Abstract
The accumulating evidence of the beneficial effects of cinnamon (Cinnamomum burmanni) in type-2 diabetes, a chronic age-associated disease, has prompted the commercialisation of various supplemental forms of the spice. One such supplement, Cinnulin PF®, represents the water soluble fraction containing relatively high levels of the double-linked procyanidin type-A polymers of flavanoids. The overall aim of this study was to utilize genome-wide mRNA-Seq analysis to characterise the changes in gene expression caused by Cinnulin PF in immortalised human keratinocytes and microvascular endothelial cells, which are relevant with respect to diabetic complications. In summary, our findings provide insights into the mechanisms of action of Cinnulin PF in diabetes and diabetic complications. More generally, we identify relevant candidate genes which could provide the basis for further investigation.
To access the supplementary material to this article: ‘Supplementary tables 1–’ please see Supplementary files under Reading Tools online.
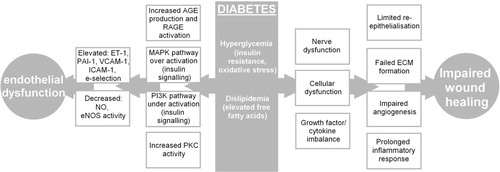
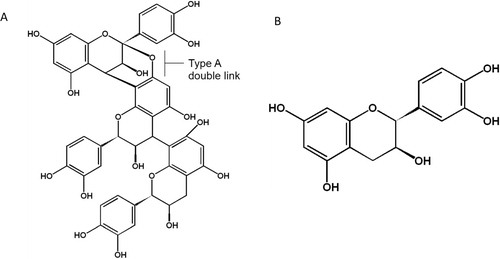
Diabetes is a highly debilitating metabolic disorder which can lead to life-threatening complications as a result of uncontrolled hyperglycemia, and is becoming an increasingly significant disease in elderly populations. Historically, many plant products have been used as treatments for diabetes, with varied success. One such plant product is cinnamon, a spice produced from the bark of trees from the genus Cinnamomum. Studies have shown that cinnamon has potent antioxidant and anti-inflammatory activities. In vitro, a 20-fold enhancement in insulin-dependent glucose metabolism following treatment with cinnamon has been shown Citation1. The active water soluble components of cinnamon have been identified as double-linked procyanidin type-A polymers, mainly trimers and tetramers () Citation1. These procyanidins are naturally occurring polymers of flavonoids such as catechin which are well known to be potent antioxidants.
Fig. 1. Double-linked type-A procyanidin polymer (trimer) (A). Comprised of the flavanoid catechin (B), which is made up of two substituted benzene rings, and a substituted dihydropyran ring.
Water soluble components of cinnamon have been shown to result in the autophosphorylation of the insulin receptor, as well in specific inhibition of the phosphotyrosine phosphatase, which dephosphorylates the insulin receptor Citation2. Findings from an in vivo study demonstrated that when an aqueous cinnamon extract was administrated orally (300 mg/kg), there was an 18% increase in phosphorylation levels of the insulin receptor-β and 33% insulin receptor substrate-1 compared to control rats Citation3. Overall, the findings suggested that the water soluble component of cinammon can increase insulin sensitivity, by increasing glucose uptake in skeletal muscle Citation3. In a clinical study, it was shown that 500 mg per day of Cinnulin PF® (CPF, a commercial cinnamon extract containing the bioactive double-linked procyanidin type-A trimers and tetramers) resulted in improvements in fasting blood sugar levels, systolic blood pressure and body composition in participants with metabolic syndrome Citation4.
Although there is accumulating evidence of the beneficial effects of cinnamon in diabetes, few studies have investigated the molecular mechanisms accounting for these observations. In particular, mechanisms related to the effects of cinnamon in diabetic complications are very limited. Major complications of diabetes include diabetic foot ulcers, which arise as a result of impaired wound healing in lower extremities and endothelial dysfunction which can lead to cardiovascular conditions such as atherosclerosis () Citation5 Citation6 Citation7 . The overall aim of this study was to investigate the effects of the water soluble component of cinnamon, using CPF, on gene expression changes by genome-wide mRNA-Seq analysis in representative, human keratinocyte and microvascular endothelial cell lines.
