Great leaps forward in our understanding of the basic biology of aging, including interventions that extend longevity, have come about from using common laboratory animal models. As we now strive to apply these findings for human benefit, a serious concern arises in how much of this research will directly translate to normal, largely healthy, and genetically varied populations of people. Laboratory animals, including rodents, are only distantly related to humans and have undergone different evolutionary pressures that likely have driven species-specific idiosyncrasies of aging. Due to our long lifespans, any outcomes of longevity interventions in human studies are unlikely to be discovered even during the research careers of current graduate students. There is then strong rationale for testing whether the interventions discovered that slow aging in laboratory rodents, such as dietary restriction, mTOR (mechanistic target of rapamycin)inhibition, or acarbose (Citation1–Citation3), will also extend the lifespan of species more closely related to humans. In this context, the calorie restriction studies utilizing non-human primates and performed by the University of Wisconsin and the National Institute on Aging are prime examples of this approach. However, the rhesus macaques used in these studies also have relatively long lifespans which required time commitment in the order of decades to accomplish the recently published final results (Citation4–Citation6).
Most non-human primates that can be kept in healthy laboratory populations have relatively long lifespans, but the small South American common marmoset (Callithrix jacchus) may offer a number of advantages over other non-human primate species, particularly for researchers interested in aging.
Short lifespan
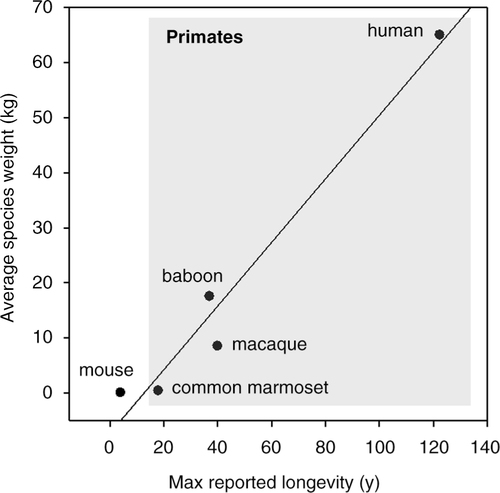
The normal lifespan of the common marmoset is the shortest of any anthropoid primate, with an average lifespan in captivity of approximately 7–8 years and maximum lifespans reported between 16 and 21 years (Citation7–Citation9). While much longer-lived than rodents, the average age of marmosets is more manageable for a designed longevity study than the 25-year average lifespan of rhesus macaques or the 70-plus average lifespan of humans (see comparison in ). In addition, marmosets in a closed colony have a natural adult mortality that drives a decline in their cumulative survival rate from about 85 to 35% that occurs between 5 and 10 years of age (Citation8). In other words, a carefully designed intervention study could occur over the time course of a single NIH R01 granting period using this non-human primate.
Size and husbandry
Marmosets are relatively small (averaging 300–500 g in body weight) compared with other primates and can be maintained as breeding pairs and family units similar to what would be found in the wild. Moreover, their small size allows for the maintenance of a relatively small vivarium footprint which is more in line with rodent research. From a husbandry standpoint, marmosets may be more amenable to staff in charge of animal care procedures due to their small size and relatively docile nature in comparison with other primate species. In addition, because of their relatively short lifespan, it is much more likely that the vivarium, husbandry, and personnel required for marmoset care can be maintained consistently throughout the course of aging studies, meaning increased likelihood of replicable longitudinal assessments of physiology, behavior, etc.
Relevance to human disease and aging
Similar to other non-human primates, the sequenced marmoset genome has high homology (>93%) with that of humans. Many of the common molecular biology tools, including antibodies, have relatively good cross-species recognition (Citation10). Marmosets have a growing track record as a non-human primate model used for a number of diseases and pathologies that are generally considered as age-related, including Parkinson's disease, respiratory diseases, and infectious diseases. Moreover, marmosets display age-related changes in pathologies associated with diabetes, cardiac disease, cancer, and renal disease similar to those seen in humans (Citation8, Citation9). Marmosets thus represent a complement to the existing non-human primate models used to study aging and, in particular, a model in which effects on longevity might be assessed in a relatively timely manner.
Despite this promising outlook, there are some potential challenges to using the common marmoset as a non-human primate model to study aging. Like other non-human primates, there is much less genetic tractability in this species relative to the mouse, which must be taken into account when designing studies on the biology of aging. However, transgenic marmosets have been previously generated (Citation11) and new technologies including CRISPR/Cas systems may lead the way in developing new, genetically modified marmoset models for the study of age-related diseases or the basic biology of aging. Pertinent to the audience of this journal, large-scale, careful pathological assessments of causes of death and the rate of progression of disease need to be performed to compare to what is known about the pathology of disease progression and mortality in mouse strains. Finally, despite being much more closely related to humans than rodents, marmosets are a New World monkey species which diverged from the Old World monkey species (including humans) 26 to 43 million years ago. All but the last issue can be dealt with by using different scientific approaches.
In summary, there are a growing number of studies addressing aging and age-related diseases using the common marmoset including intervention studies such as dietary restriction and inhibition of mTOR signaling (Citation12, Citation14). There is then growing hope that such studies will have significant impact as a representation of a first step in translating longevity and healthspan interventions from mice to humans.
Conflict of interest and funding
The author discloses no potential conflicts of interest. The lab of Adam Salmon is supported by a grant from the National Institute of Health (R01 AG050797), a grant from the American Heart Association (15BGIA23220016) and the Geriatric Research, Education and Clinical Center of the South Texas Veterans Health Care System. This material is the result of work supported with resources and the use of facilities at South Texas Veterans Health Care System, San Antonio, Texas. The contents do not represent the views of the US Department of Veterans Affairs or the US government.
References
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, etal. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014; 13(3): 468–77. [PubMed Abstract] [PubMed CentralFull Text].
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, etal. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014; 13(2): 273–82. [PubMed Abstract].
- Anson RM, Jones B, de Cabo R. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. Age (Dordr). 2005; 27(1): 17–25. [PubMed Abstract].
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, etal. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009; 325(5937): 201–4. [PubMed Abstract] [PubMed CentralFull Text].
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014; 5: 3557. [PubMed Abstract] [PubMed CentralFull Text].
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, etal. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012; 489(7415): 318–21. [PubMed Abstract].
- Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, Kitajima S. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology. 2012; 13(4): 439–43. [PubMed Abstract].
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J. 2011; 52(1): 54–65. [PubMed Abstract] [PubMed CentralFull Text].
- Ross CN, Davis K, Dobek G, Tardif SD. Aging phenotypes of common marmosets (Callithrix jacchus). J Aging Res. 2012; 2012: 567143. [PubMed Abstract] [PubMed CentralFull Text].
- Marmoset Genome Sequencing and Analysis Consortium. The common marmoset genome provides insight into primate biology and evolution. Nat Genet. 2014; 46(8): 850–7. [PubMed CentralFull Text].
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, etal. Generation of transgenic non-human primates with germline transmission. Nature. 2009; 459(7246): 523–7. [PubMed Abstract].
- Ross C, Salmon A, Strong R, Fernandez E, Javors M, Richardson A, etal. Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus). Aging. 2015; 7(11): 964–73. [PubMed Abstract] [PubMed CentralFull Text].
- Tardif S, Ross C, Bergman P, Fernandez E, Javors M, Salmon A, etal. Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci. 2015; 70(5): 577–87. [PubMed Abstract].
- Lelegren M, Liu Y, Ross C, Tardif S, Salmon AB. Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol Aging Age Relat Dis. 2016; 6: 31793. [PubMed Abstract].