Abstract
The ecosystems of the western Antarctic Peninsula, experiencing amongst the most rapid trends of regional climate warming worldwide, are important “early warning” indicators for responses expected in more complex systems elsewhere. Central among responses attributed to this regional warming are widely reported population and range expansions of the two native Antarctic flowering plants, Deschampsia antarctica and Colobanthus quitensis. However, confirmation of the predictions of range expansion requires baseline knowledge of species distributions. We report a significant southwards and westwards extension of the known natural distributions of both plant species in this region, along with several range extensions in an unusual moss community, based on a new survey work in a previously unexamined and un-named low altitude peninsula at 69°22.0′S 71°50.7′W in Lazarev Bay, north-west Alexander Island, southern Antarctic Peninsula. These plant species therefore have a significantly larger natural range in the Antarctic than previously thought. This site provides a potentially important monitoring location near the southern boundary of the region currently demonstrated to be under the influence of rapidly changing climate trends. Combined radiocarbon and lead isotope radiometric dating suggests that this location was most likely deglaciated sufficiently to allow peat to start accumulating towards the end of the 19th century, which we tentatively link to a phase of post-1870 climate amelioration. We conclude that the establishment of vegetation in this location is unlikely to be linked to the rapid regional warming trends recorded along the Antarctic Peninsula since the mid-20th century.
The Antarctic pearlwort Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) and the Antarctic hairgrass Deschampsia antarctica Desv. (Gramineae) are the only native flowering plants found on the Antarctic continent, where they are limited to the Antarctic Peninsula and Scotia arc archipelagos (South Shetland, South Orkney and, for D. antarctica only, South Sandwich Islands; ). Both have wider non-Antarctic distributions, extending through some sub-Antarctic islands and along the Andean mountain chain in South America (Convey Citation1996; Smith Citation2003; van de Wouw et al. Citation2007). Both species often co-occur, with D. antarctica usually being more abundant (Holtom & Greene Citation1967; Fowbert & Smith Citation1994). Although widely distributed in their Antarctic areas, they form only a minor component of the vegetation, which is dominated by cryptogams (bryophytes and lichens), and are usually encountered as scattered colonies rather than dense closed communities (Smith Citation1984Citation2003). However, the grass occasionally forms closed swards extending over several tens or hundreds of square metres, usually towards the north of its Antarctic range (Smith Citation2003). Both species are usually found below ca. 100 m a.s.l. in the Antarctic, although plants are occasionally encountered up to ca. 180–275 m a.s.l. (Smith Citation1982).
Fig. 1 General map of Antarctic Peninsula region and (inset) map of the region between Marguerite Bay and Alexander Island, indicating locations referred to in the text.
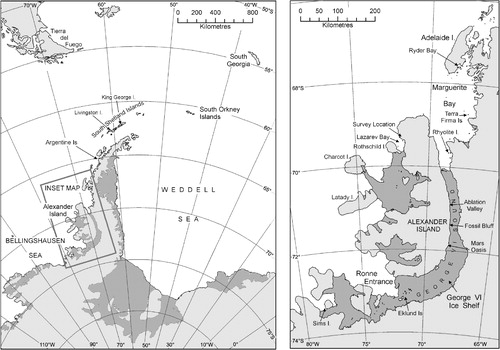
Over the last half-century, the rate of increase of mean annual air temperatures for the western Antarctic Peninsula has been amongst the most rapid anywhere in the world (Convey et al. Citation2009; Turner et al. Citation2009). This trend has been detected in instrumental data available since the 1960s as far south as Fossil Bluff (71°20′S 68°17′W) on the east coast of Alexander Island (Harangozo et al. Citation1997; , inset), and in a recent ice core record extending over the last century from the south-west Antarctic Peninsula (the Gomez ice core, 73°35′S 70°22′W; Thomas et al. Citation2009). Increases in the numbers and local extents of the two flowering plants along the Antarctic Peninsula and associated islands and archipelagoes are probably the best-known example of biological responses attributed to these climatic changes in the Antarctic (Fowbert & Smith Citation1994; Smith Citation1994; Grobe et al. Citation1997; Gerighausen et al. Citation2003). Population increases have been spectacular, for instance of more than two orders of magnitude in 30 years at the Argentine Islands between the 1960s and 1990 (Fowbert & Smith Citation1994), although with no further increase at this location between then and 2007/08 (Parnikoza et al. Citation2009). However, there is no evidence to date for southward expansion of the overall ranges of these species (Convey Citation2006), although this is a predicted consequence of environmental change (Walther et al. Citation2002; Convey Citation2003Citation2006; Convey & Smith Citation2006). These biological changes are interpreted as being underlain by increased temperature encouraging vegetative growth and spread of established plants, and increased probability of seedling establishment (Fowbert & Smith Citation1994; Smith Citation1994), increased success of sexual reproduction (Convey Citation1996), and increased recruitment by seed germination from dormant seed banks in the soils (McGraw & Day Citation1997; Ruhland & Day Citation2001).
