Abstract
Lung cancer is generally difficult to detect until the late stages of disease, when it is much more difficult to treat because of the more aggressive and invasive behavior. Advanced lung cancer is much more common in older adults making it even more challenging to treat. Adenocarcinoma belongs to a category of non-small cell lung cancers, which comprise up to 40% of all lung cancers, and about half of these have an activating K-ras mutation. Because treatment relapses are common, more effective unconventional treatment and prevention methods are needed. In this regard, the antioxidant enzyme catalase targeted to mitochondria (mCAT) has been shown to delay aging and cancer in mice, and the progression of transgenic oncogene and syngeneic tumors was suppressed, helping support the notion that attenuation of mitochondria-generated hydrogen peroxide signaling is associated with an antitumor effect. In order to determine if mCAT has any effect on naturally occurring lung cancer of the adenocarcinoma type in old mice, the tumor incidence and progression were examined in the lungs of old mCAT transgenic and wild-type (WT) mice with a CB6F1 (Balb/c X C57BL/6) background. CB6F1 mice with a WT genotype were found to have a high incidence of adenomas at 24 months of age, which progressed to adenocarcinomas at 32 months of age. CB6F1 mice with the mCAT genotype had significantly reduced incidence and severity of lung tumors at both ages. Fibroblasts isolated from the lungs of old mCAT mice, but not WT mice, were shown to secrete soluble factors that inhibited lung tumor cell growth suggesting that stromal fibroblasts play a role in mediating the antitumor effects of mCAT. The aged CB6F1 mouse, with its high incidence of K-ras mutant lung cancer, is an excellent model to further study the anticancer potential of mitochondria-targeted therapy.
Lung cancer is a devastating disease that is generally diagnosed in the late stages of tumor progression and is the leading cause of death from cancer in both men and women in the United States. Smoking is the strongest risk factor for developing lung cancer, but the insidious and protracted nature of the disease makes it a condition routinely diagnosed in older individuals. Therefore, factors associated with aging, such as the senescence-associated secretory phenotype that contributes to tumorigenesis (Citation1), must be considered in the pathogenesis and progression of the disease. Epithelial lung tumors are classified pathologically as either non-small cell lung cancer (NSCLC) or small cell lung cancer. Adenocarcinomas are NSCLC, comprising 30–40% of all lung cancers (Citation2). The extent of disease is determined by radiological exams such as magnetic resonance imaging or positron emission tomography and by lymph node biopsy to assess degree of metastasis. The result is a staging system used to stratify patients into treatment categories and to predict survival, with stage 1 being least severe and stage 4 being most severe. Chemotherapy and radiation therapy are standard treatments, but only less than 50% of stage 1 patients live after 5 years, whereas only less than 5% of stage 4 patients survive after 5 years.
A search for more effective unconventional treatment and prevention methods is clearly warranted and could be tailored to older patients. In this regard, we have previously reported that catalase targeted to mitochondria of older mice is able to delay the progression of spontaneous, transgenic, and syngeneic tumors suggesting attenuation of mitochondria-generated hydrogen peroxide signaling associated with the antitumor effect (Citation3–Citation5). These studies have suggested a role of tumor stromal cells in supporting growth and invasiveness for the devastating effects of tumor cell progression. Secretory factors such as IL6 and IL8 have been shown to be released from senescent fibroblasts that support tumor growth (Citation1). Therefore, new treatment strategies should consider the tumor microenvironment, including stromal fibroblasts, as a therapeutic target.
The testing of novel therapeutic approaches and screening strategies for lung cancer can be expedited by murine models that share some of the same molecular pathways involved in the development of lung cancer. Patient-derived tumor xenografts provide access to human cancer in immune-deficient mice but have a mixture of mouse and human stromal cell populations. Transgenic strains which express viral and cellular oncogenes, as well as chemically induced lung tumor development, provide many of the same genetic mutations as seen in human lung cancer but are generally conditions seen only in young animals. Therefore, spontaneously occurring lung cancer that develops in an age-dependent manner would be ideal as a model to study therapeutics aimed at the tumor microenvironment. Some strains of mice have a high rate of lung cancer. Moderate incidence is typical of Balb/c mice, while C57BL/6 mice have low incidence. The cross between C57BL/6 and Balb/c produces the CB6F1 hybrid cross. Similar to the Balb/c parenteral strain, CB6F1 mice have a moderate incidence of lung cancer. The cancer phenotype is adenoma and adenocarcinoma driven by a polymorphism in the second intron of K-ras (Citation6). About half of human lung adenocarcinomas contain mutations that activate K-rasCitation7. Therefore, the CB6F1 mouse might serve as a model of human lung adenocarcinoma, except that the majority of murine lung tumors have been reported histologically as early lesions such as hyperplasia and adenomas. In this paper, we describe some anatomic and molecular features of spontaneously occurring lung cancer in CB6F1 mice showing that very old mice do develop adenocarcinomas, and progression can be suppressed by stromal fibroblasts expressing mCAT.