Materials and methods
Cinnulin PF preparation
The water soluble component of cinnamon bark (Cinnamonum burmanii), Cinnulin PF® (CPF), containing 4.5% type-A polymers (trimeric and tetrameric) was a generous gift from Integrity Nutraceuticals International (USA). The powder was accurately weighed and dissolved in phosphate buffered saline without calcium and magnesium (PBS) by gentle rotation at room temperature for 30–60 minutes (∼70% soluble). The mixture was then centrifuged for five minutes at 400 ×g. The supernatant was collected, sterilized by filtration (0.22 µm) and concentrated stocks (10 mg/mL) were stored at −80°C until use.
Cell culture
Human microvasuclar endothelial, HMEC-1, cells were cultured in MCDB-131 medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS), 10 ng/mL epidermal growth factor (EGF), 5000 units/L heparin, 200 µM hydrocortisone, GlutaMAX™-1 (Invitrogen) and 20 mg/mL gentamicin.Human keratinocytes (FEP-1811) were grown in keratinocyte-SFM (K-SFM) medium (Invitrogen, serum-free medium) supplemented with L-glutamine, epidermal growth factor, bovine pituitary extract and 20 mg/mL gentamicin Citation8. Cells were grown in a humidified 5% CO2 environment at 37°C. As required, single cell suspensions were prepared by trypsinization (0.05% trypsin/EDTA solution, Invitrogen) for 5–10 minutes. Cell numbers were determined using a hemocytometer.
Cell viability and apoptosis
CellTiter-Blue® and ApoOne homogeneous Caspase 3/7® kits where used to examine the effects of CPF on cell viability and apoptosis, respectively, according to the manufacturer's instructions (Promega, Madison, WI, US). Cells were treated with CPF at a concentration range of 0–2048 µg/mL. Following a 24 hour incubation at 37°C, 5% CO2, CellTiter-Blue reagent was added and incubated at 37°C, 5% CO2 for four hours or ApoOne buffer/substrate solution was added and incubated in the dark for two hours at room temperature. Fluorescence was measured using a VICTOR 3 (PerkinElmer, Waltham MA, USA) microplate reader. The EC50 was defined as the concentration of CPF which results in 50% reduction in cell viability.
Cell cycle analysis
HMEC-1 and keratinocyte cells were treated with various concentrations of CPF (0–200 µg/mL) for 24 hours, after which cells were harvested with 0.05% trypsin, washed with PBS, resuspended in ice-cold PBS containing 2% FBS and fixed overnight with 70% ethanol by continuous rotation at 5°C. Cells were stained with an overnight incubation at 5°C in 100 µg/mL propidium iodide containing 1 µg/mL RNase A (Qiagen Inc., Valencia, CA). Samples were analyzed by flow cytometry (Facs Calibur, Becton Dickinson, Franklin Lakes, NJ, USA). Fluorescent debris were gated using forward scatter versus orthogonal side scatter and a secondary gate was placed around the single cell population using a pulse area versus pulse width dot plot. The percentage of cells in each phase of the cell cycle was analyzed using FlowJo V7.6 flow cytometry analysis software (Tree Star Inc., Ashland, OR, USA).
Migration scratch assay
HMEC-1 and keratinocytes were plated in 6-well plates. At 80% confluency and typically following a 24 hour period of supplement starvation, cells were treated with various concentrations of CPF (0–50 µg/mL) for one hour. Cells were washed with PBS and a single scratch was performed across the centre of the well using a 1 mL pipette tip. Cells were then washed twice with PBS and fresh medium was added. Digital images were acquired at defined points at the time of the scratch and after 24 hours at the same location, at a magnification of x4 using a camera (Olympus DP20) and light microscope (Olympus CKX4).
Total RNA isolation and cDNA synthesis
Cells were treated with CPF (20 and 100 µg/mL for keratinocytes, 20 µg/mL for HMEC-1 cells) for 24 hours. Total RNA was isolated using TRIZOL (Invitrogen). Cells suspended in TRIZOL were passed through a 21-gauge needle five times to ensure proper lysis, then RNA extraction was performed following the Qiagen protocol. Genomic DNA contamination was removed by DNAse treatment (Qiagen). First strand cDNA synthesis was performed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer's instructions.