To identify overall changes in range, it is axiomatic that good baseline knowledge of distributional limits is essential. This is currently not the case for the two Antarctic flowering plants or, indeed, for the typical cryptogamic communities of the region. In large part, the present gaps in distributional knowledge and uneven spatial survey coverage are a reflection of historical opportunities for survey and collection of material, and access to appropriate taxonomic expertise, in a region where fieldwork is highly constrained by logistic practicalities (e.g., Chown & Convey Citation2007). The current study took advantage of the first-ever survey opportunity to permit landings to be made on a previously known, but unvisited, isolated vegetated headland on north-west Alexander Island, west of the southern Antarctic Peninsula. Further, with the unexpected discovery of significant moss peat accumulation at this site, the opportunity was taken to apply radiometric dating techniques to material obtained in order to provide an estimate of the timing of exposure from ice cover at this location.
Methods
As part of a terrestrial biological survey of previously unexamined regions of the southern Antarctic Peninsula, an un-named low peninsula in Lazarev Bay on the north-west coast of Alexander Island (69°22.0′S 71°50.7′W) was visited for approximately 4 h on 17 February 2008 (Figs. 1, 2a). Lazarev Bay is itself uncharted, precluding access other than, as here, with the support of a marine survey vessel with ice-breaking and helicopter capability. This location has never been visited previously, although the presence of visible vegetated areas and birds had been noted during an overflight in December 1997. It consists of a low, east–west oriented headland, ca. 500 m×100 m, surrounded by 10-20-m cliffs and practically inaccessible from the sea. No specific geological data are available, but the area is included within the LeMay formation (Burn Citation1984).
Representative vegetation collections were made and samples of soil (ca. 10 g fresh weight from each of five locations) collected during the visit, and the presence and breeding status of birds noted. The vegetation collections included a single peat “monolith” of ca. 40 cm depth (to the underlying rock), removed from one of several peat accumulations under living Polytrichum strictum. Given that the previously known southern limit of such peat accumulations in the Antarctic Peninsula region is close to the Argentine Islands at about 65°S, over 500 km to the north (), it was felt that the removal of only one monolith was consistent with balancing the scientific benefit of such sampling with the clear environmental sensitivity of this exceptional and limited area location. Plant specimens were air dried on board ship before return to the UK and deposited in the UK Antarctic Herbarium, held at the British Antarctic Survey, Cambridge. The peat monolith was preserved at –20°C and returned to the UK frozen. To estimate the age of the peat accumulation, the monolith was sawn into two halves while still frozen, and sub-samples were taken from the newly exposed faces for radiocarbon (C-14) and unsupported lead-210 dating using standard analytical and calibration procedures (summarized in Tables 2, 3).
Results and discussion
The peninsula hosted a breeding population of 10–15 pairs of south polar skua (Catharacta maccormicki), with many chicks on the point of fledging and an undetermined number of non-breeding individuals.
