Abstract
Zinc plays an essential role in many biochemical pathways and participates in several cell functions, including the immune response. This review describes the role of zinc in human health, aging, and immunosenescence. Zinc deficiency is frequent in the elderly and leads to changes similar to those that occur in oxidative inflammatory aging (oxi-inflamm-aging) and immunosenescence. The possible benefits of zinc supplementation to enhance immune function are discussed.
The importance of micronutrients in nutrition and human health is unquestionable and, among them, zinc (Zn) is an essential trace element whose importance has been especially prominent in the current literature. Its deficiency can play an important role in the aging process and in the etiology of several age-related chronic illnesses such as atherosclerosis, degenerative diseases of the nervous system, immunosenescence, and cancer (Citation1). Zinc is involved in the maintenance of many homeostatic mechanisms, including efficiency of the immune system, acting as a structural and regulatory catalyst ion for the biological activity of many enzymes, proteins, and signal transcription factors, as well as cell proliferation and genome stability (Citation2).
Biological aging is a complex process that, in humans, is associated with changes in all cells, especially in the immune system. Immunosenescence is characterized by a progressive abnormal regulation of immune responses (innate, adaptive) that causes a low-grade inflammatory systemic condition, susceptibility to infections, and a lower efficacy of vaccines (Citation3, Citation4). Currently, much of the physical and biological characteristics of aging are being explained by the unbalance among oxidizing mechanisms (free radicals, oxygen reactive species), antioxidant defenses (Citation5), and inflammatory and antiinflammatory mechanisms. This results in a low-grade, pro-inflammatory state, referred to as inflammatory aging (‘inflamm-aging’) (Citation6).
The main objective of this review is to approach the recent updates on the role of zinc in human health, the aging process, and immunosenescence. The PubMed, Hinari, Ebsco, Embase, and Dinamed international data bases were searched using the descriptors zinc, zinc and immunosenescence, zinc and aging, zinc and the immune responses, and zinc supplementation. The searches were conducted between July and December 2013; 968 published articles were found, of which 106 articles that matched the required criteria for the topics included in this review were used. The searches were limited to studies published in the English language, and the priority was articles with systematic review, randomized clinical trials, and clinical reviews.
Homeostasis of zinc
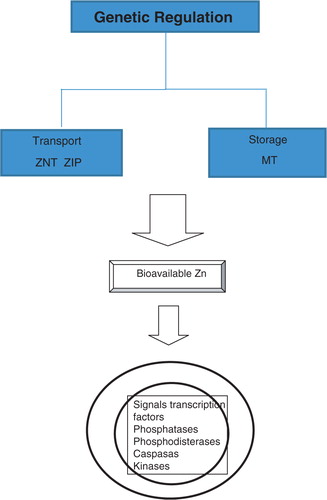
The intracellular homeostasis of Zn () is regulated by buffer proteins called metallothioneins (MT) which act as storage and transporting proteins of Zn (ZnT and ZIP families) that mediate the sign spotting of intracellular zinc, assigning this element the role of the ‘second messenger’ (Citation7). The release of ionic Zn is produced through the reduction of thiol groups in the MT molecule. The homeostasis of Zn is altered in the aging process in part as a result of nutritional deficiency common in the elderly, causing them to have decreased levels of this ion (Citation8) and, in addition, high MT levels that cause the sequestration of zinc. This leads to less availability of intracellular ionic Zn (Citation9). Zinc ions are closely linked to various structural and catalytic proteins, enzymes, and transcription factors activated by signal transduction pathways, especially in cells of the immune system. Possible cellular targets of zinc include phosphatases, phosphodiesterases, caspases, kinases, and transcription factors such as nuclear factor kappa B (NF-κB) (Citation10).
Zn activity is genetically regulated as shown by microarray analysis of the total blood ribonucleic acid (RNA) that reveals genes that respond to Zn, particularly those associated with the regulation of the cellular cycle and immune responses. The micronutrients (Zn, Cu, Fe, Mn) play a pivotal role both in maintaining and reinforcing immune and antioxidant performance and in affecting the complex system of genes implicated in encoding necessary proteins for a correct inflammatory immune response (Citation11, Citation12).
Zinc in human health
The importance of zinc for humans was acknowledged in the Middle East (Iran, Egypt), in the early 1960s, in patients with growth retardation, hypogonadism, hepatomegaly, splenomegaly, dry and wrinkled skin, and severe iron deficiency anemia (Citation13). Patients with Zn deficiency had severe immune dysfunction because of which they died from opportunistic infections before the age of 25 (Citation14). In recent years, the data about the effects of Zn deficiency and its supplementation in human health disorders has increased. Zn deficiency causes dysfunction of humoral and cell-mediated immune responses and increases susceptibility to infections. It has also been related to diarrheic diseases with therapeutic benefits being reported in acute diarrhea in children (Citation15, Citation16). A recent review (Citation17) indicates that the Zn supplementation produces reduction in diarrhea and pneumonia mortality in children aged less than 5 in developing countries. There have also been reports of benefits with regard to other illnesses such as enteropathic acrodermatitis, Wilson's disease (in asymptomatic and pre-symptomatic stages), chronic hepatitis C, shigellosis, leprosy, leishmaniasis, and the common cold (Citation18, Citation19).