Methods
Animals
CB6F1 wild-type (WT) mice were obtained from the NIA contract facility (Charles River) at age cohorts of 8, 18, 24, and 32 months. CB6F1 mice of both genders expressing mitochondrial catalase (mCAT) were generated by crossing WT Balb/c males with mCAT C57BL/6J females and followed until end of life, which ranged from 21 to 38 months. The mCAT founder line was originally generated in our laboratory and backcrossed onto the C57BL/6J background (Jackson Laboratories, Bar Harbor, ME) for more than 10 generations. The line purity was confirmed by analysis of genetic markers (Charles River Laboratories, Wilmington, MA). NSG immune-deficient mice were obtained from JAX and used as recipients for the LKR13 lung tumor cell line.
Animals were group housed (4–5 per cage, separate sexes) in ventilated cages containing Bed-O-Cob (Andersons, Maumee, OH) in a specific pathogen-free facility at the University of Washington. Mice were fed irradiated Picolab Rodent Diet 20 number 5053 (PMI Nutrition International, Brentwood, MO) and provided reverse osmosis water. All supplies entering animal rooms were autoclaved, and rooms were maintained at 70–74°F, 45–55% humidity, with 28 air changes per hour and a 12-h light/dark cycle. The University of Washington Animal Care and Use Committee approved all animal procedures.
Cell cultures
Fibroblasts were aseptically collected from the lungs of mice at 4–6 months and 24–28 months of age by sterile dissection and mincing and then washed with PBS and serum-free DMEM, placed in sterile petri dishes and mechanically dissociated and transferred to separate sterile petri dishes and DMEM with 20% FBS and 1% penicillin/streptomycin (Gibco/BRL Life Tech, Carlsbad, CA). Cells were then placed in an incubator at 37°C with 5% CO2. Medium was replaced every 2–3 days until cells were near confluence. After confluence, cells were trypsinized and passaged onto multiple petri dishes and fed DMEM with 10% FBS and 1% penicillin/streptomycin. Once cells from the first passage became confluent, they were trypsinized, passaged, and frozen with freezing medium (DMEM with 20% FBS and 10% DMSO) and stored in liquid nitrogen. Fibroblasts were identified by typical morphology and were vimentin (mesenchymal cell marker) positive and F4/80 (macrophage marker) negative.
LKR13-activated K-ras mouse lung adenocarcinoma cells were obtained from Dr. Jonathan Kurie, University of Texas at Dallas, and cultured under similar conditions.
Cell viability
Cells were plated in DMEM with 10% FBS and 1% Pen-Strep (Gibco/BRL Life Tech, Carlsbad, CA) in 96-well plates. Cell Proliferation Reagent WST-1 (BD Biosciences, Mountain View, CA) was added to each well, and cells were put back in the incubator for another 1.5 h. Plates were then shaken thoroughly on a shaker, and the absorbance of each of the wells was measured using a microplate reader at 450 nm. Wells without cells were used as a blank, and average absorbance standardized relative to this value. Viability was recorded as percent viable cells.
Cytokine assessment
Conditioned medium (CM) was collected from fibroblast cultures after 48 h with serum-free medium and tested in a Luminex 38-plex cytokine array by the Human Immune Monitoring Center at Stanford University.
Histochemistry
Fixed cells were stained with primary antibody specific for p21 (Novus Biologicals, NBP2-29463), p53 (Novus Biologicals, NB200-103), vimentin (Novus Biologicals, NB300-223), F4/80 (Novus Biologicals, NB600-404), or SMA alpha (Novus Biologicals, NB600-531), followed by a secondary antibody conjugated with chromogenic substrate 3,3’-diaminobenzidine (Santa Cruz Biotechnology). Beta galactosidase activity was assessed in viable cells with X-gal substrate (Cell Biolabs, Inc.) and subsequent staining.
