Abstract
We report the isolation and identification of bacteria that produce extracellular cold-active proteases, obtained from water samples collected near the Uruguayan Antarctic Base on King George Island, South Shetlands. The bacteria belonged to the genera Pseudomonas (growth between 4 and 30 °C) and Flavobacterium (growth between 4 and 18 °C). In all cases, extracellular protease production was evident when reaching the stationary phase at 18 and 4 °C but was not detected at 30 °C. The zymogram revealed the secretion of one extracellular protease per isolate, each with different relative electrophoretic mobility. The extracellular proteases produced at 4 °C showed thermal activity and stability at 30 °C. Both activity and stability at a temperature higher that 10 °C have no physiological meaning because the isolates do not experience such temperatures in the Antarctic environment; however, the possible ecological value of cold-active and -stable extracellular proteases is discussed.
Proteases (also called proteinases and proteolytic enzymes) are ubiquitous proteins that catalyse the cleavage of peptide bonds of other proteins (in some cases having autoproteolytic activity) and are essential for cell growth and differentiation (Rao et al. Citation1998; Gupta et al. Citation2002).
These enzymes have a large variety of applications, for example, as an ingredient in detergents and in the industrial processing of food, leather and pharmaceutical products and account for 60% of the worldwide enzymes market (Rao et al. Citation1998). The increased use of proteases in the industry has focused attention on the discovery of novel enzymes with new properties such as low-temperature performance. This is an important trait in terms of saving energy because some industrial processes could be performed at room or tap water temperature.
Due to its biochemical characteristics, the superfamily of subtilisin-like serine proteases (subtilases) is the most important among enzymes with industrial applications. Subtilases possess a serine group in their active site that is recognized by the irreversible inhibition caused by different reagents (Rao et al. Citation1998). Extracellular cold-active serine-proteases have been identified in psychrophilic and psychrotrophic bacteria such as Pseudoalteromonas (Wang et al. Citation2008), Colwellia sp. NJ341 (Wang et al. Citation2005), Flavobacterium (Morita et al. Citation1998) and Shewanella (Kulakova et al. Citation1999), among others. Cold-active enzymes have high catalytic efficiency at low temperature due to their high flexibility and higher turnover number (kcat) at the expense of Km or by optimizing both parameters (Morgan-Kiss et al. Citation2006).
The Antarctic continent experiences severe environmental conditions such as extreme cold, broad temperature fluctuations, abrupt chemical gradients and low carbon and nitrogen supply. Antarctic habitats are regarded as ecosystems of low productivity because of their oligotrophic situation and the low temperatures to which they are exposed. In winter, the level of photosynthetically active radiation is low and energy supplies are dramatically decreased. However, during the summer season, a period of high irradiance and mild temperatures, maximal biological C- and N-fixation rates are achieved, increasing carbon and nitrogen supply to the different Antarctic environments. Although the environment is inhospitable, there is a highly diverse microbial population in Antarctic soils and water samples (Niederberger et al. Citation2008). Thus, these environments constitute a suitable place for the isolation of bacteria that produce cold-active enzymes.
In this work we report the isolation and identification of microorganisms that produce extracellular cold-active proteases from water samples collected at Fildes Peninsula (King George Island, South Shetlands), focussing on the selection of serine-protease producers because this group of proteases is the most important for industrial applications (Neklyudov et al. Citation2000; Acevedo et al. Citation2008).
Materials and methods
Sampling and isolation of extracellular protease-producing bacteria
Water samples were collected using sterile flasks from Uruguay Lake, Collins Bay and two temporary gullies near the Uruguayan Antarctic Base (62° 11′4″ S, 58° 51′7″ W) on King George Island, South Shetlands. Collections were done during the summer season and the average temperature measured in air and water was −8 and 2 °C, respectively. Samples (20 ml) were transported under refrigerated condition to the laboratory and filtered using 0.45-µm membrane filters soaked with 50-mM phosphate buffer, pH 7. Then, filters were stacked in 2-ml phosphate buffer and cellular suspensions were spread on Luria-Bertani (LB) medium (10-g/l tryptone, 5-g/l yeast extract, 10-g/l NaCl) agar plates that were incubated at 4 °C. The strains were purified by repeated streaking on the same medium. Proteolytic microorganisms were identified by streaking pure cultures on agar plates containing minimal milk (MM) medium (20-fold diluted LB supplemented with 5-g/l skim milk). The plates were incubated at 4 and 30 °C and observed daily for signs of clearing of the agar around the colonies, indicating caseinolytic activity. Positive colonies were grown in liquid LB medium and stored at −80 °C with 20% glycerol.