Current investigations suggest that Zn deficiency increases the risk of neurodegenerative disorders, affecting neurogenesis and increasing neuronal apoptosis, which can cause deficiency in learning and memory. This links Zn deficiency to cerebral aging, depression, Parkinson's disease, and Alzheimer's disease (Citation20–Citation22). It has been postulated that the increased intake of inorganic copper in drinking water can be important in the pathogenesis of Alzheimer's disease. The elevation of the levels of Zn leads to a decrease of copper levels. It has been reported that the treatment with Zn offers protection to patients older than 70 years against the cognitive decline by decreasing the levels of free copper (Citation23). A clinical essay indicates more improvement of symptoms in patients suffering from depression supplemented with Zn and antidepressants than in those who were administered a placebo and antidepressants (Citation24).
Several studies have revealed an association between Zn intake and gastrointestinal cancer. A systematic review of 19 studies up to April 2013 that included 400,000 participants pointed out that a larger intake of zinc led to a reduced risk of colorectal cancer, and a small intake of this microelement was linked to a higher risk of esophageal–gastric cancer in Asia but not in Europe (Citation25). Some authors talk of a possible link between the trace elements copper and zinc (mostly an increase in their association) and the transitional cell carcinoma of the bladder (Citation26, Citation27).
Other studies indicate that Zn can be effective in decreasing the risk of incidence of ocular diseases correlated to aging (Citation28) and the progression of age-related macular degeneration (Citation29). The role of Zn in the distortion of the sense of taste in the elderly is still being discussed (Citation30). In addition, Zn supplementation has proven to be beneficial in decreasing the incidence of infections in the elderly (Citation18) and in sickle cell anemia patients (Citation31).
Abnormal T cell regulation of the inflammatory immune response in aging
The immune system develops significant changes related to age (immunosenescence), which manifest both in innate and adaptive immunity, but they are mainly reflected in alterations in the bone marrow and in the thymus that cause a change in the composition of the immune cell repertory. This is more significant in T cells (Citation32–Citation34).
Studies performed in healthy octogenarians and nonagenarians have identified an immune risk profile (IRP) of immunosenescence that is distinguished by high numbers of CD8+T cells and low numbers of CD4+T cells (inversion of the index CD4+/CD8+), an increase in the number of dysfunctional and differentiated terminal T cells that were previously exposed to antigens (effectors and memory cells) and the exhaustion of cells that are capable of recognizing and neutralizing new antigens (native or virgins cells) (Citation35–Citation37). Currently, it is being argued whether these changes arise from an intrinsic abnormal regulation of the aging process or from a response to a lifelong continued exposure of the immune system to external stimuli such as infections or other antigenic stimulants (Citation38, Citation39).
In other words, immunosenescence is explained by two hypotheses that are not mutually exclusive: first, the anatomic–physiological decrease of the immune system with aging (intrinsic abnormal regulation) produced, in particular, by the involution of the thymus gland and the decrease of the capacity of auto renovation of the cells of the bone marrow (Citation40, Citation41), and second, the decay that is produced in the immune system due to a lifelong exposure to prolonged or latent infections (Citation42–Citation44) and to a chronic antigenic stimulation (Citation45).
Oxidative inflammatory aging (oxi-inflamm-aging)
Several studies show evidence that a systemic low-grade inflammation characterizes aging with several inflammatory markers, which are significant predictors of mortality in the elderly (Citation46, Citation47). Currently, it is being postulated that immunosenescence is associated with oxidative stress in such a way that the oxidation of proteins alters functionality of immune cells; how this oxidative stress contributes to the chronic inflammatory process is known as oxi-inflamm-aging (Citation48). The oxi-inflamm-aging theory states that oxidative stress associated with aging affects all of the organism's cells, but particularly the cells of regulatory systems (nervous, endocrine, and immune). As a result, there is an inability to preserve redox balance resulting in functional losses that limit the preservation of homeostasis. In the immune system, these changes would be associated with immunosenescence. Activation of improperly regulated transcription factors stimulates the expression of genes that program the production of large quantities of oxidative and inflammatory compounds (cytokines) (Citation49–Citation51).
Other authors believe that inflammatory aging is a consequence and not a cause of immunosenescence. In this sense, it is pointed out that abnormal immune regulation of CD4+ T helper cells in advanced age has been suggested to cause an imbalance of TH1 and TH2 cytokines, with an increase in pro-inflammatory cytokines such as IL-6, IL 1, IL 8, and IL 18, and consequently a chronic systemic inflammation at ‘low intensity’ (Citation52, Citation53).