Histopathology
Tissues were collected in formalin. Formalin-fixed tumors and lungs were processed routinely and stained with Hematoxylin and Eosin (H&E). Tumor tissues stained with H&E were assessed for morphology and general appearance. Tumor grade was established on the basis of 0=no presence of tumor; 1=low number of abnormal appearing cellular features with some distortion of cellular boundaries and some increased mitotic activity; 2=Mildly increased number of abnormal appearing cellular features with increased distortion of cellular boundaries and mildly increased mitotic activity; 3=Moderately increased number of abnormal appearing cellular features with significantly increased distortion of cellular boundaries and mitotic activity, and evidence of capsular penetration; 4=Severe increase in the number of abnormal appearing cellular features with a highly significant increase in distortion of cellular boundaries and mitotic activity, and frank capsular penetration and invasion of surrounding tissue. Grades 1 and 2 were considered more benign and labeled as adenomas, whereas grades 3 and 4 were malignant and invasive adenocarcinomas.
Statistical analysis
Student's t-test was used to detect significant differences between groups for the following dependent variables: percent increase of tumor cell viability, tumor volume, immunostaining intensity, and tumor marker labeling index. Pearson's correlation analysis was used to assess relationships between mCAT mice and WT controls. Prism (Graph Pad, Version 5) was used to perform the statistical analyses. All results are presented as means±standard deviation unless stated otherwise. p value of<0.05 was used to indicate a significant difference.
Results and discussion
Lung adenomas progress to adenocarcinomas in an age-dependent manner in CB6F1 mice
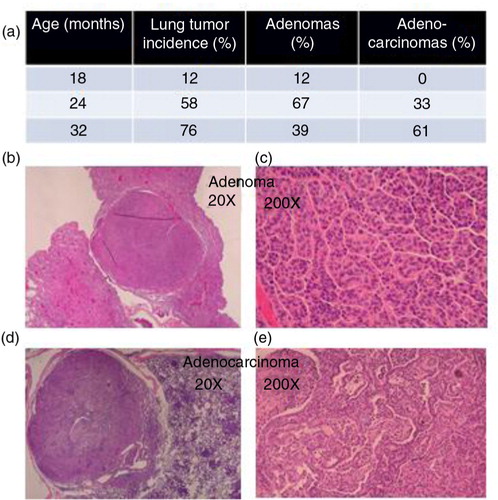
WT CB6F1 male mice at 18, 24, and 32 months of age were observed at necropsy and follow-up histopathology for evidence of lung tumors. Very few adenomas (12%) and no adenocarcinomas were seen at 18 months of age (a). The appearance of lung tumors in a large number of mice occurred between 18 and 24 months of age with an increase from 12 to 58%. The majority (67%) were benign adenomas (b and c), whereas 43% were adenocarcinomas (d and e). The incidence of lung tumors increased between 24 and 32 months of age from 58 to 76%, but the predominant tumor type was adenocarcinoma at 61% compared with 39% for adenomas. Therefore, CB6F1 mice develop lung cancer of the adenocarcinoma type similar to humans, but it occurs very late in old mice.
Fig. 1 (a) Lung adenomas progress to adenocarcinomas in male CB6F1 mice (N=18–22) in an age-dependent manner. Adenomas are characterized by a well-encapsulated clump (b) of moderately disorganized epithelial cells with features resembling normal formational architecture (c). Adenocarcinomas are characterized by invasive capsular penetration (d) and a highly disorganized cellular configuration with extensive mitotic activity (e).
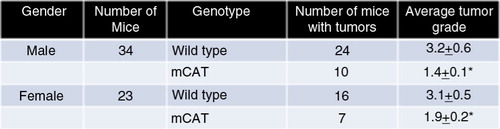
CB6F1 mice expressing mCAT have decreased incidence and severity of lung tumors at end of life
When CB6F1 mice in a lifespan study were observed at the end of life, 10 of 34 males expressing mCAT had lung tumors with an average grade of 1.4±0.1, whereas 24 of 34 WT males had lung tumors with an average grade of 3.2±0.6, p≤0.01 (). The incidence was less in females but there was still a significant difference between females expressing mCAT and WT females (1.9±0.2 vs. 3.1±0.5, respectively, p≤0.05). Therefore, expression of catalase targeted to mitochondria can prevent the progression of adenoma to adenocarcinoma in the lungs of CB6F1 mice.