16S rDNA amplification
Genomic DNA was extracted using the phenol:chlorophorm procedure described by Sambrook et al. (Citation1989). The 16S rDNA was amplified using two pairs of primers (784F–1401R and 63F–907R), which amplify an overlapping fragment of 123 base pairs (bp) based on the position in Escherichia coli (primer sequences; 784F- 5′ AAA CAG GAT TAG ATA CCC 3′; 1401R- 5′ CGG TGT GTA CAA GGC CCG GGA ACG 3′; 63F- 5′ CAG GCC TAA CAC ATG CAA GTC 3′; 907R- 5′CCG TCA ATT CCT TTR AGT TT 3). Purified polymerase chain reaction products were sequenced by Macrogen Inc. (Seoul, Korea) using the dideoxy chain-termination method. A fragment of approximately 1500 bp was reconstructed for each isolate by joining both sequences using the bl2seq specialized BLAST search of the US National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/; Altschul et al. Citation1997). The 16S rDNA gene sequence similarity searches against the NCBI database were carried out using the BLAST programme and the EzTaxon website (http://eztaxon.org/; Chun et al. Citation2007). Sequences with a high degree of similarity were aligned with CLUSTAL W programme (Thompson et al. Citation1994) and corrected manually. Similarities between sequences from the same genus were analysed with the programme GeneDoc (Nicholas et al. Citation1997).
Growth rate and protease production at different temperatures
The ability of the proteolytic isolates to grow at different temperatures (4, 18 and 30 °C) was analysed in tubes filled with 5 ml of LB medium with constant shaking (200 rpm). The experiment started with an initial optical density (OD) of 0.01 (107 cells/ml) and growth was measured by determining OD at 620 nm.
Extracellular protease production was measured as follows: tubes containing 5 ml of MM medium were cultured at different temperatures (4, 18 and 30 °C; initial OD of 0.01) and were observed daily until clarification of the medium was evident (milk coagulation; caseinolytic activity). At this time colony forming units (CFU) were determined and cultures were centrifuged (14 000 rpm, 4 °C for 15 min) and the proteolytic activity was determined in the cell-free supernatant using Azocasein as a substrate (Andrews & Asenjo Citation1986). In brief, reaction mixtures containing 250 µl of 1% (w/v) Azocasein, 250 µl of 0.5-M Tris buffer (pH 7.5) and 250 µl of culture supernatant were incubated at 30 and 4 °C for 20 min. The reaction was stopped by the addition of 500 µl of 25% (w/v) trichloroacetic acid; then each tube was centrifuged at 12 000 rpm (30 min at room temperature) and the absorbance of the supernatant was read at 340 nm. One unit of enzyme activity (U) was defined as the amount of cell-free supernatant required to increase absorbance at 340 nm by one unit under the assay conditions.
Effect of temperature on protease activity and stability
Cell-free supernatants obtained after clarification of the MM medium at 4 °C were used as substrates in proteolytic activity assays as described above but at different reaction temperatures (4 and 30 °C).
Thermal protease stability in cell-free supernatants was assayed by incubation at 4 and 30 °C for 48 h and determination of residual activity was done every 12 h.
Zymogram and identification of extracellular serine proteases
Protease profiles were studied by performing zymograms using gelatine as the copolymerized substrate (Chakrabarti et al. Citation2000) with 8% acrylamide for the resolving gel and 5% for the stacking gel. Cell-free supernatants obtained after clarification of the MM medium at 4 °C were used as samples. Gels were run at 90 V for 1 h at 4 °C. To allow gelatine digestion, gels were incubated at 4 and 30 °C for 16 h in phosphate buffered saline (PBS). Gels were stained with Coomassie Brilliant Blue R-250 and the protease band(s) became clearly visible as unstained band(s) against a blue background.
In parallel, zymograms were also done running copolymerized gels as described above but supplemented with 2-mM phenylmethilesulfonylfluoride (PMSF) and further incubation with PBS buffer containing 2-mM PMSF, a serine protease inhibitor.