Role of zinc in aging and inmunosenescence
Zn is an essential micronutrient required in many cellular processes especially in the normal performance and functioning of the immune system (Citation54). Zn deficiency causes significant decline in the innate and adaptive immune responses and promotes systemic inflammation (Citation55, Citation56). The human adult has a total body content of 2–3 g of Zn, and around 1% of it is replaced daily (Citation57). The recommendations of daily requirements of Zn vary according to different authors and regions. In Spain, 15 mg of elementary Zn for men and 12 mg for women are recommended (Citation58). The daily intake recommended by several reviews for people aged more than 70 are 11 mg for males and 8 mg for women with 40 mg being the top tolerable dosage (Citation56, Citation59). Nine European reports indicate needs of Zn that range from 7 to 14 mg in males and 4.9–9 mg in women (Citation60). Elderly people are a particularly susceptible population to zinc deficiency. Although to this day data are insufficient to determine the frequency of Zn deficiency in the elderly (Citation61), it has been estimated that people older than 65 have an intake of zinc below the 50% recommended level (Citation2). A recent study in a group of 102 elderly European people revealed that 44% of them had Zn deficiency and 20% had high Zn deficiency (Citation62).
Zn deficiency is quite common in elderly, frail people since they often avoid meats and other foods that contain this metal to avoid increasing blood cholesterol levels. In addition, they increase the consumption of refined wheat products deficient in Zn and other fiber-rich foods that contain fitates, which limit the intestinal absorption of this trace element (Citation61, Citation63). Other causes of deficiency of this micronutrient in the elderly include inadequate food chewing, intestinal malabsorption, psychosocial factors such as depression, pharmacologic interactions, and altered subcellular processes (zinc carriers, metallothioneins, divalent metal carrier-1) (Citation2, Citation64). A study performed at nursing homes detected that the lowest serum levels of zinc were found in those patients whose daily life activities were greatly compromised, such as being bed ridden, a low body mass index, and/or increased cognitive decline (Citation65).
Age-related conditions may be related to Zn deficiency by the alteration of intracellular Zn homeostasis, because metallothioneins are unable to release zinc, and transporting proteins (ZIP families) are defective resulting in a low content of intracellular Zn (Citation7). The upregulation of the ZIP genes has some influence in the entrance of Zn into cells, which is more pronounced in lymphocytes from young adults than in lymphocytes from old donors (Citation66). Metallothioneins are antioxidant proteins that release Zn ions for several proteins and enzymes implicated in antioxidant responses and in the restoration of DNA. In several models of senescent cells, a decreased expression of metallothioneins and intracellular Zn takes place (Citation67).
There is a significant parallel between changes described in immunosenescence and those that are associated with Zn deficiency, among them a reduction of thymus activity and its hormones, a deviation of T helper cells to type TH2 cell activity, a decrease of the response to vaccines, and a deterioration of innate immune cell (phagocytes and NK cells) function (Citation8). The role of Zn and metallothioneins is crucial as they affect NK/NKT development and function. In particular, some of the polymorphisms of metallothioneins are involved in maintaining the innate immune response. The availability of intracellular Zn in aging, many times decreased, compromises this response (Citation68). It has also been demonstrated that Zn deficiency decreases the thymic hormone thymuline, which is necessary for the maturation of T helper cells. This, along with the deviation of the function of TH1 to TH2, causes dysfunction of the immune response mediated by cells (Citation69). Zn is involved in the genetic expression of melatonin recipients and in the proliferation and apoptosis of cells of the thymus and the reactivation of thymuline (Citation70).
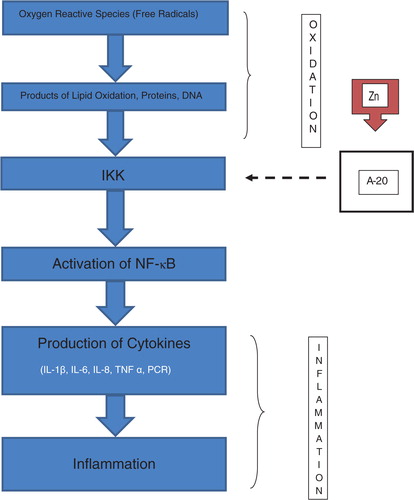
Currently, Zn is regarded as a molecule of intracellular signposting, that is, its status becomes altered in response to an extracellular stimulus, acting like a neurotransmitter (Citation71, Citation72). Two types of intracellular signaling of Zn have been identified: an early one, which is induced directly by an extracellular stimulus, and a late one, that depends on changes of expression of the zinc carriers (Citation73, Citation74). In immune cells, the signaling of intracellular Zn modulates its activity (Citation75, Citation76) through the inhibition of NF-κB, a transcription factor that can control many genes of immune response (Citation77, Citation78). In its latent state, NF-κB is sequestered in the cytosol by inhibitory protein IkB, and it is stimulated through the activation of the Ik kinase (IKK) mediated by Zn (Citation79). In cell culture studies, it has been observed that Zn leads protein A 20 that inhibits the enzyme IkB kinase (IKK). This enzyme is capable of inactivating NF-κB, causing a decrease of inflammatory cytokine generation (Citation13, Citation80).