Library construction and RNA sequencing
RNAseq libraries were prepared according to Illumina mRNAseq library preparation kit (Illumina Inc. San Diego, CA, USA, cat # RS-100-0801). Briefly, 8 µg of total RNA was taken for mRNA isolation using poly-T oligo-attached magnetic beads. Poly-A mRNA was fragmented with the presence of divalent cations under elevated temperatures. The mRNA fragments were reverse transcribed via SuperScript II (Invitrogen) with random primers. The second strand was synthesized with addition of RNaseH and DNA Pol I. Double stranded complementary DNA (cDNAs) were end-repaired, followed by 3′ adenylation using Klenow fragment (3′ to 5′ exo minus). Then Illumina paired end adapters with T- overhangs at their 3′ end were ligated to the cDNAs. Following column purification (Qiagen MinElute), to remove excess of unligated adapter, library cDNA was separated on a 2.0% agarose gel. Library cDNA in the range of 200 bp±10 bp fragments were isolated by gel purification (Qiagen) and amplified with 15 cycles of Phusion DNA polymerase mediated PCR (10s 98°C, 30s 65°C, 30s 72°C) using oligonucleotides complementary to Illumina sequencing adapters. Column purified libraries were quantified fluorometrically (Invitrogen), and visualised by MultiNA (DNA 2500 kit) electrophoresis for quality assurance. Libraries were diluted to 10 nM and stored at −20°C prior to cluster generation. Denatured DNA samples were diluted to 6 pM prior to cluster generation (v4 cat # GD-103-4001). Flow cell sequencing of 36 bp tags was conducted on a Genome Analyzer II apparatus (Illumina) according to the manufacturer's protocols using v4 sequencing reagents (cat # FC-104-4001).
Quantitive reverse transcriptase PCR (qPCR)
PCR amplification was performed using an Applied Biosystems 7500 Fast Real-Time PCR System. Primers used in the validation were HPRT-1 (F: TGACACTGGCAAAACAATGCA, R: GGTCCTTTTCACCAGCAAGCT), HMOX-1 (F: TCCCCTCGAGCGTCCTCAGC, R: TGGGGCATGCTGTCGGGTTG), VEGFA (F: TGAGGAGTCCAACATCACCA, R: TCTCTCCTATGTGCTGGCCT), VEGFB (F: CCACCAGAGGAAAGTGGTGT, R: ATGAGCTCCACAGTCAAGGG), CASP-1 (F: CCACAATGGGCTCTGTTTTT, R: CATCTGGCTGCTCAAATGAA), JUNB (F: CACCTCCCGTTTACACCAAC, R: GGAGGTAGCTGATGGTGGTC), JUND (F: CGCCTGGAAGAGAAAGTGAA, R: GTTGACGTGGCTGAGGACTT), MMP1 (F: CAGAGGGAGCTTCCTAGCTG, R: AGTGGAGGAAAGCTGTGCAT), SOD-2 (F: CAGTGTGCGGCACCAGCAGG, R: TGAGGTTCCAGGGCGCCGT), ICAM-1 (F: GGCCACGCATCTGATCTGTA, R: CACTTCCCCTCTCATCAGGC), p50 (F: ACTGCCAACAGCAGATGGCCCA, R: GCACCAGGTAGTCCACCATGGG). 5 pmoles of forward and reverse primer, cDNA template and FAST SYBR® Green Master Mix (Roche, Indianapolis, IN, USA) were mixed to a final volume of 20 µl. Reactions were incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. Analyzed genes were normalized to the level of HPRT1.
Analysis
Sequencing was performed at a depth of ∼20 million mapped reads per sample. The number of sequence reads and alignment statistics are shown in . Reads generated by the Illumina base calling software V1.6 were aligned with Burrows Wheeler Aligner to the human transcriptome database curated by RefSeq (Hg18 build). Raw counts were normalised to adjust for slightly differing data volume across lanes giving the widely used score reads per transcript per million reads. These scores were used to determine relative fold changes and the conventionally used±1.5 fold-change, which is discernable with qRT-PCR was defined a significant change in expression. The online program MetaCore (GeneGo Inc. St. Joseph, MI, USA) was used for pathway analysis. GeneGo is a commercial pathway analysis package however those interested can further investigate pathways discussed in this manuscript using freely available packages such as DAVID functional annotation tools (http://david.abcc.ncifcrf.gov/tools.jsp).