GenBank accession numbers
The 16S rDNA gene sequences of the Antarctic isolates were deposited in the NCBI's GenBank database and the following accession numbers were assigned: GU814620 (AU1), GU814621 (AU3), GU814622 (AU4), GU814623 (AU5), GU814624 (AU6), GU814625 (AU7), GU814626 (AU8), GU814627 (AU9), GU814628 (AU10), GU814629 (AU12), GU814630 (AU13), GU814631 (AU14) and GU814632 (AU15).
Results
Isolation and identification of extracellular protease-producing bacteria
After the isolation of 45 colonies on LB medium, 13 protease-producing bacteria on MM medium at 4 °C were found. These isolates were checked for growth on LB agar and for extracellular protease production on MM agar at both 4 and 30 °C. Interestingly, although 9 out of 13 isolates were able to grow at 30 °C, we did not find protease production at 30 °C by any isolate. Sequencing of approximately 1500 bp 16S rDNA allowed assessing the phylogenetic affiliation of isolates. The bacteria belonged to the classes Gammaproteobacteria (nine isolates matching the genus Pseudomonas, 97–99% nucleotide identity) and CFB group bacteria (four isolates matching the genus Flavobacterium with 97–98% identity) (). Alignment of 16S sequences among isolates belonging to the same genus showed 97–99% identity, except for AU14 and AU15 that had 100% identity suggesting that most isolates are different, a tentative result until conclusive identification using a polyphasic approach can be done.
Table 1. Identification of isolates, bacterial growth and extracellular protease production at different temperatures.
Growth rate
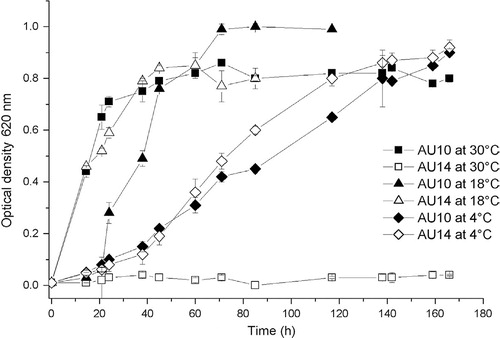
Bacterial growth at different temperatures (30, 18 and 4 °C) was monitored in liquid medium as described above ( shows representative curves; ). Pseudomonas isolates were able to grow at all the tested temperatures, showing a doubling-time on average of 3, 2 and 15 h at 30, 18 and 4 °C, respectively. Independently of the growth temperature, all these isolates reached a similar OD at stationary phase. Flavobacteria isolates did not grow at 30 °C but grew at 18 and 4 °C showing an average doubling-time of 13 and 25 h, respectively and, at the stationary phase, reached an OD similar to that of the Pseudomonas isolates.
Fig. 1. Growth curves for some of the extracellular protease producing isolates in Luria-Bertani broth medium at different temperatures. Values of 108–1010 CFU/ml of culture were achieved at stationary growth phase. Means and standard deviations (SD) were obtained from three separate measurements of a representative experiment. At least three experiments were done. Error bars (SD) were in some cases smaller than the size of the symbol.
Protease production at different temperatures in liquid medium
Proteolytic activity was demonstrated in liquid medium by coagulation of milk proteins and clarification of the milky white MM medium (a). In all cases, coagulation and clarification was evident at 18 and 4 °C after reaching the stationary phase. At this point, extracellular proteolytic activity was quantitatively determined in cell-free cultures using the method of Andrews & Asenjo (Citation1986) as described above (; see for quantitative values of proteolytic activity of cell-free supernatant obtained after growth at 4 °C). Although growth was detected (measured by CFU), coagulation and proteolytic activity were not found in liquid cultures of Pseudomonas isolates at 30 °C, even after two weeks of incubation as, demonstrated on MM plates.
Fig. 2. Evidence of proteolytic activity in liquid medium and zymogram analysis. (a) Appearance of liquid minimal milk (MM) medium before bacterial inoculation (on the left) and after bacterial growth (on the right; clarification of the milky-white MM medium). (b) Zymogram, analysis of cell-free supernatant of proteolytic isolates. Lane + is the positive control (the commercial protease Savinase from Novozymes, Bagsværd, Denmark). Isolates are indicated above the lanes. The negative picture of the electrophoretic gel is shown. (c) Identification of extracellular serine proteases. Polyacrylamide gels with and without phenylmethilsulfonylfluoride (PMSF) are shown. Isolates are indicated above the lanes.

