Abstract
We show by immunohistochemical labeling that prominent cell types in the tumor microenvironment of PyMT transgenic mice are tumor-associated macrophages (TAMs) and endothelial cells, and that both populations are decreased in the presence of mitochondrial targeted catalase (mCAT). This observation suggests that mitochondrial ROS can drive tumor invasiveness in conjunction with the presence of TAMs and increased angiogenesis. Since primary PyMT tumor cells expressing mCAT undergo increased apoptosis, mitochondrial antioxidants might be attractive anti-tumor agents.
The oxidative stress theory of aging predicts that attenuation of reactive oxygen species (ROS) would delay aging and age-related diseases. We have shown that mice with the human catalase gene targeted to their mitochondria (mCAT) have decreased cellular levels of mitochondrial ROS in association with increased lifespan Citation1 and decreased incidence and severity of age-related pathology Citation2. In particular, old mice expressing mCAT had reduced non-hematopoeitic tumor burden suggesting that age-associated malignant lesions were suppressed in the absence of excess levels of mitochondrial ROS. We showed the potential involvement of the tumor microenvironment in a follow up publication indicting that the tumor suppression by mCAT occurred mainly in the invasive phase of tumor progression and metastasis, and that cells in the tumor microenvironment were likely key players Citation3. We report in this short note that prominent cell types in the tumor microenvironment of PyMT mice were tumor-associated macrophages (TAMs) and endothelial cells, and that both populations were decreased in the presence of mCAT.
We used F4/80 and CD34 to label TAMs and endothelial cells, respectively. Formalin-fixed mammary gland sections from samples previously described were immunostained according to our established procedure Citation3. The number of F4/80 or CD34 labeled cells was determined by counting positive cells in a grid of eight squares encompassing the entire plane of view at 20× magnification per slide. The labeling index was calculated as the average per cent positive cells. As can be seen in , F4/80 is reduced in primary tumors from PyMT mCAT positive mice compared to primary tumors from PyMT wild type mice, with respective labeling indices of 58±3% and 10±2, p = 0.02 () suggesting a reduction in TAMs. A reduction in malignancy in association with a decreased TAM population is consistent with observations by Lin et al. Citation4, suggesting that TAMs play a key role in regulating tumor growth in this model. The authors went on to describe a reduction in angiogenesis associated with the decrease in TAMs and reduced tumor malignancy. We show similar findings in our tumors from mCAT positive PyMT mice, with decreased labeling for CD34 positive endothelial cells compared to tumors from wild type PyMT mice (). The difference in labeling index between 13±1 and 4±0.4, respectively, was significant at p = 0.05 (). The data suggest that mitochondrial ROS can drive tumor invasiveness via TAMs and increased angiogenesis.
Fig. 1. F4/80 labeling for macrophages (brown stain) is decreased in primary breast tumors from PyMT transgenic mice expressing mCAT compared to primary tumors from non-expressing PyMT mice.
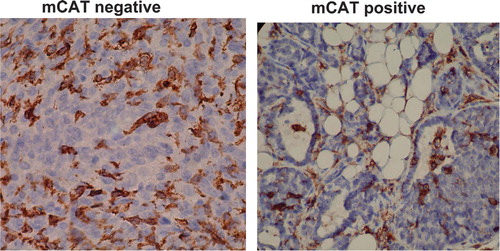
Fig. 2. The presence of mCAT in PyMT tumor cells is associated with a decreased labeling index (%) for F4/80 (macrophages) and CD34 (vascular endothelial cells) and an increased labeling index for caspase 3 (apoptosis). N=3–4 per cohort, *p = 0.05.
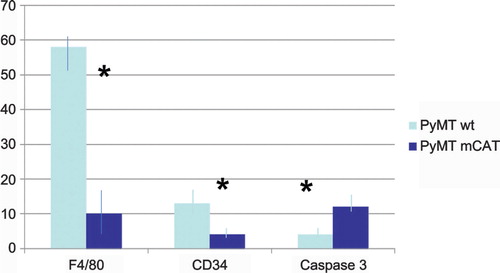
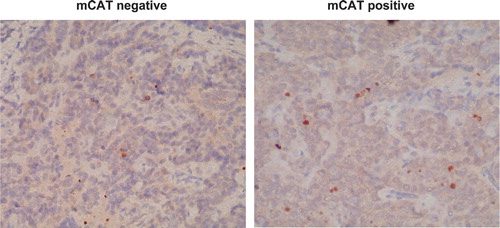
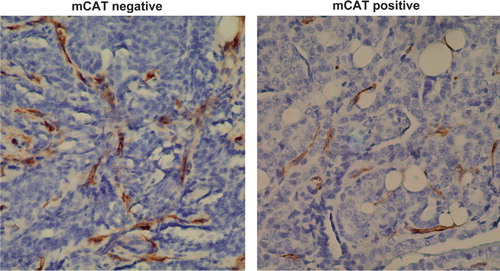
Fig. 3. CD34 labeling (brown stain) is decreased in primary breast tumors from PyMT transgenic mice expressing mCAT suggesting a decrease in angiogenesis compared to primary tumors from non-expressing PyMT mice.
We previously showed that mCAT positive PyMT tumor cells had a decreased proliferative capacity by ki-67 labeling Citation3. In order to determine if apoptosis was a contributing factor, we labeled primary tumor cells with caspase 3 as described Citation5. shows that tumors from mCAT positive PyMT mice had increased labeling, 11.8±0.8%, compared to tumors from wild type PyMT mice, 3.6±0.4%, p = 0.05, respectively, () suggesting that tumor cells expressing mCAT were adversely affected by an increase in apoptosis. We speculate that mitochondrial ROS signaling drives stromal cell pro-tumor activity and when this is attenuated by mCAT, stromal cells undergo apoptosis resulting in decreased tumor growth. The increased apoptosis would be consistent with the decrease in angiogenesis. Therefore, catalase mimetics that attenuate mitochondrial ROS Citation6 might be attractive anti-tumor agents.
Conflict of interest and funding
The authors have not recieved any funding or benefits for commercial companies to conduct this study.
Acknowledgements
Presented in part at the 2011 Nathan Shock Center Annual Conference on Aging, Inflammation in Aging and Age-related Disease, Mayan Ranch, Bandera, TX, USA. This research was supported by NIH/NCI grant R21 CA140916 (WCL) and NIA grant P30 AG13280, Project 3 (WCL).
References
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn GE, Emond M et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. 2005; 30857301909-11. 10.3402/pba.v2i0.17391.
- Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. 2008; 63: 813-22. 10.3402/pba.v2i0.17391.
- Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C et al. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. 2011; 11: 19110.3402/pba.v2i0.17391.
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ et al. Progression to malignancy in the Polyoma Middle T Oncoprotein mouse breast cancer model provides a reliable model for human diseases. 2003; 163: 2113-26. 10.3402/pba.v2i0.17391.
- Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR et al. Pancreatic ß cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum stress response gene P58IPK. 2005; 5441074-81. 10.3402/pba.v2i0.17391.
- Ladiges WC, Wanagat J, Preston B, Loeb LA, Rabinovitch PS. A mitochondrial view of aging, reactive oxygen species and metastatic cancer. . 2010(4):462–5.10.3402/pba.v2i0.17391.