Table 1. Sequence reads and alignment statistics for mRNA-seq experiments
Data is expressed as the mean±SD, and where relevant, statistical analysis was performed using a one-way Anova followed by a Dunnett's test where *p<0.05 was considered statistically significant.
Results
CPF induces a dose-dependent decrease in cell viability and increased apoptosis in HMEC-1 and keratinocytes
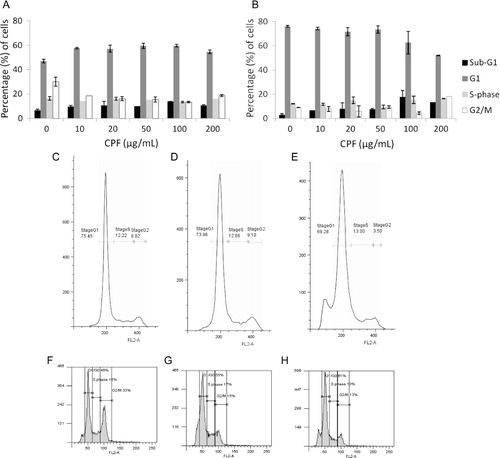
The effect of CPF on cell viability and induction of apoptosis in HMEC-1 and keratinocyte cells was assessed using the CellTiter-Blue and ApoOne Homogeneous Caspase 3/7 assay kits (). The findings indicate that CPF induces a dose-dependent decrease in cell viability in both cell lines. The EC50 values were calculated to be 416 and 663 µg/mL for HMEC-1 and keratinocyte cells, respectively (A,B). Similarly, the caspase activity data indicated a concentration-dependent increase in apoptosis in both HMEC-1 and keratinocytes (C,D). The effect of CPF on the cell cycle was accessed using PI staining and FACS analysis. Cell cycle analysis indicated that CPF had minimal effects on the different stages of the cell cycle, except for the increase seen in the percentage of cells in the sub-G1 population in both cell lines at 100 and 200 µg/mL ().
Fig. 3. Cell viability and apoptosis. Graphs showing the relative survival and EC50 (µg/mL) values following 24 hour incubations with CPF (0, 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024 µg/mL), in HMEC-1 (A) and keratinocytes (B) using the CellTiter-Blue® Assay. The results of the ApoOne homogeneous 3/7® assay indicating caspase activity in HMEC-1 (C) and keratinocytes (D) cells treated with various concentrations of CPF for 24hrs. The data is representative of at least two independent experiments and is presented as the mean±SD of triplicate determination from a single experiment. *indicates p < 0.05, compared to the control.
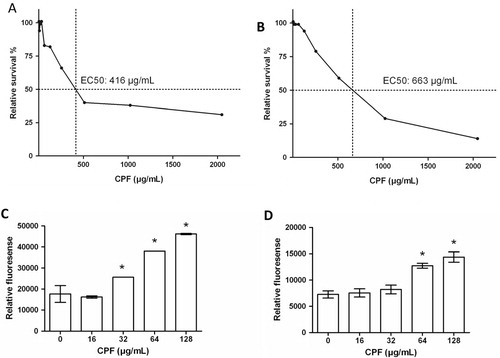
Fig. 4. Graph showing the average% of cells in the different phases of the cell cycle in HMEC-1 cells (A) and keratinocyte cells (B). Cells were incubated with CPF for 24 hours, fixed and stained with propidium iodide. The proportion of cells in the different stages of the cell cycle was determined by FACS analysis. The data is representative of two independent experiments and is expressed as the mean±SD from a single experiment performed in duplicate. C–E show graphs of the cell cycle distribution of keratinocyte cells and F–H HMEC-1 cells treated with CPF at 0 (C, F), 20 (D, G) and 100 (E, H) µg/mL.
CPF enhances migration at 20 µg/mL in both HMEC-1 and keratinocytes
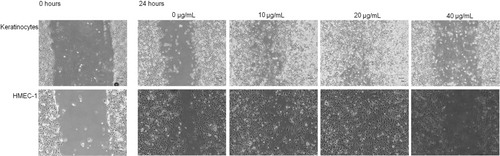
To determine the effect of CPF on cell migration in vitro, a scratch migration assay was used in HMEC-1 and keratinocytes. It was found that in both cell lines, CPF promoted cell migration at the lower concentrations investigated compared to the untreated controls following a one hour treatment and 24 hour incubation, with the effect being most profound at 20 µg/mL in both the HMEC-1 cells and keratinocytes (). However, CPF was not effective at promoting cell migration at higher concentrations.