Table 2. Effect of temperature on protease activity. Isolates were grown until clarification of the liquid minimal milk medium at 4 °C and the cell-free supernatants were used in protease activity assays at 4 and 30 °C. Means and standard deviations were obtained from three separate measurements of a representative experiment.
Effect of temperature on protease activity and stability
Isolates were grown at 4 °C until clarification of the liquid MM medium and cell-free supernatants were used in protease activity assays at 4 and 30 °C (). All isolates produced extracellular proteases active at both 4 and 30 °C and, in most cases, the activity was slightly higher at 30 °C than at 4 °C.
Interestingly, when the protease stability of cell-free supernatant obtained after growth at 4 °C was analysed at 4 and 30 °C, it was found that the extracellular protease maintained similar enzymatic activity even after 48 h incubation at either temperature (data not shown).
Protease zymogram and inhibition of proteolytic activity
To analyse whether the isolates produced different extracellular proteases, zymograms were performed as described above. In all cases, the analysis revealed the presence of only one band per isolate but with different relative electrophoretic mobilities between them. Similar zymograms were obtained at both temperatures of incubation (4 and 30 °C), suggesting that a single extracellular protease is secreted per isolate (b, showing representative isolates).
In order to select isolates producing extracellular serine-proteases, zymograms were performed as described above, adding PMSF to the polyacrylamide gel and to the PBS reaction buffer during the incubation step. Serine-proteases were identified by a decrease in band intensity in PMSF-polyacrylamide gels. Two Pseudomonas isolates were identified as extracellular serine-protease producers: AU5 and AU6 (c shows representative isolates).
Discussion
As part of a project aimed at the selection of cold-adapted bacteria expressing biotechnologically relevant enzymes, 13 microorganisms that grow and produce proteases at 4 °C were isolated from water samples collected at King George Island, South Shetlands. These microorganisms were identified as Pseudomonas and Flavobacterium strains. Isolation of cold-adapted protease-producing bacteria belonging to those genera from Arctic and Antarctic regions has been previously reported (Morita et al. Citation1998; Brambilla et al. Citation2001; Groudieva et al. Citation2004; Männistö & Häggblom Citation2006; Olivera et al. Citation2007; Liu et al. Citation2009).
Our Antarctic isolates produced active extracellular proteases on MM medium when growing at 4 and 18 °C but not at 30 °C, even though Pseudomonas isolates were able to grow at this high temperature. The temperature dependence of enzyme production during growth of Arctic and Antarctic isolates has been previously reported (Zeng et al. Citation2003; Groudieva et al. Citation2004). For example, Groudieva et al. (Citation2004) reported high levels of α-amylase and β-galactosidase production by Arctic bacterial isolates at growth temperatures between 4 and 10 °C but almost no production at higher temperatures (20–30 °C).
Results for all isolates revealed that each produced only one extracellular protease active at both 4 and 30 °C, showing thermal stability at 30 °C after 48 h of incubation. Olivera et al. (Citation2007) reported the identification of some Antarctic bacterial isolates that produce thermo-stable extracellular proteases when growing in a skim milk supplemented liquid medium like our MM medium. It is already known that low temperatures strongly reduce the rate of chemical reactions and that cold-adapted microorganisms maintain the rate of enzyme-catalysed reactions synthesizing cold-active, but heat-labile, enzymes (Feller & Gerday Citation2003). Kinetic and thermodynamic experiments as well as X-ray crystallography analysis support the weak conformational stability of cold-active enzymes. However, these experiments have been done using purified enzymes. Our study of protease stability, as well as the study by Olivera et al. (Citation2007), was done using the cell-free supernatant obtained after bacterial growth in a medium supplemented with skim milk.