The zinc carrier (ZIP 8) is a transcriptional target of NF-κB and negatively regulates the inflammatory response through modulation of the activity of IKK (Citation80). Recent opinions suggest that during evolution, host defenses and the aging process have been intimately linked with the mechanisms and defensive factors related to the NF-κB system suggesting a common point between aging and immunosenescence (Citation81). As part of new techniques of cell therapy, the induction of metallothioneins provides better protection of the cells because they act as antioxidant, antiinflammatory, and anti-apoptotic agents, increasing the transcriptional regulation of genes implicated in proliferation and cell differentiation (Citation82). Experimental studies in mice, in particular mice with Zn deficiency, have consistently proven that expression of ZIP 8 is remarkably induced in immune innate cells at the beginning of infections with a concomitant increase of signals mediated by NF-κB. This was associated with increased inflammation and an increase in the severity of sepsis (Citation83).
A system of signals has been described in monocytes where a complex interaction occurs among Zn, nitric oxide, cyclic nucleotide signaling, and the IL-1 receptor associated with kinase-1. These act against the production of inflammatory cytokines (Citation84). Also, the function of dendritic cells is affected by disorders in the homeostasis of Zn implicating the transporting proteins (ZIP) during the upregulation induced by lipopolysaccharides and stimulating molecules (Citation85). A study on the effect of Zn ions in the promonocytic leukemia HL-CZ cell line showed that treatment with Zn caused the release of chemokine and inflammatory cytokines through activation of multiple transcription factors related to the immune response (Citation86). illustrates the role of Zn as a signal molecule in the immune system.
Fig. 2 Zinc, through the activation of protein A-20 inhibits (discontinuous arrow) the IkB kinase (IKK) causing inhibition of the inflammatory cascade. In zinc deficiency, the protein A-20 is ‘free’, and the activation of NF-κB and the generation of inflammatory cytokines are induced.
Immune cells show a decline in function when there is a decrease in Zn content. Cytotoxicity decreases in monocytes, phagocytosis is reduced in neutrophils, and in B cells apoptosis increases and standard functions of T cells deteriorate, but auto reactivity increases. This has been demonstrated in cell culture studies where the concentrations of Zn vary from 100 to 500 mmol (Citation87). B and T cells with zinc deficiency reveal different patterns of reaction compared to Zn-sufficient cells, consistent with a strong proliferative response followed by stimulation of IL-6 and IL-2 in the first case, with less proliferation followed by stimulation of IL-4 in the second case (Citation88). The closest link among aging, immunosenescence, and Zn deficiency seems to be through the oxi-inflamm-aging process. It has been documented that Zn deficiency increases oxidative stress and causes the generation of inflammatory cytokines, such as IL-1β IL-2, IL-6 and TNF α (Citation1, Citation10, Citation13, Citation14, Citation19, Citation53, Citation61, Citation62, Citation89, Citation90), both in vitro and in vivo.
Bao et al., using models of sepsis in small animals, noticed that Zn deficiency increased the number of bacteria and the activity of NF-κB in vital organs, including the lungs, with an increase in inflammation, pulmonary damage, and mortality. Zn supplementation right after the initiation of sepsis reversed these effects (Citation91). Wong et al. also found in their studies on aged rats that Zn deficiency, particularly the reduction of intracellular Zn in immune cells, was associated with an increase in inflammation with increasing age (Citation92).
Zinc supplementation in humans
There have been few studies on the effects of Zn supplementation on the human immune response. A clinical trial that included 55 people of both genders (aged between 57 and 87) who were supplemented daily with 45 mg of oral elementary zinc for 12 months reported that the incidence of infections and the generation of oxidative stress markers and inflammatory cytokines were remarkably lower in the supplemented group than in the control group (Citation93). Zn deficiency is common in adults suffering from sickle cell anemia. In a study that included 36 sickle cell patients, half received 25 mg of oral Zn once a day for 3 months and the rest a placebo. In the supplemented group, the incidence of infections and the induction of inflammatory cytokines (TNF α, IL-1 β) decreased, while the antioxidant power increased (as shown by a decrease in plasmatic nitrate and nitrite and the products of oxidation of lipids and DNA) (Citation31).
An observational study performed including 420 residents of nursing homes in Boston compared the ones who had low levels of plasmatic Zn (<70 µg/dL) with those with normal levels (≥70 µg /dL). In those who had normal concentrations of zinc, the incidence of pneumonia was lower and of decreased duration, with less prescription of new antibiotics, fewer days with the use of antibiotics, and reduction of all causes mortality, compared with the low level group (Citation94). To demonstrate the antiinflammatory and antioxidant effects of Zn, a double-blind randomized trial was performed in 40 people aged 56–83, distributed in a group that received 45 mg of oral Zn gluconate for 6 months and another that received a placebo. In the supplemented group, the inflammatory cytokines (PCR, IL 6) and the markers of oxidative stress (secretory phospholipase A, malondialdehyde, hydroxyalkenals) decreased (Citation95). In a study of long-term hemodialysis patients who had concentrations of serum Zn below the normal (<80 mg/dL), those who were supplemented with Zn for 8 weeks showed remarkably larger percentages of CD4 and CD19 lymphocytes, a higher proportion of CD4/CD8, and a decrease in the proportion Cu/Zn and of the oxidative stress and the inflammatory response (Citation96).