The vegetation present was exceptional in three respects. First, it included well-developed populations of both species of Antarctic flowering plant (b, c), with individual plants of both species scattered across much of the area of its gently rolling surface, and closed grass swards of up to 5 m2 extent present in several shallow gullies and terraces on the northern side of the peninsula. These gullies and terraces contained non-contiguous mineral and organic deposits that constituted a thin (1–3 cm) soil with organic C content of 2.4% by mass (SD = 2.4%; n=5), total N content of 0.33% (SD = 0.26%; n=5), and pH of 6.9 (SD = 0.54; n=5). The swards were comparable in size with those on islands in Ryder Bay near Rothera Point, ca. 250 km to the north (Convey & Smith Citation1997). Second, there were several areas of significant peat bank development underlying the moss P. strictum (d). The isolation and potential sensitivity of this location prohibited extensive probing of these banks, but the monolith obtained confirmed a depth of at least 40 cm. Third, although relatively limited bryophyte diversity (15 species) was present, a number of the species identified are unusual at this latitude in the region, including five whose southern range limit is now extended to this location and seven that represent new species records for Alexander Island ().
Fig. 2 Flowering plants at the new southern limit of their global distribution. (a) Oblique aerial photograph of the un-named peninsula on north-west Alexander Island. (b) Example of a well-developed grass sward found during the survey. (c) Example of a mixed community with both species of flowering plant, including fruiting Colobanthus quitensis. (d) Polytrichum strictum peat bank of >30 cm depth.

Table 1 Bryophytes recorded from the un-named peninsula in Lazarev Bay, north-west Alexander Island, during the current survey.
Both flowering plants included individuals with abundant mature seed heads produced in the current and previous seasons. Previous Antarctic studies have shown that both species produce flowers in most years (Holtom & Greene Citation1967; Edwards Citation1974), although the seeds frequently do not develop to maturity. Our observations continue to confirm previous findings that mature seed can be formed at all locations within their Antarctic range. In the 1960s and early 1970s the proportion of years in which mature seed could be produced was small (Corner Citation1971; Greene & Holtom Citation1971; Edwards Citation1974). By the early 1990s, this proportion had increased considerably (Convey Citation1996), putatively linked with regional climate amelioration (Convey & Smith Citation2006). The presence of mature seed in both species, combined with that of a significant depth of accumulated moss peat, strongly suggests that this location provides particularly suitable micro-environmental conditions for their establishment and growth.
The timing of exposure of this location from ice cover during glacial retreat is unknown. Comparing the depth of peat and development of vegetation sward with other areas in Antarctica and the Arctic provides circumstantial evidence that plant communities could have been present for a considerable period. The base of moss peats of the same species of up to 2 m depth on Signy Island (60°S, South Orkney Islands) and 3 m on Elephant Island (62°S, South Shetland Islands) has been radiocarbon dated to ca. 5500 years before present (yr BP) (Fenton Citation1982; Björk et al. Citation1991). On the Fildes Peninsula, King George Island (South Shetland Islands) peat deposits have been dated at 3265 + 120 yr BP, with an annual growth rate of 0.195 mm yr–1 in their upper layers (Ye & Rongquan Citation1999). Peat beds beneath another commonly co-occurring moss, Chorisodontium aciphyllum (Hook. f. & Wils.) Broth., of 111 cm depth from Signy Island have a radiocarbon age of 1754 + 32 yr BP, equating to 0.63 mm yr–1 annual growth (E. Roads, pers. comm.).
Peat deposits of up to 40 cm depth from Arctic Svalbard (a location with a considerably warmer summer climate than anywhere in the maritime Antarctic) have been dated at 300–1300 years (van der Knaap Citation1991). Several estimates of growth rate based on morphological measurements made in the top few centimetres of moss banks are also available for P. strictum from sub-Antarctic and Antarctic locations (Longton & Greene Citation1967; Longton Citation1970). South Georgia (sub-Antarctic, 54°S) data indicate a shoot growth rate range of 2–8 mm y–1, most commonly 3–5 mm y–1, while rates decreased considerably with increasing latitude in the more extreme conditions of the maritime Antarctic. Using these previous estimates of growth and accumulation rates, the depth of the P. strictum banks on north-west Alexander Island would represent an age range between a minimum of 50–200 years BP (based on sub-Antarctic growth rates) and 630–2050 years BP (based on Signy Island and South Shetland Islands rates).