The cell-free supernatant is a hydrolysate of milk proteins with a complex composition. It usually contains peptides of a wide range of molecular weights and even free amino acids (Neklyudov et al. Citation2000). Hydrolysis of peptide bonds causes changes in the properties of proteins including structural molecular changes that liberate the hydrophobic moiety of the protein molecule, exposing it on the surface of the oligopeptides formed during the process (Neklyudov et al. Citation2000). The interactions between the hydrophobic moieties and the enzymatic protein are crucial for cold-adapted enzyme stability (Marx et al. Citation2007). Thus, the milk hydrolysate environment is a complex medium with increased hydrophobicity that may favour protease stability. Interestingly, a review by Arnold et al. (Citation2001) describes directed evolution experiments with mesophilic subtilisin proteases in which they can evolve to thermo-stable and highly active enzymes at low temperature. Among other experiments, the introduction of a Pro residue into and extended loop of S41 (psychrophilic subtilisin from Antarctic Bacillus TA41) contributed to the enzyme's adaptation to high temperature, showing that minimal amino acid substitutions can generate large increases in thermo-stability (Arnold et al. Citation2001).
In their natural environment, bacteria and the extracellular enzymes they produce are surrounded by a heterogeneous physical/chemical environment that probably allows a wide range of enzyme stability. Microbial Antarctic mats are a major feature of waterbodies (lakes, meltwater ponds and gullies) and are established as endemic microbiota in Antarctica, making a significant contribution to carbon fixation (Laybourn-Parry & Pearce Citation2007). The mats create different internal chemical environments and are usually held together by slimy substances secreted by the microorganisms. In this scenario, bacteria trapped in the mat are enmeshed in a complex environment rich in polymeric substances where cold-active enzymes could be stabilized.
The activity and stability of the extracellular proteases at temperatures higher than 10 °C has no physiological meaning since the isolates do not have to deal with warmer environments on King George Island, Antarctica. We agree with Yu et al. (Citation2009), who indicated that the evolutionary progress of enzyme adaptation to cold temperatures is not consistent with that of bacterial physiology and metabolism; however, the entire environment must be taken into account. In the Antarctic environment, the organic matter in lakes and gullies plays a central role in the geochemical cycle of bioelements through their production, transformation, transport and/or decomposition. During the processes involved in carbon turnover, cold-adapted microorganisms play a fundamental role through the production of extracellular hydrolases. Among extracellular hydrolases, cold-active proteases participate in the carbon flux associated with mats in aquatic ecosystems. These systems show variations in physical and chemical conditions that fluctuate with the seasons. In this scenario, the production of a set of proteases active at a wide range of temperatures and possessing thermal stability constitutes an advantage for a bacterial community dealing with episodic changes in temperature and food supplies.
Future work will deal with identification of the serine-protease genes and their heterologous expression, biochemical characterization of the protein product and exploitation for potential biotechnological products.
Acknowledgements
This work was partially supported by Uruguay's Basic Science Development Programme (PEDECIBA) and the Uruguayan Antarctic Institute. Dr Valerie Dee revised linguistic aspects of the manuscript.
References
- Acevedo J.P. Reyes F. Parra L.P. Salazar O. Andrews B.A. Asenjo J.A. Cloning of complete genes for novel hydrolytic enzymes from Antarctic sea water bacteria by use of an improved genome walking technique. Journal of Biotechnology. 2008; 133: 277–286.
- Altschul S.F. Madden T.L. Schäffer A.A. Zhang J.H. Zhang Z. Miller W. Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997; 25: 3389–3402.
- Andrews B.A. Asenjo J.A. Synthesis and regulation of extracellular β (1–3) glucanase and protease by Cytophaga sp. in batch and continuous culture. Biotechnology and Bioengineering. 1986; 28: 1366–1375.
- Arnold F.H. Wintrode P.L. Miyazaki K. Genshenson A. How enzymes adapt: lessons from directed evolution. Trends in Biochemical Sciences. 2001; 26: 100–106.
- Brambilla E. Hippe H. Hagelstein A. Tindall B.J. Stackebrandt E. 16S rDNA diversity of cultured and uncultured prokaryotes of a mat sample from Lake Fryxell, McMurdo Dry Valleys, Antarctica. Extremophiles. 2001; 5: 23–33.
- Chakrabarti S.K. Matsumura N. Ranu R.S. Purification and characterization of an extracellular alkaline serine protease from Aspergillus terreus (IJIRA 6.2). Current Microbiology. 2000; 40: 239–244.
- Chun J. Lee J-H. Jung Y. Kim M. Kim S. Kim B.K. Lim Y.W. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. International Journal of Systematic and Evolutionary Microbiology. 2007; 57: 2259–2261.
- Feller G. Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nature Review Microbiology. 2003; 1: 200–208.