The nutritional state of Zn can influence response to vaccination. A cross-sectional study of 80 elderly people who were inoculated with pneumococcal vaccine revealed that in those who prior to the vaccination had higher levels of Zn, the immune response (IgM) to the vaccine increased by over 10% (Citation97). The supplementation of Zn in children with heart disorders vaccinated against influenza had beneficial effects in decreasing their discomfort (Citation98), which is one of the adverse effects of this vaccine, and also decreased the serum levels of TNF-α. However, in another clinical study in which 140 elder people were vaccinated against hepatitis B, those who were supplemented with Zn sulfate did not show benefits in increasing immunity levels (Citation99).
In an updated systematic review of Zn fortification in diet, only 11 studies were found including 771 participants, mainly children, none on the elderly, with reports of growth rate increase, mostly in those with low weight at birth (Citation100). Efforts have been made to determine the effects of Zn supplements in diarrhea and pneumonia in children where they have demonstrated beneficial effects (Citation18, Citation101).
Other difficulties arise in planning the supplementation of Zn in the elderly, the daily needs vary among populations, the therapeutic dosages are not standardized, and over-administration of Zn can cause toxic effects and side effects on immunity (Citation102). Zn toxicity is relatively unusual. High dietary intake can cause nausea, vomiting, epigastric pain, lethargy, fatigue, and a decline in the immune response, hypocupremia, neutropenia, macrocytic anemia, and sideroblastic anemia (Citation59, Citation103). Currently, it is thought that the evaluation of nutritional Zn is complex due to the fact that the biochemical measurements of this element have limitations in sensitivity and specificity (Citation57). Recently, the measurement of Zn concentrations in fingernails has been used in a unique study in the elderly with and without Alzheimer's disease, but without finding significant differences between these two groups (Citation104). Some authors consider that the selection of elderly people for the supplementation of Zn must be based on low blood concentrations. It is necessary to take into account the genetic background in relation to the polymorphisms of metallothioneins and IL-6. Elderly people who are bearers of the genotype GG (called C negative) in locus IL 6-147G/G develop high levels of IL-6 and metallothioneins, low contents of intracellular Zn, and deterioration of immunity and are the most adequate for Zn supplementation. The bearers of genotypes GC and CC (named C positive) have a satisfactory content of intracellular Zn, and its supplementation can be inappropriate (Citation2, Citation7, Citation105).
Areas of uncertainty for further investigations
Based on what has been investigated thus far, some gaps remain which might be approached in future investigations.
A wide field is open relating to nutritional aspects that involve Zn as an essential trace element, and the clinical and biochemical aspects of its deficiency that contribute to a better evaluation and more precise determination of plasma concentrations.
The research on the pathogenic mechanisms of Zn deficiency in aging, frailty, immunosenescence, and age-related chronic and degenerative diseases should be continued, in addition to the search for possible therapeutic targets.
Clinical trials related to the effects of Zn and its deficiency in the health of the elderly are needed.
There is a need for more precise determination of elderly people at risk for Zn deficiency and compounds and ideal dosage for the supplementation of this element.
Further studies are needed to determine the efficiency of Zn in fighting infections in the elderly.
Conclusion
Zn is an essential micronutrient for human health in general, and particularly for the elderly, with evidence in cell culture experiments of its involvement as a signaling molecule in the oxidation and inflammatory process that occurs in cell senescence and in immune system dysfunction. Although the body of proof is limited in determining the efficacy of the supplementation of Zn in the prevention and treatment of infections and degenerative processes that accompany old age, promising perspectives are open in the homeostatic regulation of this trace element as a therapeutic objective and an attractive field of anti-aging research. Current evidence suggests that educating the elderly to consume an appropriate diet with foods that contain the necessary Zn requirements would be a health-promoting activity. Clinically, we should not lose sight of the nutritional demands of geriatric patients, taking into account the elements discussed in this review, which possess indisputable practice value.
Conflict of interest and funding
The author has not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Chasapis CT, Loutsidou AC, Spilipoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012; 86(4): 521–34.
- Mocchegiani E, Romeo J, Malavolta M, Costarelli L, Giacconi R, Díaz LE, etal. Zinc: dietary intake and impact of supplementation on immune function in elderly. Age (Dordr). 2013; 35(3): 839–60.
- Alonso P, De la Fuente M. Role of the immune system in aging and longevity. Curr Aging Sci. 2011; 4(2): 78–100.
- Lang PO, Govind S, Michel JP, Aspinall R, Mitchell WA. Immunosenescence: implications for vaccination programs in adults. Maturitas. 2011; 68(4): 322–30.
- Oliveira BF, Nogueira JA, Chaves MM. The role of oxidative stress in the aging process. ScientificWorldJournal. 2010; 10: 1121–8. 10.1100/tsw.2010.94.
- Candore G, Caruso C, Colonna G. Inflammation, genetic background and longevity. Biogerontology. 2010; 11(5): 656–73.
- Mocchegiani E, Malavolta M, Costarelli L, Giacconi R, Cipriano C, Piacenza F, etal. Zinc, metallothioneins and immunosenescence. Proc Nutr Soc. 2010; 69(3): 290–9.
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009; 6: 9–26. 10.1186/1742-4933-6-9.
- Mocchegiani E, Costarelli L, Giacconi R, Piacenza E, Basso A, Malavolta M. Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenic approach. Biogerontology. 2011; 12(5): 455–65.