Quantitative radiocarbon (C-14), Pb-210, and Cs-137 dating data (–, ) suggest growth rates of 1–4 mm y–1 for Lazarev Bay P. strictum. On average, these are consistent with sub-Antarctic growth rates, if slightly slower, rather than those previously published from the maritime Antarctic. This is again consistent with this location experiencing a particularly favourable microclimate for plant growth. Although Pb-210 concentrations were an order of magnitude lower than expected, and mostly below the detection limit, ages calculated using the constant rate of supply (CRS) model for Pb-210 to the surface are broadly consistent, if slightly “older” than the radiocarbon “bomb” calibrated dating data, at least in the upper 13 cm (, ).
Fig. 3 Summary of radiocarbon (14C) and lead isotope (210Pb) chronological data for the Polytrichum strictum peat monolith obtained from Lazarev Bay. Radiocarbon data have depth errors of ±0.25 cm; Pb-210 depth errors are ±1 cm; methods and calibration as described in Tables 2 and 3.
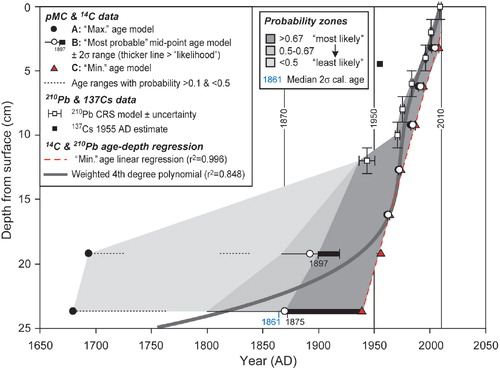
Table 2 Radiocarbon age data for the Lazarev Bay Polytrichum strictum peat monolith. Summary of methods: samples of P. strictum were removed from seven points in the frozen peat below the green zone at ca. 2 cm depth using sterile disposable scalpels. Samples were sent to Beta Analytic (Miami, FL) for accelerator mass spectrometry (AMS) radiocarbon dating, where standard radiocarbon dating procedures were used. Samples were gently crushed and dispersed in de-ionized water, followed by a hot HCl acid wash and a NaOH alkali wash to remove carbonates and secondary organic acids, with a final acid rinse to neutralize the resulting solution; absolute percentage of modern carbon (pMC) data were corrected according to 13C/12C isotopic ratios from measured pMC, where the “modern” (i.e., 1950) pMC value is 100 and the present day (2010) pMC value is 107.5.
The Pb-210 chronology indicates a possible increase in the rate of peat accumulation since ca. 1943, when accounting for compaction perhaps as much as 3.7 mm y–1 over the last decade. However, this interpretation should be treated with caution given the uncertainties on the low excess Pb-210 massic activities measured. The mean accumulation rate for the profile based on Pb-210 age alone is estimated to be 2.5 mm y–1. The Cs-137 data show some inconsistency with the CRS Pb-210 and radiocarbon age estimates (, –). The Cs-137 concentration was greatest in the 0–6 cm layer and below detection limits beyond 10 cm depth. If related to nuclear weapons testing in the 1950s, it suggests accumulation of around 6 cm in 60 years, or 1 mm y–1.
As Pb-210 and Cs-137 data are at or near detection limits and Cs-137 is less mobile in frozen soil compared to saturated organic soils, we consider the radiocarbon chronology to be more reliable overall. Radiocarbon “bomb calibrated” dating data indicate that the age of all material above 16 cm is firmly post-1960 AD. The only traditional radiocarbon age obtained suggests 24 cm depth could be as old as ca. 1800 AD, which is broadly consistent with extrapolated Pb-210 age estimate. Due to the nature of the current radiocarbon calibration curve in this time period, we cannot rule out ages as old as ca. 1800 AD or as young as ca. 1954 AD (). Since the conventional radiocarbon age at 24 cm depth is 100±40 years () and its median calibrated C-14 age is 1861 AD (), we consider the “most likely” colonization age to be post ca. 1870 AD (, ). Overall, growth rates from the most probable C-14 age range of 1870–2005 AD average ca. 1.5 mm y–1, consistent with, but lower than the Pb-210 based estimate, and slightly faster than the Cs-137 based growth rates.
