all pointed out the importance of molecular pathways that control activity-dependent synaptic remodeling in the pathology of autism (Citation24). Notably, a transient reduction in the number of microglia within the early postnatal mouse brain impairs synapse elimination (Citation25), and disrupts functional brain connectivity and social behavior (Citation26). Moreover, once activated, for example, by diverse immune stimuli or environmental factors, glial cells produce a wide range of immune signaling molecules, including cytokines, chemokines, and other inducible factors such as nitric oxide, all of which may profoundly influence neural function (Citation27). These data suggest a mechanism in which either a disruption of normal microglial development or their activation by abnormal immune stimuli or environmental factors will cause aberrant synaptic pruning by these cells, leading to neural circuit dysfunction and ASD-like behaviors.
The role of inflammation on brain function
A second argument that supports the view of autism as a pandemic associated with immune dysfunction is based on the causal relationship between inflammation and brain dysfunction: since modern culture has led to immune system dysfunction and an overly inflammatory state, and since inflammation profoundly affects brain development and function, then many neurodevelopmental abnormalities, perhaps including autism, might be a result of modern culture. Given the many ways in which the immune system is important for normal brain development, the capacity for immune-inducing events to influence the long-term trajectory and function of these processes is likely profound during fetal development, perhaps more so than at any other stage of life (Citation28). The induction of autism-like symptoms in mice by stimulation of the maternal immune system during pregnancy (Citation29) is an extremely direct and relevant demonstration of the susceptibility of the fetal brain to inflammation. Further, early-life infection can permanently alter vulnerability to cognitive and neuropsychiatric disorders, including Alzheimer's disease, Parkinson's disease, schizophrenia, and autism in humans Citation30–(Citation33) , and can have permanent effects on the sensitivity of the brain to inflammatory stimuli in laboratory animal models Citation34–(Citation36) .
Non-epidemiological evidence that autism is a modern pandemic: shared features with pandemics of inflammatory disease
A third line of reasoning suggesting that autism is a modern pandemic associated with immune dysfunction is based on the observation that autism shares many common features of diseases known to be modern pandemics of immune dysfunction. This line of reasoning is the weakest, but it has merit. Becker's compelling argument that autism is a modern pandemic was based on epidemiological, morphometric, molecular, and genetic features shared in common between autism and asthma (Citation37). Further, like autism, autoimmune diseases affecting the nervous system such as lupus and multiple sclerosis are characterized by diverse arrays of symptoms, with substantial individual variation in both phenotype and progression of disease. In addition, autism shares some immunological features with schizophrenia (Citation38), a neurological disease associated with both modern culture and inflammation. Finally, autism and immune diseases share some of the same risk factors. These shared risk factors, which include vitamin D deficiency and chronic stress, are known destabilizers of immune function. Further, both autism and autoimmune disease can be ‘triggered’ by inflammatory events that include acute infections. Although this list of apparent similarities between autism and immune disease provides only circumstantial evidence of relatedness, the broad nature of this evidence adds compelling credence to the deductive arguments cited above.
Non-epidemiological evidence that autism is a modern pandemic: the recent introduction of triggers for autism
Pandemics of non-infectious immune disease in post-industrial society are underpinned by such ubiquitous culture-based factors as biome depletion. However, other factors, particularly genetics, epigenetics, and specific triggers, clearly play a role in the initiation and progression of disease. Ragweed pollen, for example, serves as a stimulus for the development of hayfever. With this in mind, a particularly informative observation, now about 7 years old, was made when the use of acetaminophen was found to be associated with the development of autism with an odds ratio of about 8 (95% CI 2.08–33.7, p=0.003) compared to ibuprofen (Citation39). The odds ratio climbed to a factor of about 20 when only those cases of children with regression were considered. This original study has been subsequently supported by other studies (Citation40, Citation41) and plausible mechanisms explaining the role of the drug in the pathogenesis of autism have been put forth (Citation39, Citation42) (Citation43). Thus, the potentially strong and very plausible association of acetaminophen but not ibuprofen with autism, coupled with the very recent introduction of acetaminophen into Western culture, provides extremely compelling evidence that autism is, at least in part, a modern pandemic.