Fig. 5. CPF promotes cell migration at low concentrations. Images are of keratinocytes (top) and HMEC-1 cells (bottom) treated with various concentrations of CPF (0, 10, 20 and 40 µg/mL) for 1 hour. Scratches were performed immediately following the incubation and images were taken at 0 hrs (representative images shown) and 24 hrs post scratching; magnification x4. Data is representative of five independent experiments for keratinocytes and two independent experiments for HMEC-1.
CPF alters the expression levels of a finite number of genes
To determine the effect of CPF on genome-wide gene expression, mRNA sequencing was performed by next generation sequencing (NGS), using keratinocytes treated at 20 and 100 µg/mL and HMEC-1 cells treated at 20 µg/mL for 24 hours; gene expression levels were compared to the relevant untreated controls. The 20 most up and down regulated genes for each treatment group is indicated in and more detailed characteristics of each of the genes are described in supplementary Tables . The mRNA-Seq findings indicate that the greatest changes in genes expression levels was found in keratinocytes treated with 100 µg/mL CPF, with 8% percent of genes up regulated and 11% down regulated (A). By comparison, 4% of genes were up regulated and 3% down regulated in keratinocytes treated with 20 µg/mL, and 6% up regulated and 5% down regulated in HMEC-1 cells treated with 20 µg/mL CPF (A). A comparison of the mRNA-Seq data for the two cell lines indicated that there was very little overlap between the genes up and down regulated in keratinocyte and HMEC-1 cells treated with 20 µg/mL CPF (Bi). As expected a large overlap was observed between the up and down regulated genes for the low and high CPF concentrations in keratinocytes however, there are certain genes which are up or down regulated at 20 µg/mL which are either unchanged at 100 µg/mL or regulated in the opposite direction (Bii). RT-PCR was used to validate mRNA-Seq findings and positive correlation was identified for validated genes ().
Fig. 6. (A) The% of transcripts up/down regulated for the three treatment groups. HMEC-1 cells were incubated with 20 µg/mL CPF and keratinocytes were incubated with 20 and 100 µg/mL for 24 hours. Significant gene changes were based on a fold change of ±1.5 compared to the relevant untreated control. (B) Venn diagrams showing the number of genes up and down regulated in HMEC-1 and keratinocyte cells treated with CPF (20 µg/mL) for 24 hours (i) and in keratinocytes treated with 20 and 100 µg/mL CPF for 24 hours (ii).
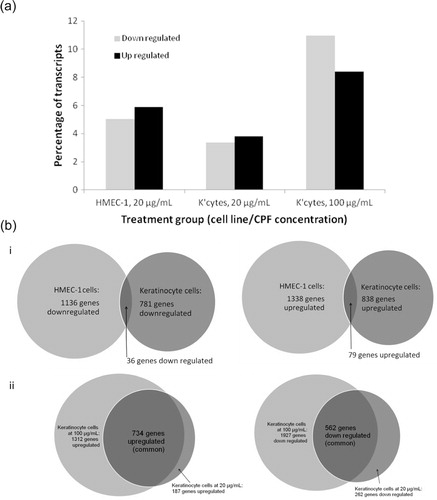
Fig. 7. RT-PCR (white, left) was used to validate the mRNA-Seq results (grey, right). Keratincoye (A) and HMEC-1 (B) cells were treated with 20 µg/mL CPF for 24 hours. RNA was then isolated from the cells, and the RNA of a selected number of genes was then amplified by RT-PCR. The results are expressed as the fold change in mRNA expression compared to untreated cells. RT-PCR results represent the mean±SD from a single experiment performed in triplicate.