Table 3 Calibrated radiocarbon age data for the Lazarev Bay Polytrichum strictum peat monolith. Calibration: samples were calibrated using CALIBomb SH1, a compilation of Southern Hemisphere data sets (Hua & Barbetti Citation2004), from absolute percentage of modern carbon (pMC) data, corrected according to 13C/12C isotopic ratios from measured pMC, and where the “modern” (i.e., 1950) pMC value is 100 and the present day (2010) pMC value is 107.5. Radiocarbon age data were initially calibrated using the CALIB v6 SHCAL04 Southern Hemisphere atmosphere data set. This is applicable directly to samples <1175 14C yr BP and has an average offset of 43±13 years from the INTCAL09 data set. For samples >ca. 1 ky BP, a random effects model is used to account for variation in this offset through time compared to the INTCAL09 calibration data set (McCormac et al. Citation2004; Reimer et al. Citation2009; http://calib.qub.ac.uk/calib/calib.html). Calibrated ages have been rounded to the nearest year, which may be too precise; hence, likely ages have been rounded to the nearest five years in the discussion. Results from the INTCAL09 calibration were compared to the CALIB v6 SHCAL04. The latter has fewer data points for the last 500 years and, therefore, produces a broader age range of 1805–1954 with a median age of 1861 AD and probability p=0.842 for the LAZ23.5–24 sample compared to the INTCAL09 data set; hence, we consider INTCAL09 is better able to determine the most likely age range of this sample, but have retained both for completeness. The 1951–1954 age range for the LAZ23.5–24 sample is not considered likely in the INTCAL09 data set. Data in boldface are considered “most likely”; calibrated age ranges not in stratigraphic order (and therefore on the “incorrect” side of the “bomb” C-14 peak), and pMC calibration data with probabilities less than 0.1 have been omitted for clarity; where shown, the figure in brackets is the probability excluding calibrated ages which are out of stratigraphic order; combined errors on growth rates are typically 5–10%; + indicates date of sample collection, not date of measurement. Age model A contains the "most probable" age; B is a maximum age model; C is a minimum age model.
Therefore, in conclusion, combined radiocarbon and lead isotope radiometric dating suggests that this location was most likely deglaciated sufficiently to allow peat to start accumulating at least towards the end of the 19th century. We tentatively link initial peat colonization in Lazarev Bay to a phase of post-1870 climate amelioration. Initially, growth rates were ca. 1–2 mm y–1, with a more sustained period of growth with enhanced growth rates of ca. 3–4 mm y–1 in the latter decades of the 20th century (), perhaps related to the well-documented warming trend established across the Antarctic Peninsula since ca. 1950 AD (Bentley et al. Citation2009).
Table 4 Pb-210 and Cs-137 count and dating data. Samples were extracted from 2 cm depth intervals, dried at 80°C and ground/homogenized. Samples were palletized and standard laboratory efficiency calibrations used, derived from NPL RO8–04 Mixed Radionuclide Spike and ISOTRACK RBZ-B44. All absolute efficiency calibrations have been corrected for variations in sample density and thickness. All testing was performed at room temperature and tested against international standard reference materials. Unsupported Pb-210 estimates are derived from the constant rate of supply (CRS) method (Appleby & Oldfield Citation1978).
The previously reported global southern limit to the distribution of both flowering plant species is the Terra Firma Islands, southern Marguerite Bay (68°43′S 67°31′W; , inset) (Smith Citation1982; Smith & Poncet Citation1987), where there is low-lying, ice-free ground with adequate liquid water during the summer. Further south, there are relatively few areas of ice-free ground at low altitudes that might potentially host these species. Neither species is recorded on Rhyolite Island, the southernmost partially ice-free island in the entrance to George VI Sound (69°40′S, 68°35′W). Between 71 and 72°S, there are several relatively benign ice-free locations between Ablation Valley and Mars Oasis along the south-eastern coast of Alexander Island adjacent to the George VI Ice Shelf, where environmental conditions permit the development of bryophyte communities that are exceptional for the latitude, but neither higher plant is currently found at these locations (Smith Citation1988; Convey & Smith Citation1997). Similarly, neither is present on Charcot Island, the only biologically surveyed ice-free location west of northern Alexander Island (Convey et al. Citation2000). The bryophyte community of Charcot Island, in an analogous fashion to that recorded here, consists of an unusual combination of species, with several at each location also representing their current known southern distributional limit. These unusual species assemblages most likely are indicative of the stochastic consequences of rare colonization events at these extremely isolated locations.