Immune dysfunction in modern society
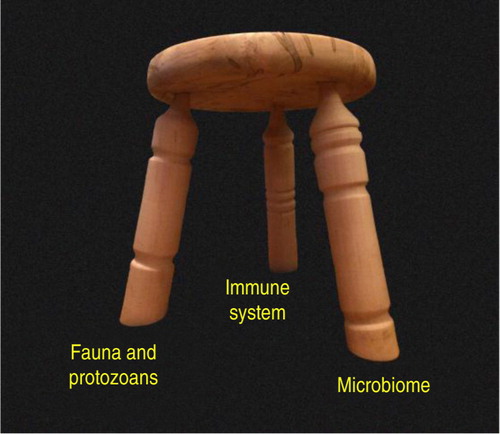
The factors present in modern society that destabilize immune function are no mystery. These factors, which arise directly from common cultural advances such as food processing equipment, water treatment facilities, and artificial indoor lighting, include inflammatory diets, insufficient exercise, chronic psychological stress, and vitamin D deficiency. However, foremost among the factors that cause modern immune dysfunction is ‘biome depletion’ Citation44–(Citation46) , a condition induced by a wide range of technological advancements, including indoor plumbing, hot water heaters, water treatment facilities, and refrigerated storage. In this context, the biome is defined as all life present within the ecosystem of the human body. The immune system and the biome are essentially co-dependent, with the immune system providing support for the biome, while the biome, in turn, provides stimulus necessary for proper immune system development. Importantly for this discussion, the biome can be divided roughly into two components that are, to an extent, independent from one another. One of these two components, the microbial flora, is now referred to universally as the microbiome, although this term obscures the fact that the human biome contains additional components. A second major component of the human biome is the fauna, or animal life, normally present in the intestines and, often less innocuously, in other organs of the body such as the liver and the blood. In viewing the ecosystem of the human body, the analogy of a three-legged stool is helpful (). In this model, the immune system and two components of the biome, the microbial flora and the fauna, interact together, providing stability for the ecosystem as a whole. Protozoans are included with the fauna, since these organisms share some similarities with the fauna in regards to their effects on immune function, and since protozoans are not generally considered in modern studies of the human microbiome. In this model, the loss or substantial alteration of any of the three legs threatens the stability of the entire system.
Fig. 1 The three-legged stool analogy. The ecosystem of the human body is stabilized by immune function in concert with distinct and independent components of the biome. In this model, protozoans are placed with the fauna rather than the microbiome because (a) they exert some of the same effects on the immune system as the fauna, and (b) they are not normally considered in most modern studies of the microbiome, being virtually eliminated from the human biome by Western culture. Loss or substantial alteration of any one leg has the potential to destabilize the entire system, although current evidence suggests that the fauna/protozoan leg exists naturally without requirements for specific species. That is, the fauna/protozoan leg is highly variable in the human population in terms of its species composition, although the complete absence of this leg apparently has very detrimental consequences for the ecosystem as a whole (Citation46). The microbiome leg, in contrast, contains many species that are required for normal function, whereas other species may vary from individual to individual depending on diet and other factors. The three-legged stool in the photograph was designed and created by Kim Turk.
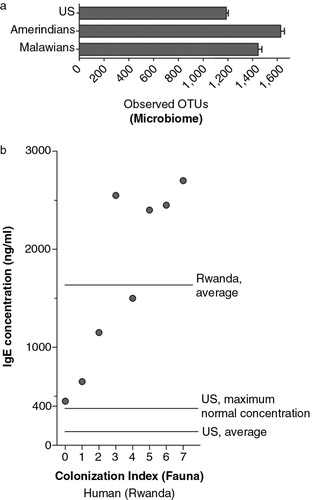
There is little doubt that alteration of the microbial flora using broad-spectrum antibiotics can destabilize immune function, leading to a range of inflammatory conditions that include allergic and autoimmune diseases. Indeed, the failure to preserve the microbial flora via use of probiotics, autotransplants, and, when necessary, allotransplants during medical interventions that place the microbiome at risk (e.g. use of broad-spectrum antibiotics or delivery by C-section) probably represents one of the most costly and most easily avoidable failures of modern medicine. However, evaluation of the biome in modern versus pre-industrial cultures suggests that the effects of modern culture on the microbial flora pale in comparison to the effects on the fauna (). Although the average diversity of the microbial flora in the US society was 15–30% less than that found in two pre-industrial societies, the differences between individuals were greater than the differences between populations (Citation47). More importantly, most of the differences between populations could be accounted for by diet and the known effects of diet on the microbial flora (Citation47). In stark contrast, using a biomarker for the presence of fauna, it is apparent that the fauna in modern society has been virtually annihilated (). This destruction of a large swath of the biome was essential in order to prevent the spread of infectious diseases, but a large body of research, including numerous studies in animal models and several clinical studies, points toward this biome depletion as the leading cause of immune dysfunction in modern society Citation44–(Citation46) . As such, the failure to enrich the biome in order to compensate for this depletion is probably the single most costly shortcoming of modern medicine.