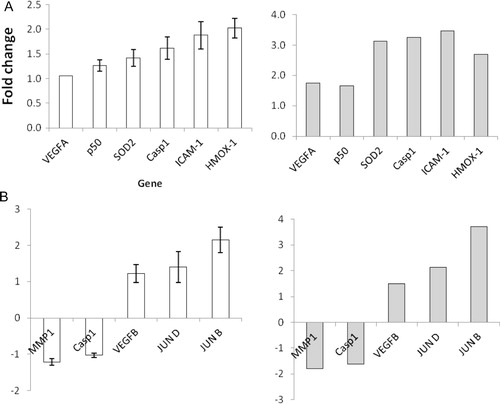
Table 2. The top 20 most up/down regulated genes by CPF in the three treatment groups
CPF alters the expression of genes related to the cell cycle, DNA replication and repair and apoptosis at a high concentration
Down regulation of genes involved in cell cycle regulation, DNA replication and repair was seen in keratinocytes treated with 100 µg/mL of CPF (). For example, there was extensive down regulation of cyclins and cyclin dependent kinases, which are proteins essential for the regulation of the different stages of the cell cycle. Further, down regulation of important DNA replication genes such as DNA polymerase and topoisomerase were also observed. Down regulation of genes such as BRCA-1 and p53 results in impaired DNA repair. There was also a corresponding increase in genes involved in apoptosis. For example, BAX is involved in initiating the mitochondrial apoptotic pathway, while BIM, BIK and PUMA block proteins required for normal mitochondrial function ().
Table 3. Regulation of genes involved in DNA replication and repair, the cell cycle and apoptosis, following treatment of keratinocytes and HMEC-1 cells with CPF
CPF alters the expression of genes involved in proliferation, migration and wound healing at 20 µg/mL in both HMEC-1 and keratinocytes
Up regulation of genes involved in proliferation and cellular migration was observed in both HMEC-1 cells and keratinocyte cells treated with 20 µg/mL CPF (). For example, in keratinocytes there is up regulation of the genes expressing the EGF-R ligands amphiregulin and HB-EGF. In HMEC-1 cells, there was up regulation of the transcription factors c-FOS and c-JUN, as well as PDGF-B. Also, in keratinocytes up regulation of genes involved in migration, including integrins, amphiregulins and HB-EGF was observed. In HMEC-1 cells, genes changes associated with migration included the up regulation of PDGF-B and integrins ().
Table 4. The fold change of the expression of mRNA in genes involved in various processes, following treatment of keratinocytes and HMEC-1 cells with CPF
Further, changes in the expression of inflammation-associate genes were observed in both HMEC-1 and keratinocytes treated with 20 µg/mL CPF (). TGF-β1 was up regulated in HMEC-1 cells while the receptor was also up regulated in keratinocyte cells at 20 µg/mL. While production of IL-6 was increased in keratinocyte cells, it was decreased in HMEC-1 cells. CCL2 (macrophage chemotactic protein) was also down regulated in HMEC-1 cells. In addition, a number of gene expression changes were observed in both cell lines at 20 µg/mL CPF which may be beneficial for the promotion of wound healing (). For example, in HMEC-1 cells there was up regulation of TIMPs, with a corresponding, general decrease in MMP expression levels, which is important for extracellular matrix formation and re-epithelialisation. There was also up regulation of genes involved in angiogenesis, including PDGF-B in HMEC-1 cells, and HIF-1α and VEGF in keratinocytes ().
CPF induces changes in the expression of genes related to endothelial cell function
Up regulation of superoxide dismutase was observed in keratinocyte cells (). In HMEC-1 cells the expression of numerous genes which may be either detrimental or beneficial to endothelial cell function was following treatment with 20 µg/mL CPF. Beneficial changes include the upregulation of eNOS, while potentially detrimental changes include the up regulation of genes encoding for endothelin-1, protein kinase C and PAI-1 ().
Discussion
We investigated the effects of a water soluble cinnamon extract, CPF, in the context of diabetic complications, by examining gene expression changes in human keratinocytes and HMEC-1 cells by mRNA-Seq. When examining the mRNA-Seq data it is important to consider that the main aim of this study was to identify potential gene targets for further investigation. Much of the following discussion is speculative on the basis of the gene expression analysis in the context of diabetic complications. Only changes in gene expression and not the functional endpoint of gene expression – i.e. synthesis of a functional protein, post-translational modifications – were examined in this study. Further issues when using crude extracts are related to modifications which occur in vivo (e.g. conjugation reactions) afnd bioavailability of the active components in cells. The uptake of polyphenolic compounds have been examined in numerous studies, and a number of studies have investigated the effects various cinnamon extracts in cell culture systems indicating the intracellular absorption of relevant compounds Citation9 Citation10 Citation11 Citation12 Citation13 Citation14 . In this context, in vivo bioavailability is also an important consideration. Although studies have reported that procyanidins are bioavailable in rodents and humans, the issue remains largely controversial and requires further exploration.