A recent molecular phylogeographic study of D. antarctica in the maritime and sub-Antarctic (van de Wouw et al. Citation2007) supported the hypothesis that maritime Antarctic populations had established and differentiated following post-Pleistocene glacial retreat and exposure of ice-free habitats. Edwards (Citation1972), working in the South Orkney Islands, considered that D. antarctica colonized new sites primarily by vegetative dispersal, often assisted by birds, particularly through nesting brown skuas fragmenting tillers during nest construction. Fowbert & Smith (Citation1994) proposed that colonization by C. quitensis is likely to be via seeds. Given the geographical isolation of the newly discovered site on north-west Alexander Island, and the presence of a significant skua breeding population, it is plausible that skuas were responsible for the initial dispersal of both species to this location.
Clearly, there remains a possibility of further extension to the known range in this largely unsurveyed and unknown region. However, no other comparable low-lying, ice-free areas were apparent during flights over the land surrounding the study location and uncharted islands in Lazarev Bay, and in particular no significant concentrations of skuas or other birds. Thus, while further small plant populations may be present at locations elsewhere around Lazarev Bay, their presence would only marginally alter the newly described distributional limits. At larger scale, it is unlikely that they occur elsewhere on northern and central Alexander Island, other than the parts of the coast of Lazarev Bay described here, because these areas are extremely mountainous and heavily glaciated with little exposure of ice-free ground other than steep cliffs. The peninsulas of western Alexander Island and Latady Island are covered by permanent ice piedmonts, which cannot support a terrestrial flora. Progressing southwards, the next low altitude, ice-free land encountered is on the Eklund Islands (Ronne Entrance, 73°09′S 71°50′W) and Sims Island (Carroll Inlet, English Coast, 73°21′S 78°19′W; , inset). Both have breeding skua populations. Parts of the Eklund Islands were examined on 12 February 2008, finding very limited moss but no flowering plants, and well-developed if low diversity lichen communities. Sims Island, which has a much larger ice-free exposure, was overflown on 13 February 2008, but the density of breeding birds (south polar skuas and Adélie penguins) meant that no landing could be made. Although isolated by a considerable distance from north-west Alexander Island, Sims Island would appear to offer the next and most southern realistic possibility in this region for locating significant populations of either the two higher plants or cryptogamic communities.
The presence of closed grass swards and significant peat accumulation supports their presence at this site for an extended period. We conclude that the establishment of these plant communities on north-west Alexander Island likely predates the initiation of the current warming trends along the Antarctic Peninsula, and thus is not indicative of a response to recent climate warming in the region, even though significantly extending southwards and westwards the distributions of these higher plants and peat-forming moss. Although still in practical terms very inaccessible, this site also provides a potentially important monitoring location near the southern boundary of the region currently demonstrated to be under the influence of rapidly changing climate.
Acknowledgements
We thank the Captain and Ship's Company of HMS Endurance, particularly the flight and hydrographic survey personnel. R.I.L. Smith commented on an earlier manuscript version. We thank C. Ruhland and an anonymous referee for their encouraging and constructive suggestions. Figure 1 was prepared by P. Fretwell. DWH was funded under the Natural Environment Research Council Antarctic Funding Initiative (NE/D00893X/1). This paper contributes to the research programmes Polar Science for Planet Earth (British Antarctic Survey) and Evolution and Biodiversity in Antarctica (Scientific Committee on Antarctic Research). We thank R. Ochyra for bryophyte identification, S. Bradley for assistance with the 210Pb and 137C counting and P.G. Dennis for the soil analyses. Plant specimens are deposited with the Antarctic Herbarium, held at the British Antarctic Survey, Cambridge, UK.
References
- Appleby P.G. Oldfield F. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena. 1978; 5: 1–8.
- Bentley M.J. Hodgson D.A. Smith J.A. Cofaigh C.O. Domack E.W. Larter R.D. Roberts S.J. Brachfeld S. Leventer A. Hjort C. Hillenbrand C.D. Evans J. Mechanisms of Holocene palaeoenvironmental change in the Antarctic Peninsula region. The Holocene. 2009; 19: 51–69.