Fig. 2 Independent alteration of the two compartments of the biome by modern culture. Depletion of biome diversity is much less profound when assessing the microbial flora (a) than the fauna (b). Data from the microbiome (a) compared the US population with two pre-industrial populations, and are taken from Yatsunenko et al. (Citation47, Citation48) The average number of ‘operational taxonomic units’ (OTUs), an indicator of biological diversity, is shown on the X-axis. In graph b, IgE concentrations (shown on the Y-axis) are plotted against an index of measured colonization with helminths and protozoans (X-axis). Although high levels of IgE in post-industrial populations are indicative of allergy, IgE can be used as a biomarker for helminth and protozoal colonization in pre-industrial populations. The colonization index and IgE concentrations are taken from Scaglia et al. (Citation48), who assessed IgE levels in pre-industrial groups from Rwanda. Scaglia's colonization index was calculated as the sum of parasitosis scores for individual parasites (both helminths and protozoans), and the population (n=161) was divided into eight groups according to their colonization index. The juxtaposition of these two diagrams illustrates how two important parts of the biome are depleted essentially independently of each other.
Anatomy of the pandemic of autism
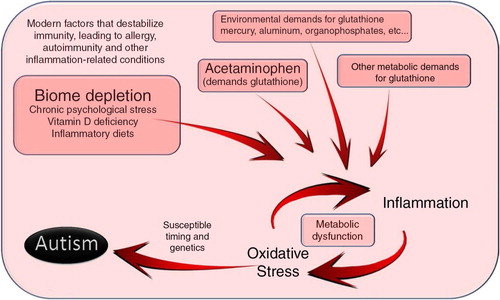
The proposed mechanism by which acetaminophen triggers autism involves depletion of glutathione, a critical factor that moderates the impact of oxidative stress. With this idea in view, the connections between immune instability, oxidative stress, and autism come into focus (). In this model, immune system destabilization by environmental factors interacts with oxidative stressors, causing inflammation that, under the appropriate conditions, can lead to autism (). In this model, a potential role of the microbial flora as a source of oxidative stress during the pathogenesis of autism becomes evident. Other information regarding the cause of autism also falls into place given the view presented in . For example, the gender bias associated with autism is understandable given the observations that testosterone ‘depresses resistance to oxidative stress’ in an animal model (Citation52) and may be deleterious for brain function under conditions of high oxidative stress in humans (Citation53). Further, the association between autism and use of the drug valproic acid (Citation54), which causes microglia activation in cultured glial cells (Citation55), is also consistent with the view presented in .
Fig. 3 A model for the induction of autism, with a central focus on glutathione metabolism. Induction of autism occurs along pathways that strongly resemble those seen in allergy, autoimmune disease, and cancer. Indeed, this diagram is similar to that proposed by others for the induction of autism (Citation49), with the exception of the emphasis on the biome. Autism, in particular, apparently involves oxidative stress at an early age of development. Although the glutathione pathway is emphasized in this diagram, other pathways involved with oxidative stress, including the cP450 system (Citation50, Citation51), are probably also involved in pathology under the influence of chronic inflammation. This model provides at least two means by which the microbiome might be involved in the induction of autism: (a) alteration of the microbiome could destabilize the ecosystem of the human body, leading to a much greater propensity for inflammatory reactions, and (b) aberrant interactions between the microbiome and the immune system could lead to increased inflammation and oxidative stress, with metabolic dysfunction via glutathione insufficiency or cP450 system downregulation (Citation50, Citation51) exacerbating the problem. Thus, the microbiome could be involved in more distal and more proximal aspects of autism induction, respectively.