Our initial experiments indicate that CPF promotes migration at concentrations between 10 and 20 µg/mL in both keratinocytes and HMEC-1 cells (). Although we performed detailed time-course studies, using cell viability, apoptosis and migration, we specifically chose to closely investigate treatment with 20 µg/mL at 24 hours to reflect a non-toxic/non-apoptotic concentration of the formulation in both cell lines. mRNA-Seq analysis indicated that numerous genes associated with diabetic wound healing were regulated by CPF (). For example, certain integrins which are transmembrane glycoproteins involved in cell migration by interacting with components of the extracellular matrix such as laminin 1, fibronectin and collagen (types I, II and IV), were up regulated by CPF in both cell lines. Further, platelet derived growth factor B (PDGF-B) was up regulated 2.18 fold in HMEC-1 cells and fibroblast growth factor-2 (FGF-2) was found to be increased 1.75 fold in keratincoytes treated with 20 µg/mL CPF (). FGF-2 has been shown to induce keratinocyte migration in vitro and has also been strongly associated with promoting angiogenesis Citation15. Other proteins involved in migration pathways that were found to be up regulated by CPF include CDC42, Fyn and PAK1. Furthermore, up regulation of the epidermal growth factor receptor (EGF-R) ligands amphiregulin and heparin binding epidermal growth factor (HB-EGF) in keratinocyte cells.
An aspect which leads to impaired diabetic wound healing is the prolonged inflammatory phase which prevents the progression of the healing process. This is associated with decreased expression of cytokines, including IL-10, IL-6, TGF-β1 and receptors TGF-βR-I and TGF-βR-II Citation16 Citation17 Citation18 Citation19 . It was found that CPF increased the expression of TGF-β1 in HMEC-1 cells but not in keratinocytes. Furthermore, TGF-βR-I mRNA expression was increased in keratinocyte cells treated with 20 µg/mL CPF, while expression of both receptors was decreased in HMEC-1 cells ().
Further, extracellular matrix (ECM) formation is impaired in diabetic wound healing and increased expression of matrix metalloprotienases (MMPs) has been implicated Citation20. Although the expression of most MMPs was not changed, expression of MMP-1 and MMP-14 were both down regulated in HMEC-1 cells (). This was associated with a corresponding increase in the expression of some tissue inhibitors of MMPs (TIMPs) in HMEC-1 cells. Angiogenesis, a process which is essential for wound healing and the delivery of nutrients and oxygen to the newly forming and highly active granulation tissue, is also impaired in diabetes. One reason for this is the decreased expression of hypoxia inducible factor-1-alpha (HIF-1α) in response to glucose. HIF-1α leads to the production of growth factors needed for angiogenesis such as vascular endothelial growth factor (VEGF) Citation21 Citation22. CPF may improve angiogenesis by increasing HIF-1α expression in keratinocytes and by the up regulation of VEGF-A, VEGF-B and VEGF-C observed in the cell lines ().
Endothelial dysfunction involves the altered phenotype of endothelial cells as a result of a number of factors including decreased bioavailability of nitric oxide (NO), increased oxidative stress, a pro-inflammatory environment and increase in prothrombotic factors Citation23. In the endothelium, NO is produced by endothelial NO synthase (eNOS). It plays an important role in normal vascular homeostasis and vasodilation and is helps to protect the blood vessels from endogenous injury by mediating a number of events. For example, eNOS prevents leukocyte and platelet interaction with the endothelial cells and inhibits VSMCs from proliferating and migrating Citation24 Citation25 Citation26 . In endothelial dysfunction, NO is degraded by ROS, and activation of the RAGE receptor by increased AGE levels, resulting in decreased eNOS activation. Although CPF lead to an increase in the expression of the antioxidant enzyme superoxide dismutase (SOD) in keratinocytes and decreased expression of RAGE, the same effects were not observed in HMEC-1 (). However, eNOS expression in HMEC-1 cells was increased, which could potentially lead to increased expression of NO.