- Björk S., Malmer.N., Hjort C., Sandgren P., Ingólfsson O., Wallén B., Smith R.I.L. & Jónsson, B.L. 1991. Stratigraphic and palaeoclimatic studies of a 5500-year-old moss bank on Elephant Island, Antarctica. Arctic and Alpine Research 23, 361–374..
- Burn R.W. The geology of the LeMay Group, Alexander Island. British Antarctic Survey Scientific Reports 109. British Antarctic Survey. Cambridge, 1984
- Chown S.L. Convey P. Spatial and temporal variability across life's hierarchies in the terrestrial Antarctic. Philosophical Transactions of the Royal Society of London, Series B. 2007; 362: 2307–2331.
- Convey P. Reproduction of Antarctic flowering plants. Antarctic Science. 1996; 8: 127–134.
- Convey P. Maritime Antarctic climate change: signals from terrestrial biology. Antarctic Peninsula climate variability: historical and palaeoenvironmental perspectives. Domack E. et al.. American Geophysical Union. Washington D.C., 2003; 145–158.
- Convey P. Antarctic climate change and its influences on terrestrial ecosystems. Trends in Antarctic terrestrial and limnetic ecosystems: Antarctica as a global indicator. Bergstrom D.M. et al.. Springer. Dordrecht, 2006; 253–272.
- Convey P. Bindschadler R.A. di Prisco G. Fahrbach E. Gutt J. Hodgson D.A. Mayewski P. Summerhayes C.P. Turner J. Antarctic climate change and the environment. Antarctic Science. 2009; 21: 541–563.
- Convey P. Smith R.I.L. The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander Island, maritime Antarctic. Antarctic Science. 1997; 9: 12–26.
- Convey P. Smith R.I.L. Responses of terrestrial Antarctic ecosystems to climate change. Plant Ecology. 2006; 182: 1–10.
- Convey P. Smith R.I.L. Peat H.J. Pugh P.J.A. The terrestrial biota of Charcot Island, eastern Bellingshausen Sea, Antarctica: an example of extreme isolation. Antarctic Science. 2000; 12: 406–413.
- Corner R.W.M. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv: IV. Distribution and reproductive performance in the Argentine Islands. British Antarctic Survey Bulletin. 1971; 26: 41–50.
- Edwards J.A. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv: V. Distribution, ecology and vegetative performance on Signy Island. British Antarctic Survey Bulletin. 1972; 28: 11–28.
- Edwards J.A. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv: VI. Reproductive performance on Signy Island. British Antarctic Survey Bulletin. 1974; 39: 67–86.
- Fenton J.H.C. The rate of peat accumulation in Antarctic moss banks. Journal of Ecology. 1982; 68: 211–228.
- Fowbert J.A. Smith R.I.L. Rapid population increase in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arctic and Alpine Research. 1994; 26: 290–296.
- Gerighausen U. Bräutigam K. Mustafa O. Peter H.-U. Expansion of vascular plants on an Antarctic island—a consequence of climate change?. Antarctic biology in a global context. Huiskes A.H.L. et al.. Backhuys Publishers. Leiden, 2003; 79–83.
- Greene D.M. Holtom A. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv. III. Distribution, habitats and performance in the Antarctic botanical zone. British Antarctic Survey Bulletin. 1971; 26: 1–29.
- Grobe C.W. Ruhland C.T. Day T.A. A new population of Colobanthus quitensis near Arthur Harbor, Antarctica: correlating recruitment with warmer summer temperatures. Arctic and Alpine Research. 1997; 29: 217–221.
- Harangozo S.A. Colwell S.R. King J.C. An analysis of a 34-year air temperature record from Fossil Bluff (71°S 68°W), Antarctica. Antarctic Science. 1997; 9: 355–363.
- Holtom A. Greene S.W. The growth and reproduction of Antarctic flowering plants. Philosophical Transactions of the Royal Society of London, Series B. 1967; 252: 323–337.
- Hua Q. Barbetti M. Review of tropospheric bomb C-14 data for carbon cycle modeling and age calibration purposes. Radiocarbon. 2004; 46: 1273–1298.
- Longton R.E. Growth and productivity of the moss P. alpestre Hoppe. in Antarctic regions. Antarctic ecology. Holdgate M.W. Academic Press. London, 1970; 818–837.