The epidemiology of autism
Intuitively, epidemiology might seem the most direct and incisive way to determine if a particular disease is an epidemic (or pandemic). Indeed, in the original Greek meaning of the terms, epidemiology was the study of epidemics. One typical epidemiological approach used to determine whether a disease is an epidemic is, of course, to monitor and document the incidence of that disease within a population over time. In the case of non-infectious diseases such as allergic and autoimmune disorders, this documentation can be tricky because the incidence of the diseases usually increases over the timespan of several human generations. Although it is generally difficult or even impossible for a single individual to document such long-term changes in disease rate over time, very senior investigators often note changes over a lifetime of work, especially in the first decades following the appearance of the disease in the population. Thus, the work of Charles Harrison Blackley (Citation56) and of Arthur Keith (Citation57) at the turn of the 19th century provides valuable insight into the epidemic nature of hay fever and of appendicitis, respectively. With this in mind, it is potentially useful to examine the works of Kanner and of Asperger in the 1940s, when they first described autism. At first glance, the answer regarding the epidemic nature of autism is evident. To quote one of their colleagues:
It is certainly true that we all thought in the 1960s that autism was an extremely rare condition. (Uta Frith, September 2014; personal communication to WP, quoted with permission)
I have met clinicians who are personally convinced that the actual incidence of cases has gone up. However, this could be very subjective. In objective terms there are many factors that can explain a greater prevalence, and not necessarily a greater incidence. These factors include greater awareness, different and more lenient diagnostic criteria, the vanishing of a ‘mental retardation’ diagnosis, the desire to obtain an autism diagnosis to obtain services, the tendency to medicalise even very mild cases, and so on. So far I have not been persuaded that there is an actual increase in cases, as all these factors together seem to provide sufficient reasons for the increase in diagnosed cases. The diagnostic criteria today seem to me to be pretty lax and to act now as a ‘catch-all’: There are very few other diagnostic categories that could be used for children affected by neuro-developmental disorders. However, there remains some doubt: There could be a real increase over and above the increase in prevalence. (Uta Frith, September 2014; personal communication to WP, quoted with permission)
Another standard tool of epidemiological research is to probe the incidence of disease in different populations. Indeed, such work has been helpful in corroborating clinical studies that point toward acetaminophen as a trigger in autism (described above). However, to answer questions regarding the possibility that autism results from post-industrial immune dysfunction using this approach, it is necessary to determine the incidence of autism in pre-industrial populations and compare those results with results from Western populations. Unfortunately, due to the relatively small size and inaccessibility of pre-industrial populations, such studies are difficult. Indeed, none currently exist (Citation64). Further, substantial cultural differences associated with child rearing potentially complicate efforts to compare the prevalence of autism in pre-industrial and post-industrial cultures.
Another factor that complicates the study of autism in the field of epidemiology is the fact that autism is not a single disorder. Just as cancer and allergy (general terms) are both comprised of a range of cancers and allergies, so is autism (general term) comprised of many autisms that exist on a spectrum. This issue does not generally confound epidemiologic studies in the fields of cancer and allergy research since these disease types are subdivided into reasonably discrete entities, and these entities have been well established for some time. Thus, it is apparent when one type of allergy, for example, hayfever, remains constant in prevalence, while another type of allergy, for example, allergy against nuts, begins to increase in prevalence. Unfortunately, discrete categories of autism have not been established and monitored for an extended period of time, dramatically complicating the epidemiology of the disease spectrum. Indeed, autism was widely considered to be a consequence of aberrant parenting skills for more than 20 years following its discovery (Citation65, Citation66), and regressive autism was only recognized as a real entity about 10 years ago (Citation65). Thus, tracking the incidence of different ‘types’ of autism for an appreciable period of history is not possible. To add further to the difficulty, the changing face of inflammatory disease in the past 30 years, combined with changes during the same time period in levels of exposure to at least one oxidative stressor implicated in the impairment of cognitive function (e.g. acetaminophen), suggests the possibility that cognitive disorders in children have been a moving target for quite some time. Indeed, the fact that the research community has been so slow to recognize different ‘types’ of autism may be due, in part, to the increasing complexity of the milieu of disease now classified as autism. With this in mind, the idea that the prevalence of disease described and defined as autism in 1943 has remained fairly unchanged during the past 70 years, as suggested by Dr. Frith and many others, does not preclude the most reasonable interpretation of the available data: The milieu of inflammation-associated neurodevelopmental disorders, much of which is currently classified as autism, and the number of people affected by those disorders have profoundly increased over the past 70 years. Given the dramatically increasing impact of a wide range of inflammation-related diseases on Western culture in recent decades, this scenario seems highly likely. Perhaps most importantly, whether today's high burden of inflammation-associated neurodevelopmental disorders currently classified as autism is due to an increase in autism as it was defined in 1943 or to an increase in other diseases may be inconsequential and even a diversion from matters of importance. The single-minded goal of research in this field of medicine is to prevent if possible and to treat if necessary inflammation-associated neurodevelopmental disorders. We argue that this goal is entirely attainable despite our lack of understanding regarding the history of the disease.