- Groudieva T. Kambourova M. Yusef H. Royter M. Grote R. Trinks H. Antranikian G. Diversity and cold-active hydrolytic enzymes of culturable bacteria associated with Arctic sea ice, Spitzbergen. Extremophiles. 2004; 8: 475–488.
- Gupta R. Beg Q.K. Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Applied Microbiology and Biotechnology. 2002; 59: 15–32.
- Kulakova L. Galkin A. Kurihara T. Yoshimura T. Esaki N. Cold-active serine alkaline protease from the psychrotrophic bacterium Shewanella strain ac10: gene cloning and enzyme purification and characterization. Applied Environmental Microbiology. 1999; 65: 611–617.
- Laybourn-Parry J. Pearce D.A. The biodiversity and ecology of Antarctic lakes: models for evolution. Philosophical Transactions of the Royal Society B. 2007; 362: 2273–2289.
- Liu Y. Yao T. Jiao N. Kang S. Huang S. Li Q. Wang K. Liu X. Culturable bacteria in glacial meltwater at 6,350 m on the East Rongbuk Glacier, Mount Everest. Extremophiles. 2009; 13: 89–99.
- Männistö M.K. Häggblom M.M. Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Systematic and Applied Microbiology. 2006; 29: 229–243.
- Marx J-C. Collins T. D'Amico S. Feller G. Gerday C. Cold-adapted enzymes from marine Antarctic microorganisms. Marine Biotechnology. 2007; 9: 293–304.
- Morgan-Kiss R.M. Priscu J.C. Pocock T. Gudynaite-Savitch L. Huner N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiology and Molecular Biology Reviews. 2006; 70: 222–252.
- Morita Y. Hasan Q. Sakaguchi T. Murakami Y. Yokoyama K. Tamiya E. Properties of a cold-active protease from psychrotrophic Flavobacterium balustinum P104. Applied Microbiology and Biotechnology. 1998; 50: 669–675.
- Neklyudov A.D. Ivankin A.N. Berdutina A.V. Properties and uses of protein hydrolysates. Applied Biochemistry and Microbiology. 2000; 36: 452–459.
- Nicholas K.B. Nicholas H.B. Jr. Deerfield D.W. II. GeneDoc: analysis and visualization of genetic variation. EMBNET. news. 1997; 4: 14–18.
- Niederberger T.D. McDonald I.R. Hacker A.L. Soo R.M. Barrett J.E. Wall D.H. Cary S.C. Microbial community composition in soils of northern Victoria Land, Antarctica. Environmental Microbiology. 2008; 10: 1713–1724.
- Olivera N.L. Sequeiros C. Nievas M.L. Diversity and enzyme properties of protease-producing bacteria isolated from sub-Antarctic sediments of Isla de Los Estados, Argentina. Extremophiles. 2007; 11: 517–526.
- Rao M.B. Tanksale A.M. Ghatge M.S. Deshpande V.V. Molecular and biotechnological aspects of microbial proteases. Microbiology and Molecular Biology Reviews. 1998; 62: 597–635.
- Sambrook J. Fritsch E.F. Maniatis T. Molecular cloning: a laboratory manual2nd edn. Cold Spring Harbor Laboratory. Cold Spring Harbor NY, 1989
- Thompson J.D. Higgins D.G. Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Research. 1994; 22: 4673–4680.
- Wang Q.-F. Hou Y.-H. Xu Z. Miao J.-L. Li G.-Y. Purification and properties of an extracellular cold-active protease from the psychrophilic bacterium Pseudoalteromonas sp. NJ276. Biochemical Engineering Journal. 2008; 38: 362–368.
- Wang Q.-F. Miao J.-L. Hou Y.-H. Ding Y. Wang G.-D. Li G.-Y. Purification and characterization of an extracellular cold-active serine protease from the psychrophilic bacterium Colwellia sp. NJ341. Biotechnology Letters. 2005; 27: 1195–1198.
- Yu Y. Li H. Zeng Y. Chen B. Extracellular enzymes of cold-adapted bacteria from Arctic sea ice, Canada Basin. Polar Biology. 2009; 32: 1539–1547.
- Zeng R. Zhang R. Zhao J. Lin N. Cold-active serine alkaline protease from the psychrophilic bacterium Pseudomonas strain DY-A: enzyme purification and characterization. Extremophiles. 2003; 7: 335–337.