Expression of mCAT in lung fibroblasts from old mice enhances a senescence phenotype and secretion of soluble antitumor factors
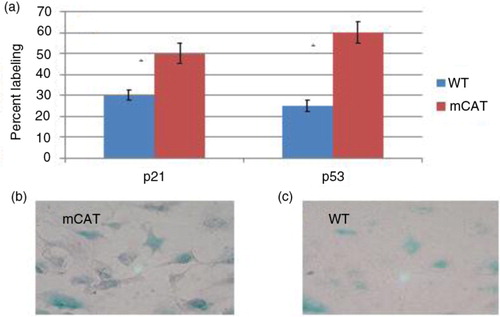
Since both tumor cells and stromal cells expressed catalase in the mCAT transgenic mice in the lifespan study, it was of interest to determine the mechanism and cell type contributing to the tumor cell resistance. Because a decreased incidence and severity of syngeneic tumors was seen in mCAT-positive recipients in a previously published report (Citation8), there was a possibility that stromal cells could be playing a major role in suppressing lung tumor growth. In an attempt to determine if stromal fibroblasts were involved, these cells were isolated from the lungs of old WT mice or old mice expressing mCAT and used as primary cultures to assess phenotype and activation status. The expression of the tumor suppressor proteins p21 and p53 was increased in lung fibroblasts from mice expressing mCAT compared with lung fibroblasts from WT mice (a). These proteins are commonly associated with a senescence phenotype. In order to provide additional evidence of a senescence phenotype, cells were stained with beta galactosidase, with mCAT-positive fibroblasts staining more intensely (c) than WT fibroblasts (b). This observation suggests that the enhanced senescence seen in mCAT-positive fibroblasts may be associated with antitumor activity. However, published studies have shown that senescent fibroblasts are capable of secreting soluble factors that actively support tumor growth (Citation1).
Fig. 3 (a) Lung fibroblasts from old mice expressing mCAT have increased labeling of p21 and p53 compared with lung fibroblasts from old WT mice, p≤0.02. Lung fibroblasts from old mice expressing mCAT have increased labeling for beta galactosidase (c) compared with lung fibroblasts from old WT mice (b), p≤0.05.
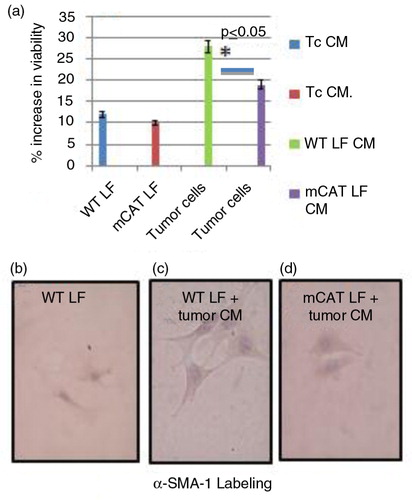
Experiments were therefore conducted to see if the presence of mCAT had any effect on secretion of factors in CM from fibroblast cultures that might have antitumor activity. When CM from LKR13 lung tumor cells was added to lung fibroblast cultures, there was no difference in activation of lung fibroblasts from mice expressing mCAT compared with lung fibroblasts from WT mice (a). When CM from lung fibroblast cultures from old mCAT and WT mice was added to mouse LKR13 lung tumor cells, the viability and cell division of the tumor cells were decreased with mCAT lung fibroblast CM but not with WT lung fibroblast CM (a). There was no difference in staining intensity for SMA alpha1, a marker for tumor-associated fibroblasts, between mCAT and WT lung fibroblasts when cultured with LKR13 tumor cell CM (c and d) suggesting that mCAT has no effect on the ability of tumor cell signaling to convert stromal fibroblasts to a ‘cancer-associated fibroblast’ phenotype. However, the expression of mCAT in lung fibroblasts from old mice appears to trigger and/or enhance the secretion of antitumor factors, possibly in a p53-dependent manner.
Fig. 4 (a) There was no difference in activation of lung fibroblasts from mice expressing mCAT compared with lung fibroblasts from WT mice when conditioned medium (CM) from LKR13 lung tumor cells was added to respective lung fibroblast cultures. CM from lung fibroblasts from old mCAT mice but not old WT mice suppressed tumor cell viability, *p≤0.05 for average percent activity of three different experiments. WT and mCAT lung fibroblasts cultured with tumor cell CM stained with increased but similar intensity (c and d respectively) compared with lung fibroblasts cultured with control medium (b).
In order to see if there was any difference in cytokine secretion in CM from lung fibroblast cultures from old WT mice compared with lung fibroblast cultures from old mCAT mice, a 26-panel cytokine assay (Stanford University) was used to test CM from 48 h growth cultures in serum-free media. Interestingly, CM from lung fibroblasts from old mCAT mice had significantly increased levels of IL6, CCL2, CCL7, and VEGF, compared with CM from lung fibroblasts from old WT mice. These are generally considered as growth factors, so the significance seems paradoxical. However, it is possible that these are capable of decreasing tissue hypoxia, thus, shutting down HIF1 alpha-driven tumor growth (Citation9). We have shown that HIF1 alpha is down-regulated in mCAT-positive mice with oncogene-driven breast cancer (Citation10). It must be pointed out that senescent mouse fibroblasts grown in 20% oxygen may undergo senescence with a different kinetics and secrete different sets of soluble factors compared with those grown in 3% oxygen and induced to senescence by irradiation as described by Coppé et al. (Citation1). Therefore, further in vitro studies of antitumor mechanisms of mCAT may require different cultural condition.