Although autism might be a moving target through time, with changing pathophysiology and behavioral features, it is still difficult to understand why physicians in pre-industrial populations did not describe in detail the distinctive social impairment characteristic of autism, especially in light of the high prevalence of the disease. Perhaps one of the most compelling arguments used to explain this lack of commentary is based on the lack of historical commentary regarding fetal alcohol syndrome. The argument has been put forth that since humans have been drinking alcohol for thousands of years in a wide range of cultures, and since fetal alcohol syndrome is not documented in the ancient literature, then physicians prior to the modern era simply did not document childhood disorders very well. In fact, fetal alcohol syndrome went essentially unrecognized until 1973, only 40 years ago (Citation67). This seems extremely odd, indeed inexplicable, in light of the fact that physicians prior to 1973 documented a variety of illnesses in great detail, including various infectious diseases that primarily affect children. A critical clue that provides a solution to this quandary was recently published; an anti-inflammatory drug blocked the development of fetal alcohol syndrome in mice (Citation68). This finding and the well-established evidence associating alcoholism and inflammation (Citation69, Citation70) point toward fetal alcohol syndrome as yet another post-industrial pandemic associated with immune dysfunction. That is, the pervasive immune dysfunction in post-industrial society has potentially resulted in alcohol becoming a trigger for inflammation-associated birth defects, just as ragweed pollen has become a trigger for allergy. The only other explanation for the lack of history of the syndrome is a selective blindness on the part of the medical community prior to 1973. This explanation ignores the role of inflammation in alcohol-mediated injury and is thus unsatisfactory.
If autism in not a modern pandemic, then …
The prognosis is less than ideal. If autism is a hallmark of humanity rather than a culturally induced pandemic, then the disease may be an evolutionary trade-off with some human-specific brain function. It may prove extremely difficult to intervene in such fundamental biological processes. On the other hand, if autism is indeed a modern pandemic, we can expect that the disease is readily preventable if we compensate for the factors in our culture that induce the disease.
The connection between aberrant immune responses and autism is inexplicable. If the immune system is aberrant because of modern culture (an undisputed conclusion), how can that aberrant response be associated with autism, but yet autism itself is not associated with modern culture?
The connection between acetaminophen and autism, which has not been disputed, must be in error. The subsequent studies that support that work must also be in error.
The association between asthma and autism, as described by Becker (Citation37), would be difficult to explain.
The association between schizophrenia and autism (Citation38) would be difficult to explain.
The biological basis of autism lacks an explanation. Why would such a high percentage of the human population lack basic social skills that are far more ancient in origin than the human species, and indeed more ancient than mammals? It has been argued that individuals with a moderate autistic phenotype might have had an advantage at some functions in pre-industrial human societies. However, tasks associated with hunter–gatherer and agrarian societies, even tasks requiring monotonous work and extensive social isolation, have long been performed adeptly by neurotypical individuals. Thus, it remains unclear what skills might be ‘enhanced’ by an autistic phenotype in a pre-industrial society. More importantly, it is difficult to imagine an environment in which a deficiency of social skills and awareness provides a significant advantage for mate selection and reproduction.
Why hasn't this disorder been found to spontaneously occur in animals other than humans, despite a long history of animal husbandry and use of animals in laboratory settings? The syndrome, if present, should be quite visible in very social animals, and indeed is quite visible when experimentally induced in laboratory rats (Citation71, Citation72).
The lack of commentary prior to 1940 on such a common disorder defies explanation. Attempted explanations utilizing parallels with fetal alcohol syndrome, a disorder first described in the 1970s, are fatally flawed, since both autism and fetal alcohol syndrome share inflammatory underpinnings.
Conclusion: the best way to determine if autism is a preventable, inflammation-associated pandemic is to see if autism can be prevented
It seems unlikely that further scientific study will ever resolve the remaining conflicts surrounding the epidemiology of autism. Immunological science provides a very clear indication of the pandemic nature of autism, although the evidence is indirect. At the same time, epidemiologic studies provide direct assessments of the question, but have limitations that may prove difficult or even impossible to circumvent to the satisfaction of everyone. However, the nature of autism can be resolved decisively by conducting one experiment – Remove potential triggers for autism, normalize immune function in the human population, and monitor the levels of cognitive disease. If the early evidence is correct, a reasonable and easily implemented first step would be the removal of acetaminophen from the list of approved children's medications. This reduction in oxidative stress during development might dramatically reduce the levels of autism, particularly forms of autism associated with regression. Second, population-wide normalization of immune function will, if the model suggested by a vast body of scientific study is correct, entirely eliminate most inflammation-associated neurodevelopmental disorders, probably including most of the diseases now classified as autism. To conduct this experiment, the effect of biome depletion must be eliminated by population-wide biome enrichment; a goal that we have argued is easily achievable Citation44–(Citation46) . Further, the focus of primary care physicians and public health efforts must be directed toward additional factors (vitamin D deficiency, chronic psychological stress, inflammatory diets, lack of exercise) which impair immune function. If this experiment is conducted, the worst case scenario is that the incidence of autism will be unchanged despite a dramatic reduction in a wide range of allergic, autoimmune, and other inflammatory related conditions, probably including heart disease and cancer. However, this worst case scenario is virtually unimaginable. Given the vast body of scientific literature connecting impaired cognitive development and function with inflammation, it seems almost certain that normalization of immune function and reduction of inflammatory disease in the human population will result in dramatically decreased levels of a wide range of cognitive disorders, including autism.