- Haase H, Rink L. Zinc signals and immune function. Biofactors. 2014; 40: 27–40. 10.1002/biof.1114.
- Ryu MS, Langkamp B, Chang SM, Shankai MN, Cousins RJ. Genomic analysis, cytokine expression and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci USA. 2001; 108(2): 20970–5.
- Mochegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, Malavolta M. Micronutrient (Zn, Cu, Fe)–gene interactions in ageing an inflammatory age-related diseases: implications for treatment. Ageing Res Rev. 2012; 11(2): 297–319.
- Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008; 43(5): 370–7.
- Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008; 14(5–6): 353–7.
- Tuerk MJ, Fazel N. Zinc deficiency. Curr Opin Gastroenterol. 2009; 25(2): 136–43.
- Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013; 4(2): 176–90.
- Yakoob MY, Theodoratou E, Jabeen A, Imdab A, Eisele TP, Ferguson J, etal. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011; 11(Suppl 3): 3–23.
- Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr. 2009; 28(3): 257–65.
- Hui J, Tang NL. Wilson's disease: a review of treatment options with a focus on zinc therapy. Orphan Drugs. 2012; 2: 35–45.
- Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front Ageing Neurosci. 2013; 5: 33. 10.3389/fnagi2013.00033.
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010; 7(4): 1342–65.
- Brewer GJ, Kanzer SH, Zimmerman EA, Molho ES, Celmins DF, Heckman SM, etal. Subclinical zinc deficiency in Alzheimer's disease and Parkinson's disease. Am J Alzheimers Dis Other Demen. 2010; 25(7): 572–5.
- Brewer GJ, Kaur S. Zinc deficiency and zinc therapy efficacy with reduction of serum free copper in Alzheimer's disease. Int J Alzheimers Dis. 2013; 2013: 586365.
- Ranjbar E, Kasaei MS, Mohammad-Shirazi M, Nasrollahzadeh J, Rashidkhani B, Shams J, etal. Effects of zinc supplementation in patients with major depression: a randomized clinical trial. Iran J Psychiatry. 2013; 8(2): 73–9.
- Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, etal. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin Nutr. 2014; 33: 415–20. 10.1016/j.clnu.2013.10.001.
- Golabek T, Darewicz B, Borawska M, Socha K, Markiewicz R, Kudelski J. Copper, zinc, and Cu/Zn ratio in transitional cell carcinoma of the bladder. Urol Int. 2012; 89(3): 342–7.
- Mao S, Huang S. Zinc and copper levels in bladder cancer: a systematic review and meta-analysis. J Trace Elem Res. 2013; 153(1–3): 5–10.
- Rasmussen HM, Johnson EJ. Nutrients for the aging eye. Clin Interv Aging. 2013; 8: 741–8.
- Visharanathan R, Chung M, Johnson EJ. A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54(6): 3985–98.
- Aliani M, Udenigure CC, Girgih AT, Povenall TL, Bugera JL, Eskin MN. Zinc deficiency and taste perception in the elderly. Crit Rev Food Sci Nutr. 2013; 53(3): 245–50.
- Bao B, Prasad AS, Beck FW, Snell D, Suneya A, Sarkar FH, etal. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cells disease patients. Transl Res. 2008; 152(2): 67–80.
- Arnold CR, Wolf J, Brunner S, Heindler D, Gurbeck B. Gain and loss of T cells subsets in old-age-related reshaping of the T cell repertoire. J Clin Immunol. 2011; 31(2): 137–46.
- Aw D, Palmer DB. It's not all equal: a multiphasic theory of thymic involution. Biogerontology. 2012; 13(1): 77–81.
- Henson SM, Akbar AN. Memory T cell homeostasis and senescence during aging. Adv Exp Med Biol. 2010; 684: 187–97.
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Löfgren S, etal. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005; 60: 556–65.
- Wikby A, Nilsson BD, Forsey R, Thompson J. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006; 127: 695–704.
- Romero AJ, Amores L, Fernández E. Immunosenescence and frailty: a current glance. Med Int Mex. 2013; 29(6): 605–11.
- Pawelec G. Hallmarks of human “immunosenescence”: adaptation or dysregulation?. Immun Ageing. 2012; 9(1): 15.
- Le Saux S, Weyand CM, Goranzy JJ. Mechanisms of immunosenescence: lessons from models of accelerated immune aging. Ann N Y Acad Sci. 2012; 1247: 69–82.
- Sada-Ovalle I, Gorocica P, Lascurain R, Zenteno E. Immunological aspects of aging. Rev Inst Nac Enf Resp Mex. 2004; 17: 293–300.
- Gruver A, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007; 211: 144–56.
- Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E. The impact of CMV infection on survival of older humans. Curr Opin Immunol. 2012; 24(4): 507–11.
- Wills M, Akbar A, Beswick M, Bosch JA, Caruso C, Colonna-Romano G, etal. Report from the second cytomegalovirus and immunosenescence workshop. Immun Ageing. 2011; 8: 10. 10.1186/1742-4933-8-10.
- Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and HIV-mediated immunosenescence. Aging Dis. 2011; 2(5): 382–97.
- Lindstrom TM, Robinson WH. Rheumatoid arthritis: a role for immunosenescence?. J Am Geriatr Soc. 2010; 58: 1565–75.
- Candore G, Caruso C, Jirillo E, Magrone T, Vasto S. Low grade inflammation as a common pathogenic denominator in age-related diseases: novel drug targets for anti-aging strategies and successful ageing achievement. Curr Pharm Des. 2010; 16(6): 584–96.
- Hubbard RE, O'Mahoney MS, Calver BL, Woodhouse KW. Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008; 64: 895–900.
- Cannizo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflam-aging and immunosenescence. J Proteomics. 2011; 74(11): 2313–23.
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflam-aging. Curr Pharm Des. 2009; 15(26): 3003–26.
- De la Fuente M. Role of the immune system in aging. Inmunology. 2008; 27(4): 176–91.
- Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Bacalini MG, etal. Immune system, cell senescence, aging and longevity: inflam-aging reappraised. Curr Pharm Des. 2013; 19: 1675–9.
- Franceschi C, Capri M, Monti D, Giunta S, Oliveiri F, Serrini F, etal. Inflammaging and anti-inflammaging: a systematic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007; 128: 92–105.
- Lang PO, Mitchell WA, Lapenna A, Pitts D, Aspinall R. Immunological pathogenesis of main age-related diseases and frailty: role of immunosenescence. Eur Geriatr Med. 2010; 1: 112–21.
- Nishida K. New knowledge from past decade: role of zinc in immune system. Nihon Eiseigaku Zasshi. 2013; 68(3): 145–52.
- Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res. 2012; 56(1): 77–87.
- Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009; 12(6): 646–52.
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006; 20(1): 3–18.
- Muñoz C, Vázquez C, De Cos AI, Gómez C, Reuss JM. Alimentación en el anciano sano. Manual de recomendaciones nutricionales en pacientes geriátricos. 2004; Madrid: Novartis Consumer Health, SA. 99–106.
- Trinch C, Morley JE, Thomas DR. Immunity and nutrition. Geriatric nutrition. Boca Ratón. 2007; FL: CRC Press. 69–102.
- Doets EL, Cavelaars AE, Dhonukshe-Rutten RA, van't Veer P, de Groot LC. Explaining the variability in recommended intakes of folate, vitamin B12, iron and zinc for adults and elderly people. Public Health Nutr. 2012; 15(5): 906–15.
- Joshi S, Morley JE, Pathy J, Sinclair AJ, Morley JE. Vitamins and minerals in the elderly. Principles and practice of geriatric medicine. 2006; 4th ed, Chichester, West Susex: Wiley. 329–46.
- Madej D, Borowska K, Bylinowska J, Szybalska A, Pietruszka B. Dietary intakes of iron and zinc assessed in a select group of the elderly: are they adequate?. Rocz Panstw Zakl Hig. 2013; 64(2): 97–104.
- Vasto S, Mocchegiani E, Malavolta M, Cuppari I, Listi F, Nuzzo D, etal. Zinc and inflammatory/immune response in ageing. Ann N Y Acad Sci. 2007; 1100: 111–22.
- Roohani N, Huvell R, Kelishadi R, Schulin R. Zinc and its importance for human health: an integrative review. J Res Med Sci. 2013; 18(2): 144–57.
- Kosaka K, Yamashita S, Ando C, Endo Y, Taniguchi K, Kikunaga S. Relationships among body mass index, activities of daily living and zinc nutritional status in disabled elderly patients in nursing facilities. J Nutr Sci Vitaminol (Tokyo). 2013; 59(5): 420–30.
- Giacconi R, Malavolta M, Costarelli L, Busco F, Galeazzi R, Bernardini G, etal. Comparison of intracellular zinc signals in nonadherent lymphocytes from young and elderly donors: role of zinc transporters (Zip family) and proinflammatory cytokines. J Nutr Biochem. 2012; 23(10): 1256–63.
- Mocchegiani E, Costarelli L, Basso A, Giacconi R, Piacenza F, Malavolta M. Metallothioneins, ageing and cellular senescence: a future therapeutic target. Curr Pharm Des. 2013; 19(9): 1753–64.
- Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009; 29(4): 416–25.
- Prasad AS. Zinc: mechanisms of host defense. J Nutr. 2007; 137(5): 1345–9.
- Mocchegiani E, Malavolta M, Costarelli L, Giacconi R, Piacenza F, Lattanzio F, etal. Is there a possible single mediator in modulating neuroendocrine–thymus interaction in aging?. Curr Aging Sci. 2013; 6(1): 99–107.
- Hirano T, Murakami M, Fukada T, Yamasaki S, Susuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008; 97: 149–76.
- Yu M, Lee WW, Tomar D, Pryschep S, Czesnikiewicz M, Lamar DL, etal. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011; 208(4): 775–85.
- Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc and signaling in health and diseases: zinc signaling. J Biol Inorg Chem. 2011; 16(7): 1123–34.
- Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals. 2009; 22(1): 149–57.
- Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009; 29: 133–52.