Lung fibroblasts expressing mCAT have in vivo antitumor activity
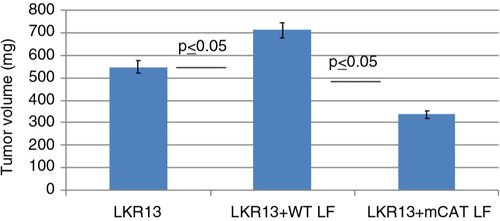
Lung fibroblasts from old WT or mCAT-positive mice were coinjected with the mouse lung tumor cell line, LKR13, into immune-deficient NSG mice as a way to determine if mCAT-positive lung fibroblasts could affect in vivo tumor cell progression. NSG mice were used because they are depleted of macrophages and NK cells, which allowed a more stringent environment for studying fibroblast activity. Mice receiving mCAT-positive LF had decreased tumor burden compared with mice receiving WT LF suggesting that stromal fibroblasts expressing mCAT can prevent or delay tumor cell growth ().
Fig. 5 Lung fibroblasts expressing mCAT have in vivo antitumor activity. Five times 10 to the third lung fibroblasts, from either WT or mCAT mice, were mixed with five times 10 to the fifth LKR13 lung tumor cells, and the comixture was injected into the right flank of 4-month-old male NSG mice. An additional cohort of NSG mice was injected with LKR13 tumor cells only. Mice were euthanized 3 weeks later and tumors assessed for volume to determine the tumor burden.
In conclusion, lung fibroblasts from mice with a senescence phenotype were programmed to secrete soluble factors capable of suppressing tumor cell growth by using the human antioxidant catalase transgenically expressed in mitochondria. The antitumor activity seen in naturally occurring lung cancer in old CB6F1 mice appears to be driven by increased expression of p53 and increased secretion of soluble antitumor factors. This could likely explain some of the antitumor effects by as yet unidentified factors, but the increase in concentrations of protumor growth cytokines in CM from old mCAT lung fibroblasts was unexpected. It is possible that these cytokines may be involved in signaling pathways that indirectly suppress tumor cell growth such as by increased oxygenation and down-regulation of HIF1 alpha. Mitochondria-targeted therapy with antioxidant enzymes or mimetics in tumor stromal fibroblasts should be considered in further studies to develop more effective treatments for lung cancer in older people.
Conflict of interest and funding
This work was supported by R21 CA 140916, NIH/NCI and P01 AG01751 and P30 AG013280, NIH/NIA.
References
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010; 5: 99–118. doi: http://dx.doi.org/10.1146/annurev-pathol-121808-102144.
- Travis WD, Linder J, Mackay B. Pass HI, Mitchell JB, Johnson DH, Turrisi AT, Minna JD. Classification, histology, cytology and electron microscopy. Lung cancer: principles and practice. 2000; Philadelphia, PA: Lippincott Williams & Wilkins. 453–95.
- Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, etal. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008; 63(8): 813–22. [PubMed Abstract].
- Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C, etal. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer. 2011; 11: 191. doi: http://dx.doi.org/10.1186/1471-2407-11-191 [PubMed Abstract] [PubMed CentralFull Text].
- Fatemie S, Goh J, Pettan-Brewer C, Ladiges W. Breast tumors in PyMT transgenic mice expressing mitochondrial catalase have decreased labeling for macrophages and endothelial cells. Pathobiol Aging Age Relat Dis. 2012; 2: 17391–3. doi: http://dx.doi.org/10.3402/pba.v2i0.17391.
- Tuveson DA, Jacks T. Modeling human lung cancer in mice: similarities and shortcomings. Oncogene. 1999; 18(38): 5318–24. [PubMed Abstract].
- Malkinson AM. Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp Lung Res. 1998; 24(4): 541–55. [PubMed Abstract].
- Goh J, Tsai J, Bammler TK, Farin FM, Endicott E, Ladiges WC. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS One. 2013; 8(11): e80123. doi: http://dx.doi.org/10.1371/journal.pone.0080123 [PubMed Abstract] [PubMed CentralFull Text].
- Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, etal. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015; 107(5): pii: djv040. doi: http://dx.doi.org/10.1093/jnci/djv040.
- Goh J, Kirk EA, Lee SX, Ladiges WC. Exercise, physical activity and breast cancer: the role of tumor-associated macrophages. Exerc Immunol Rev. 2012; 18: 158–76. [PubMed Abstract].