Not only will the experiment potentially lead to decreases in a wide range of inflammatory conditions, it may be necessary to preserve the integrity of one of modern medicine's most successful developments: Increasingly unstable immune systems in Western culture, possibly in combination with drugs (e.g. acetaminophen) that trigger cognitive dysfunction, may lead to an increase in adverse reactions either due to particular vaccines (Citation73) or associated with the administration of vaccines (Citation39, Citation58) (Citation74). Indeed, post-industrial-associated inflammatory diseases entail an increase in adverse reactions to a very wide variety of environmental antigens, and there is no reason to expect that vaccines would be immune to this problem. Given the necessity of vaccines for living in crowded (modern, urban) conditions, risk to this asset of modern medicine is not tenable. This fact underscores the urgency of resolving issues regarding immune function in modern society. Importantly, the best way to evaluate potential solutions is to conduct the necessary experiments. Fortunately, the experiments are very feasible.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Acknowledgements
The authors thank Julie Daniels for careful reading of the manuscript and helpful commentary on the epidemiologic aspects of the paper.
Notes
This paper is part of the Supplement: The Microbiome in Autism Spectrum Disorder. More papers from this supplement can be found at http://www.microbecolhealthdis.net
References
- Becker KG . Autism, asthma, inflammation, and the hygiene hypothesis. Med Hypotheses. 2007; 69: 731–40.
- Brimberg L , Sadiq A , Gregersen PK , Diamond B . Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatr. 2013; 18: 1171–7.
- Braunschweig D , Ashwood P , Krakowiak P , Hertz-Picciotto I , Hansen R , Croen LA , etal. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology (Amsterdam). 2008; 29: 226–31.
- Frye RE , Sequeira JM , Quadros EV , James SJ , Rossignol DA . Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 2013; 18: 369–81. [PubMed Abstract] [PubMed CentralFull Text].
- Braunschweig D , Krakowiak P , Duncanson P , Boyce R , Hansen RL , Ashwood P , etal. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013; 3: 277.
- Ashwood P , Wakefield AJ . Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006; 173: 126–34. [PubMed Abstract].
- Ashwood P , Wills S , Van de Water J . The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006; 80: 1–15. [PubMed Abstract].
- Blaylock RL . A possible central mechanism in autism spectrum disorders, part 2: immunoexcitotoxicity. Altern Ther Health Med. 2009; 15: 60–7. [PubMed Abstract].
- Blaylock RL , Strunecka A . Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr Med Chem. 2009; 16: 157–70.
- Derecki NC , Privman E , Kipnis J . Rett syndrome and other autism spectrum disorders – brain diseases of immune malfunction?. Mol Psychiatr. 2010; 15: 355–63.
- Enstrom AM , Onore CE , Van de Water JA , Ashwood P . Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010; 24: 64–71.
- Maezawa I , Jin LW . Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010; 30: 5346–56.
- Pardo CA , Vargas DL , Zimmerman AW . Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005; 17: 485–95. [PubMed Abstract].
- Atladottir HO , Pedersen MG , Thorsen P , Mortensen PB , Deleuran B , Eaton WW , etal. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009; 124: 687–94. [PubMed Abstract].
- Keil A , Daniels JL , Forssen U , Hultman C , Cnattingius S , Soderberg KC , etal. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010; 21: 805–8. [PubMed Abstract] [PubMed CentralFull Text].
- Croen LA , Grether JK , Yoshida CK , Odouli R , Van de Water J . Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005; 159: 151–7. [PubMed Abstract].
- Mouridsen SE , Rich B , Isager T , Nedergaard NJ . Autoimmune diseases in parents of children with infantile autism: a case-control study. Dev Med Child Neurol. 2007; 49: 429–32.
- Vargas DL , Nascimbene C , Krishnan C , Zimmerman AW , Pardo CA . Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005; 57: 67–81. [PubMed Abstract].
- Streit WJ . Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002; 40: 133–9.