- Longton R.E. Greene S.W. The growth and reproduction of Polytrichum alpestre Hoppe on South Georgia. Philosophical Transactions of the Royal Society of London Series B. 1967; 232: 295–322.
- McCormac F.G. Hogg A.G. Blackwell P.G. Buck C.E. Higham T.F.G. Reimer P.J. SHCal04 Southern Hemisphere calibration, 0-11.0 cal kyr BP. Radiocarbon. 2004; 46: 1087–1092.
- McGraw J.B. Day T.A. Size and characteristics of a natural seed bank in Antarctica. Arctic and Alpine Research. 1997; 29: 213–216.
- Parnikoza I. Convey P. Dykyy I. Trakhimets V. Milinevsky G. Tyschenko O. Inozemtseva D. Kozeretska I. Current status of the Antarctic herb tundra formation in the central Argentine Islands. Global Change Biology. 2009; 15: 1685–1693.
- Reimer P.J. Baillie M.G.L. Bard E. Bayliss A. Beck J.W. Blackwell P.G. Bronk Ramsey C. Buck C.E. Burr G.S. Edwards R.L. Friedrich M. Grootes P.M. Guilderson T.P. Hajdas I. Heaton T.J. Hogg A.G. Hughen K.A. Kaiser K.F. Kromer B. McCormac F.G. Manning S.W. Reimer R.W. Richards D.A. Southon J.R. Talamo S. Turney C.S.M. van der Plicht J. Weyhenmeyer C.E. IntCal09 and Marine09 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon. 2009; 51: 1111–1150.
- Ruhland C.T. Day T.A. Size and longevity of seed banks in Antarctica and the influence of ultraviolet-B radiation on survivorship, growth and pigment concentrations of Colobanthus quitensis seedlings. Environmental and Experimental Botany. 2001; 45: 143–154.
- Smith R.I.L. Farthest south and highest occurrences of vascular plants in the Antarctic. Polar Record. 1982; 21: 170–173.
- Smith R.I.L. Terrestrial plant biology of the sub-Antarctic and Antarctic. Antarctic ecology. Laws R.M. Academic Press. London, 1984; 1: 61–162.
- Smith RI.L. Bryophyte oases in ablation valleys on Alexander Island, Antarctica. Bryologist. 1988; 91: 45–50.
- Smith R.I.L. Vascular plants as indicators of regional warming in Antarctica. Oecologia. 1994; 99: 322–328.
- Smith R.I.L. The enigma of Colobanthus quitensis and Deschampsia antarctica in Antarctica. Antarctic biology in a global context. Huiskes A.H.L. et al.. Backhuys Publications. Leiden, 2003; 234–239.
- Smith R.I.L. Poncet S. Deschampsia antarctica and Colobanthus quitensis in the Terra Firma Islands. British Antarctic Survey Bulletin. 1987; 74: 31–35.
- Thomas E.R., Dennis P.F., Bracegirdle T.J. & Franzke C. 2009. Ice core evidence for significant 100-year regional warming on the Antarctic Peninsula. Geophysical Research Letters 36, L20704, doi: 10.1029/2009GL040104..
- Turner J., Bindschadler R. Convey P., di Prisco G., Fahrbach E., Gutt J. Hodgson D., Mayewski P. & Summerhayes C. (eds.) 2009. Antarctic climate change and the environment. Cambridge: Scientific Committee on Antarctic Research..
- Walther G.-R. Post E. Convey P. Parmesan C. Menzel M. Beebee T.J.C. Fromentin J.-M. Hoegh-Guldberg O. Bairlein F. Ecological responses to recent climate change. Nature. 2002; 416: 389–395.
- van der Knaap W.O. Palynology of peat sections from Spitsbergen covering the last few centuries. Nordic Journal of Botany. 1991; 11: 213–223.
- van de Wouw M. van Dijk P. Huiskes A.H.L. Regional genetic diversity patterns in Antarctic hairgrass (Deschampsia antarctica Desv.). Journal of Biogeography. 2007; 35: 365–376.
- Ye Z. Rongquan L. 14C dating of moss peat banks in Fildes Peninsula, Antarctica. Chinese Science Bulletin. 1999; 44: 1817–1819.