It should be noted that CPF also increased the expression of a number of genes which could promote endothelial dysfunction for example, endothelin-1 (ET-1), a potent vasoconstrictor up-regulated in diabetes. Further, insulin resistance results in the up regulation of plasminogen activator inhibitor type-1 (PAI-1) and adhesion molecules such as VCAM-1, ICAM-1 and E-selectin via the MAPK pathway, which may contribute to endothelial dysfunction Citation27 Citation28. CPF did not alter the expression of the adhesion molecules however there was an increase in the expression of PAI-1. Furthermore, up regulation of a number of proteins involved in the MAPK pathway in HMEC-1 cells – MAPK1/3 and MAP2K2 – which is already up regulated in the diabetes, was observed.
Overall, our findings may provide some insights into the mechanisms of action of the water soluble cinnamon extract, CPF, in the context of diabetic complications. Our sequencing data provides numerous gene targets which will provide the basis for further interrogation. Finally, this study was performed in transformed human cell lines in normoglycemic conditions and further experimentation will incorporate the use of normal cell lines and hyperglycaemic conditions. Our analysis is comprehensive with respect to diabetic wound healing and further examination in cell culture systems will involve the use of other relevant cell lines such as fibroblasts and cells of the immune system. Given limitations associated with cell culture studies further it is preferable that further studies are conducted using appropriate in vivo models and isolation of relevant cells for analysis.
Conflict of interest and funding
TCK was the recipient of AINSE awards. Epigenomic Medicine and Human Epigenetics Labs are supported by the National Health and Medical Research Council of Australia. HR is supported by an Australian post-graduate and Baker IDI bright spark awards. KV is supported by a Baker IDI post-graduate scholarship. Supported in part by the Victorian Government's Operational Infrastructure Support Program.
Supplementary Table 1
Download PDF (102.5 KB)Supplementary Table 2
Download PDF (113.4 KB)Supplementary Table 3
Download PDF (112.2 KB)Acknowledgements
The support of the Australian Institute of Nuclear Science and Engineering is acknowledged.
References
- Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. 2004; 52: 65-70.
- Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebritsen TS, Anderson RA et al. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. 1998; 50: 177-82.
- Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. 2003; 62: 139-48.
- Ziegenfuss TN, Hofheins JE, Mendel RW, Landis J, Anderson RA. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. 2006; 3: 45-53.
- Blakytny R, Jude EB. Altered molecular mechanisms of diabetic foot ulcers. 2009; 8: 95-104.
- Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. 2005; 366: 1725-35.
- Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. 2008; 37: 685-711, ix–x.
- Hurlin PJ, Kaur P, Smith PP, Perez-Reyes N, Blanton RA, McDougall JK. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. 1991; 88: 570-4.
- Panickar KS, Polansky MM, Anderson RA. Cinnamon polyphenols attenuate cell swelling and mitochondrial dysfunction following oxygen-glucose deprivation in glial cells. 2009; 216: 420-7.
- Schoene NW, Kelly MA, Polansky MM, Anderson RA. Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. 2005; 230: 134-40.
- Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. 2007; 459: 214-22.
- Cao H, Urban JFJr, Anderson RA. Cinnamon polyphenol extract affects immune responses by regulating anti- and proinflammatory and glucose transporter gene expression in mouse macrophages. 2008; 138: 833-40.
- Cao H, Urban JFJr, Anderson RA. Insulin increases tristetraprolin and decreases VEGF gene expression in mouse 3T3-L1 adipocytes. 2008; 16: 1208-18.
- Cao H, Graves DJ, Anderson RA. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. 2010; 17: 1027-32.
- Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. 2002; 8: 483-9.
- Fahey TJ 3rd., Sadaty A, Jones WG 2nd., Barber A, Smoller B, Shires GT Diabetes impairs the late inflammatory response to wound healing. . 1991;50:308–13..
- Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. 2006; 14: 558-65.
- Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. 1995; 3: 408-18.
- Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. 2009; 85: 9-14.
- Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. 2002; 45: 1011-6.
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). 2006; 70: 1469-80.
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. 2004; 36: 1-12.
- Drexler H. Endothelial dysfunction: clinical implications. 1997; 39: 287-324.
- Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. 1987; 148: 1482-9.
- Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. 1996; 78: 225-30.
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. 1991; 88: 4651-5.
- Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. 2002; 277: 1794-9.
- Mukai Y, Wang CY, Rikitake Y, Liao JK. Phosphatidylinositol 3-kinase/protein kinase Akt negatively regulates plasminogen activator inhibitor type 1 expression in vascular endothelial cells. 2007; 292: H1937-42.