- Eroglu C , Allen NJ , Susman MW , O'Rourke NA , Park CY , Ozkan E , etal. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009; 139: 380–92.
- Eroglu C , Barres BA . Regulation of synaptic connectivity by glia. Nature. 2010; 468: 223–31. [PubMed Abstract].
- Schafer DP , Lehrman EK , Kautzman AG , Koyama R , Mardinly AR , Yamasaki R , etal. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012; 74: 691–705. [PubMed Abstract] [PubMed CentralFull Text].
- Schafer DP , Stevens B . Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013; 23: 1034–40. [PubMed Abstract] [PubMed CentralFull Text].
- Gilman SR , Iossifov I , Levy D , Ronemus M , Wigler M , Vitkup D . Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011; 70: 898–907. [PubMed Abstract] [PubMed CentralFull Text].
- Paolicelli RC , Bolasco G , Pagani F , Maggi L , Scianni M , Panzanelli P , etal. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011; 333: 1456–8. [PubMed Abstract].
- Zhan Y , Paolicelli RC , Sforazzini F , Weinhard L , Bolasco G , Pagani F , etal. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014; 17: 400–6. [PubMed Abstract].
- Streit WJ , Conde JR , Fendrick SE , Flanary BE , Mariani CL . Role of microglia in the central nervous system's immune response. Neurol Res. 2005; 27: 685–91. [PubMed Abstract].
- Bilbo SD , Schwarz JM . The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012; 33: 267–86. [PubMed Abstract] [PubMed CentralFull Text].
- Malkova NV , Yu CZ , Hsiao EY , Moore MJ , Patterson PH . Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012; 26: 607–16. [PubMed Abstract] [PubMed CentralFull Text].
- Hornig M , Weissenbock H , Horscroft N , Lipkin WI . An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999; 96: 12102–7.
- Nelson KB , Willoughby RE . Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000; 13: 133–9.
- Rantakallio P , Jones P , Moring J , Von Wendt L . Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997; 26: 837–43.
- Shi L , Fatemi SH , Sidwell RW , Patterson PH . Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003; 23: 297–302. [PubMed Abstract].
- Bilbo SD . Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010; 94: 57–64. [PubMed Abstract] [PubMed CentralFull Text].
- Bilbo SD , Biedenkapp JC , Der-Avakian A , Watkins LR , Rudy JW , Maier SF . Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005; 25: 8000–9. [PubMed Abstract].
- Williamson LL , Sholar PW , Mistry RS , Smith SH , Bilbo SD . Microglia and memory: modulation by early-life infection. J Neurosci. 2011; 31: 15511–21. [PubMed Abstract] [PubMed CentralFull Text].
- Becker KG , Schultz ST . Similarities in features of autism and asthma and a possible link to acetaminophen use. Med Hypotheses. 2010; 74: 7–11.
- Meyer U , Feldon J , Dammann O . Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation?. Pediatr Res. 2011; 69: 26R–33R.
- Schultz ST , Klonoff-Cohen HS , Wingard DL , Akshoomoff NA , Macera CA , Ji M . Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder. The results of a parent survey. Autism. 2008; 12: 293–307.
- Brandlistuen RE , Ystrom E , Nulman I , Koren G , Nordeng H . Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol. 2013; 42: 1702–13.
- Shaw W . Evidence that increased acetaminophen use in genetically vulnerable children appears to be a major cause of the epidemics of autism, attention deficit with hyperactivity, and asthma. J Restorative Med. 2013; 2: 1–16.
- Good P . Did acetaminophen provoke the autism epidemic?. Altern Med Rev. 2009; 14: 364–72. [PubMed Abstract].
- Schultz ST . Can autism be triggered by acetaminophen activation of the endocannabinoid system?. Acta Neurobiol Exp (Wars). 2010; 70: 227–31. [PubMed Abstract].
- Bilbo SD , Wray GA , Perkins SE , Parker W . Reconstitution of the human biome as the most reasonable solution for epidemics of allergic and autoimmune diseases. Med Hypotheses. 2011; 77: 494–504. [PubMed Abstract].
- Parker W , Ollerton J . Evolutionary biology and anthropology suggest biome reconstitution as a necessary approach toward dealing with immune disorders. Evol Med Public Health 2013. 2013; 89–103.
- Parker W , Perkins SE , Harker M , Muehlenbein MP . A prescription for clinical immunology: the pills are available and ready for testing. Curr Med Res Opin. 2012; 28: 1193–202. [PubMed Abstract].