- Morgan CI, Ledford JR, Zhou P, Page K. Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. J Inflamm (Lond). 2011; 8: 36. 10.1186/1476-9255-8-36.
- Kriskova S, Ryvolona M, Hrabeta J, Adam V, Stiborova M, Eckschlager T, etal. Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metab Rev. 2012; 44(4): 287–301.
- Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A-20-mediated inhibition of nuclear factor-κB. Nutrition. 2011; 27(7–8): 816–23.
- Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013; 42: 443–68.
- Liu MJ, Bao B, Gálvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlowicz RE, etal. ZIP 8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013; 3(2): 386–400.
- Balistreri C, Candore G, Accardi G, Colonna-Romano G, Lio D. NF-κB pathway activators as potential ageing biomarkers: targets for new therapeutic strategies. Immun Ageing. 2013; 10: 24–40.
- Sharma S, Rais A, Sandhu R, Nel W, Ebadi M. Clinical significance of metallothioneins in cell therapy and nanomedicine. Int J Nanomed. 2013; 8: 1477–88.
- Knoell DL, Liu MJ. Impact of zinc metabolism on innate immune function in the setting of sepsis. Int Z Vitam Ernahrungsforsch Beih. 2010; 80(4–5): 271–7.
- Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007; 20(3–4): 579–85.
- Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007; 28(1): 1–4.
- Tsou TC, Chao HR, Yeh SC, Tsai FY, Lin HJ. Zinc induces chemokine and inflammatory cytokine release from human promonocytes. J Hazard Mater. 2011; 196: 335–41.
- Ibs RH, Rink L. Zinc-altered immune function. J Nutr. 2003; 133(5 Suppl 1): 1452–65.
- Gruber K, Maywald M, Rosenbranz E, Haase H, Plumakers B, Rink L. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. J Biol Regul Homeost Agents. 2013; 27(3): 661–71.
- Suwendi E, Iwaya H, Lee JS, Hara H, Ishizuka S. Zinc deficiency induces dysregulation of cytokine productions in an experimental colitis of rats. Biomed Res. 2012; 33(6): 329–36.
- Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012; 4(7): 676–94.
- Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, etal. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NK-κB. Am J Physiol Lung Cell Mol Physiol. 2010; 298(6): L744–54.
- Wong CP, Magnusson KR, Ho E. Increased inflammatory response in aged mice is associated with age-related zinc deficiency and zinc transporter dysregulation. J Nutr Biochem. 2013; 24(1): 353–9.
- Prasad AS, Beck FWJ, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, etal. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative estrés. Am J Clin Nutr. 2007; 85: 837–44.
- Meydani SN, Barnett JB, Dollal GE, Fine BC, Jacques PF, Leka LS, etal. Serum zinc and pneumonia in nursing home elderly. Am J ClinNutr. 2007; 86: 1167–73.
- Bao B, Prasad AS, Beck F, Fitzgerald JT, Snell D, Bao CW, etal. Zinc decreases C-reactive protein, lipid peroxidation and inflammatory cytokines in elderly subjects. A potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010; 91: 1634–41.
- Guo CH, Wang CL. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int J Med Sci. 2013; 10(1): 79–89.
- Hamza SA, Mousa SM, Taha SE, Adel LA, Samaha HE, Hussein DA. Immune response of 23-valent pneumococcal polysacharide vaccinated elderly and its relation to frailty indices, nutritional status and serum zinc levels. Geriatr Gerontol Int. 2012; 12(2): 223–9.
- Yalçın SS, Engür-Karasimav D, Alehan D, Yurdakök K, Ozkutlu S, Coşkun T. Zinc supplementation and TNF-α levels in vaccinated cardiac patients. J Trace Elem Med Biol. 2011; 25(2): 85–90.
- Afsharian M, Vaziri S, Janbakhsh AR, Sayad B, Mansouri F, Nourbakhsh J, etal. The effect of zinc sulfate on immunologic response to recombinant hepatitis B vaccine in elderly: zinc sulfate and immunologic response to recombinant hepatitis B vaccine. Hepat Mon. 2011; 11(1): 32–5.
- Das JK, Kumar R, Salam RA, Bhutta ZA. Systematic review of zinc fortification trials. Ann Nutr Metab. 2013; 62(Suppl 1): 44–56.
- Penny ME. Zinc supplementation in public health. Ann Nutr Metab. 2013; 62(Suppl 1): 31–42.
- Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012; 3(1): 91–129.
- Sheqwara J, Alkhatib Y. Sideroblastic anemia secondary to zinc toxicity. Blood. 2013; 122(3): 311.
- Kuyumcu ME, Yesil Y, Ozturk ZA, Cankurtaran M, Ulger Z, Halil M, etal. An alternative way for the evaluation of zinc status in the elderly; nail zinc levels and relationship with Alzheimer's disease. Eur Rev Med Pharmacol Sci. 2013; 17(11): 1467–71.
- Mocchegiani E, Basso A, Giacconi R, Piacenza F, Costarelli L, Pierpaoli S, etal. Diet (zinc)–gene interaction related to inflammatory/immune response in ageing: possible link with frailty syndrome?. Biogerontology. 2010; 11(5): 589–95.