- Yatsunenko T , Rey FE , Manary MJ , Trehan I , Dominguez-Bello MG , Contreras M , etal. Human gut microbiome viewed across age and geography. Nature. 2012; 486: 222–7. [PubMed Abstract] [PubMed CentralFull Text].
- Scaglia M , Tinelli M , Revoltella R , Peracino A , Falagiani P , Jayakar SD , etal. Relationship between serum IgE levels and intestinal parasite load in African populations. Int Arch Allergy Appl Immunol. 1979; 59: 465–8. [PubMed Abstract].
- Strickland AD . Prevention of cerebral palsy, autism spectrum disorder, and attention deficit-hyperactivity disorder. Med Hypotheses. 2014; 82: 522–8. [PubMed Abstract].
- Renton KW . Alteration of drug biotransformation and elimination during infection and inflammation. Pharmacol Therapeut. 2001; 92: 147–63.
- Morgan ET . Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997; 29: 1129–88. [PubMed Abstract].
- Alonso-Alvarez C , Bertrand S , Faivre B , Chastel O , Sorci G . Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc Biol Sci. 2007; 274: 819–25. [PubMed Abstract] [PubMed CentralFull Text].
- Cunningham RL , Singh M , O'Bryant SE , Hall JR , Barber RC . Oxidative stress, testosterone, and cognition among Caucasian and Mexican–American men with and without Alzheimer's disease. J Alzheimer's Dis. 2014; 40: 563–73.
- Christensen J , Gronborg TK , Sorensen MJ , Schendel D , Parner ET , Pedersen LH , etal. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013; 309: 1696–703. [PubMed Abstract].
- Dambach H , Hinkerohe D , Prochnow N , Stienen MN , Moinfar Z , Haase CG , etal. Glia and epilepsy: experimental investigation of antiepileptic drugs in an astroglia/microglia co-culture model of inflammation. Epilepsia. 2014; 55: 184–92. [PubMed Abstract].
- Blackley CH . Experimental researches on the causes and nature of catarrhus aestivus, hay fever or hay asthma. 1873; London: Bailliere, Tindall, and Cox.
- Keith A . The functional nature of the caecum and appendix. Br Med J. 1912; 2: 1599–602.
- Nevison CD . A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environ Health. 2014; 13: 73.
- Treffert DA . Epidemiology of infantile autism. Arch Gen Psychiatry. 1970; 22: 431–8.
- Lotter V . Epidemiology of autistic conditions in young children. I: prevalence. Soc Psychiatr. 1966; 1: 124–37.
- Tanino S . The prevalence of autistic children and suspected autistic children in Toyama Prefecture. Jpn J Child Psychiatr. 1971; 12: 150–8.
- Brask BH . A prevalence investigation of childhood psychoses. Nordic symposium on the comprehensive care of the psychotic children. 1972; Oslo: Barnpsykiatrist Forening. 145–53.
- Fombonne E . Epidemiology of pervasive developmental disorders. Pediatr Res. 2009; 65: 591–8.
- Baxter AJ , Brugha TS , Erskine HE , Scheurer RW , Vos T , Scott JG . The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2014; 1–13.
- Werner E , Dawson G . Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry. 2005; 62: 889–95. [PubMed Abstract].
- Bettelheim B . The empty fortress: infantile autism and the birth of the self. 1967; New York: Free Press.
- Armstrong EM , Abel EL . Fetal alcohol syndrome: the origins of a moral panic. Alcohol Alcohol. 2000; 35: 276–82. [PubMed Abstract].
- Zheng D , Li Y , He L , Tang Y , Li X , Shen Q , etal. The protective effect of astaxanthin on fetal alcohol spectrum disorder in mice. Neuropharmacology. 2014; 84: 13–18. [PubMed Abstract].
- Ambade A , Mandrekar P . Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol 2012. 2012; 853175.
- Wang HJ , Zakhari S , Jung MK . Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010; 16: 1304–13.
- Thomas RH , Meeking MM , Mepham JR , Tichenoff L , Possmayer F , Liu S , etal. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012; 9: 153. [PubMed Abstract] [PubMed CentralFull Text].
- MacFabe DF . Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012; 23 19260, doi: http://dx.doi.org/10.3402/mehd.v23i0.19260 .
- Winstone AM , Stellitano L , Verity C , Andrews N , Miller E , Stowe J , etal. Clinical features of narcolepsy in children vaccinated with AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in England. Dev Med Child Neurol. 2014; 56: 1117–23. [PubMed Abstract] [PubMed CentralFull Text].
- Shoenfeld Y , Agmon-Levin N . ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011; 36: 4–8.
